Abstract
Objective
To compare the image quality of computed tomography pulmonary angiography (CTPA) obtained with the injection of various low doses of contrast medium (CM) with different injection-related factors.
Methods
A total of 90 patients (42 females, 48 males; 54.3 ± 18.6 years) undergoing CTPA were included. Three CM protocols, each containing 30 patients, were created. Protocols 1, 2 and 3 consisted of a CM of 60 ml, 55 ml and 50 ml, and a bolus trigger level of 120 HU, 90 HU and 75 HU, respectively. Injection was uniphasic for protocols 1 and 2 (flow rate 5 ml/s), and biphasic for protocol 3 (flow rates 5 and 4 ml/s); with saline flushing afterwards. Enhancement was measured in three central and six peripheral pulmonary arteries.
Results
The mean attenuation value for pulmonary arteries was over 250 HU for all protocols. There was no difference between the attenuation levels with the protocols (p > 0.05). The percentage of pulmonary arteries exceeding optimal attenuation (≥250 HU) showed that protocols 2 and 3 were 90–100% successful (p < 0.05).
Conclusion
The use of proper injection-related factors during CTPA, such as a low trigger level and a high flow rate with saline injection following a decreased CM volume (55 ml or 50 ml), will enable adequate pulmonary artery contrast enhancement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography pulmonary angiography (CTPA) is a routine technique used safely for the diagnosis of patients suspected of having pulmonary embolism [1–3]. The purpose of this technique is to determine an attenuation difference between the pulmonary artery and the thrombus to ensure that the thrombus is directly visible by increasing the enhancement in the pulmonary arteries by means of contrast medium (CM). An adequate amount of contrast enhancement in the pulmonary arteries is therefore essential for the success of CTPA and is affected by many factors such as the CT device, CM injection-related factors and volume [1, 4–13]. One of the goals during CTPA is to achieve maximum enhancement using minimum CM, as this can help reduce CM-induced side effects as well as prevent the economic burden of this increasingly popular method [14].
Our hypothesis in this prospective study was that optimal enhancement of the pulmonary arteries can be achieved by decreasing the CM volume used in CTPA acquired with 64-slice CT scanner. Therefore, we created three different CM protocols, each containing different injection-related factors suitable for decreased CM volumes in the CTPA performed with 64-slice CT. We then investigated the effects on enhancement and optimal enhancement both in the central and peripheral pulmonary arteries, and the effects on the global image quality.
Materials and methods
Patient populations
A total of 90 patients (22 female, 48 male; mean age, 54.3 ± 18.6 years) who applied to our CT unit with suspected pulmonary embolism and underwent CTPA between January 2009 and July 2009 were included. Known allergy to contrast medium, renal insufficiency, pregnancy and age under 18 years were considered contraindications for participating in the study. Before the examination, informed consent was obtained from the participants of this prospective study that had the approval of the Institutional Review Board.
CTPA
All imaging was performed by 64-slice CT (Aquillion 64, Toshiba Medical Systems, Otowara, Japan) with the automatic dose modulation technique (Real Exposure Control, Toshiba Medical System). Patients were examined in a supine position with both arms extended above the head. The CTPA was obtained in the caudocranial direction and imaging length ranged from the level of the diaphragm to the lung apices. A standard collimation of 0.5 mm was used with a gantry rotation speed of 0.5 s, and a pitch factor of 0.8. For post-processing, thin-slice reconstruction was performed with a slice thickness of 0.5 mm, and an increment of 0.3 mm.
CM administration
Following the CM with a concentration of 300 mg iodine per millilitre administration, 20-ml saline injection was performed by a dual-head power injector. The type (peripheral or central venous line) and side (left or right arm) of the peripheral venous access site were noted. The imaging delay for the individual contrast optimisation was based on a bolus tracking technique (Sure Start, Toshiba Medical System) in the main pulmonary artery (MPA) using different trigger levels for each protocol. For this purpose, a single unenhanced low-dose CT was first obtained at the MPA level for use as the reference image. A region of interest (ROI) was then set in the lumen of the MPA (by a CT technologist) on the basis of this image. A series of dynamic low-dose monitoring images were obtained to measure early enhancement 2 s after initiation of the CM injection. The patient was instructed to hold his/her breath as soon as the signal density in this ROI reached exact threshold values (bolus trigger time) and the patient was prepared for the CT data acquisition while the CT table moved. This transit time took 2–4 s depending on the distance needed for the CT table to move from the level used for bolus tracking. Finally, the delay before initiating the CT data acquisition (which is from the start of the CM injection to the start of the diagnostic investigation) was calculated as the sum of the bolus trigger time plus the transit time, and the total imaging time to cover the entire thorax was calculated using the delay period plus the duration of the CT data acquisition.
The patients were allocated into three groups and a different CM protocol (1, 2 or 3) was implemented in each group of 30 patients (Table 1).
Image interpretation
Quantitative CT image analysis
All CT were loaded on a clinical workstation (Vitrea 2 version 4.0 Workstation, Toshiba Medical Systems, Japan) for the post-processing of the volumetric MSCT data sets. Measurements were performed by a radiologist (S.U.R. with 10 years’ experience in interpreting CT images of the chest), who was blinded to the patient groups.
Imaging length was determined by the distance between the table positions of the first and the last slices. Quantitative analysis was performed with ROI measurements in the axial MIP images at 0.5-mm slice thickness after investigating thrombus presence in the central and segmentary pulmonary arteries. Attenuation measured along the following vessels: central pulmonary arteries [MPA, right pulmonary artery (RPA), left pulmonary artery (LPA)], peripheral pulmonary arteries [right upper lobe artery (RULA), right middle lobe artery (RMLA), right lower lobe artery (RLLA), left upper lobe artery (LULA), left lingular artery (LLA), left lower lobe artery (LLLA)], ascending aorta and subclavian vein.
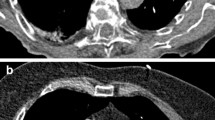
An ROI cursor covering nearly the whole vessel diameter in the axial images (Fig. 1) was placed by a radiologist (S.U.R.). The mean attenuation was recorded for each measurement. Measurement was limited to unaffected vessels if pulmonary embolism was present. It has been reported that the optimal attenuation value should exceed 250 HU in the MPA to achieve diagnostic adequacy in a CTPA [4]. The success of the attenuation values of three central and six segmentary pulmonary arteries in exceeding 250 HU was, therefore, investigated in order to objectively analyse the adequacy of each protocol. While performing this comparison, individual analyses were performed for each of the three central pulmonary arteries and a total comparison was made by adding those with attenuation values exceeding 250HU and those with attenuation values lower than 250 HU for each protocol in six segmentary pulmonary artery branches.
Qualitative CT image analysis
A subjective evaluation was performed by two radiologists (P.N.K. with 15 years’ experience in chest CT and S.U.R.). The radiologists had no previous knowledge of the injection protocol used and they independently graded the studies for perivenous artefacts and subjective image quality.
Evaluation of the perivenous artefacts associated with dense CM was performed in the superior vena cava (SVC) on axial MIP images with 0.5-mm slice thickness. For evaluation, perivenous artefacts adjacent to the SVC were graded using a four-point scale: grade 0 (no artefact), grade 1 (negligible artefacts), grade 2 (moderate artefacts but all vessel portions diagnostic), and grade 3 (severe or extensive artefacts that might lead to misinterpretation in some vessel portions).
Final subjective global image quality with regard to pulmonary vessel contrast was based on axial and coronal MIP images with a slice thickness of 10 mm. A three-point scale (1 non-diagnostic, 2 diagnostic and 3 excellent) was applied for evaluation of subjective image quality (Fig. 2).
Statistical analysis
Statistical analysis was performed using the SPSS for Windows, version 15.0, statistical package. Relations between the categorical variables were analysed with the chi-square test. Age, weight, and difference in the imaging lengths between the protocols were detected with one-way analysis of variance following Tukey’s HSD test. Pearson’s correlation coefficient was used to determine the association between the continuous variables. The relationship between the attenuation values and the protocols (adjusted by weight) was investigated with multivariate analysis of covariance. Interobserver agreement was verified with Spearman’s correlation coefficient. A p value less than 0.05 was considered significant.
Results
CTPA was performed without complications in all patients, and none of the studies had to be repeated.
Patient characteristics
Demographic characteristics of the patients are listed in Table 2. Mean imaging delay time, mean imaging duration, and mean total imaging time of the protocols are shown in Table 3. CM venous access was obtained from the peripheral venous line [from the right arm in 77 patients and left arm in 11 patients] in 88 (97.8%) patients while it was achieved from the central catheter in two (2.2%) patients. Pulmonary embolism was present in 12 patients (13.3%), while it was not detected in 78 patients (86.7%).
No difference was detected between the patients in the three protocols in terms of age, sex, venous CM access, the side the access was achieved if it was performed via the peripheral venous route, imaging length, and thrombus presence (Table 2). However, the mean weight of the patients in the third group was lower than that of the other two groups (p = 0.001). Therefore, we compared the differences in attenuation values in the measured vessels among the protocols taking weight into account.
Image interpretation
Quantitative CT image analysis
Mean attenuation values of the measured pulmonary arteries, ascending aorta and subclavian vein are summarised in Table 4. Mean pulmonary artery attenuation values were well over 250 HU in all three protocols. No difference was detected among the three groups when mean attenuation values in each protocols were compared taking weight into consideration (p > 0.05).
The weight of the patient was found to be an effective parameter for enhancement in all pulmonary arteries (p < 0.007) and it was inversely proportional to the enhancement levels (r = –0.36, p < 0.001). However, other characteristics of the patients, such as age, sex, location of venous access, imaging length and thrombus presence, did not have any effect on enhancement in the pulmonary arteries (p > 0.05). CM protocol type, or the other demographic characteristics of the patients did not have any effect on the enhancement of the ascending aorta and the subclavian vein (p > 0.05).
The success rates when the criterion to get an adequate CTPA was accepted as achieving an attenuation level of 250 HU or over for the pulmonary arteries are summarised in Table 5. The higher number of patients displaying optimal enhancements in MPA, RPA and LPA in protocols 2 and 3 was statistically significant (p = 0.024, p = 0.006 and p = 0.010, respectively). When peripheral pulmonary arteries were examined, a total of 180 segmentary pulmonary artery attenuation values were measured in each protocol. Areas of optimal enhancement in the segmentary arteries were found in 148 (82%), 169 (94%) and 173 (96%) patients in protocols 1, 2 and 3, respectively. The higher optimal enhancement rates in protocols 2 and 3 were statistically significant (p < 0.001) (Table 5).
Qualitative CT image analysis
No interobserver difference was detected between the two investigators in terms of SVC perivenous artefact and subjective image quality (r = 0.72 and r = 0.63, respectively; p < 0.001).
Perivenous artefact of SVC could not be evaluated in two subjects in protocol 3 because of central venous catheter usage. A perivenous artefact was observed in 24 (80%), 24 (80%) and 26 (90%) patients in protocols 1, 2 and 3, respectively (Table 6). However, none of these artefacts affected the evaluation of the pulmonary artery. No statistical difference was detected among the three groups in the comparison performed for perivenous artefact (p = 0.090).
None of the examinations was evaluated as non-diagnostic in the subjective global image quality evaluation (Table 7). Image quality was evaluated as diagnostic in 21 (23%) patients and excellent in 69 (77%) subjects. Subjective image quality was better in protocols 2 and 3 than in protocol 1 (p = 0.009).
Discussion
CTPA has now become an examination with high sensitivity and specificity that is widely used in patients with suspected pulmonary embolism [1, 2, 15–17]. The conspicuity of the thrombus during evaluation of CTPA depends on the density difference between the thrombus and the contrast-enhanced pulmonary artery. The significance of the enhancement level in the pulmonary arteries has, therefore, led to the requirement for creating various CTPA protocols according to CT technology [5, 7–9, 16]. The CTPA protocols in the literature generally belong to the 16-slice and older CT scanners and these protocols cannot be transferred directly to examinations performed with 64-slice CT. The short imaging duration of CTPA with 64-slice CT (4.4–5.7 s in our study) indicates the requirement for arranging the CM protocols accordingly. Additionally, the common view of the authors is that 64-slice CT examination can be performed using less CM without compromising arterial enhancement, although there are limited experiments with 64-slice CT [4, 6].
Various patient-related factors, such as weight, age, sex and imaging length, were investigated by different authors [4, 6, 15, 16, 18–20]. Similar to our study, enhancement in the pulmonary arteries was found to be negatively affected by the patient’s weight in most of these studies [4, 6, 16, 18, 21]. The effect of patient age on the pulmonary arterial enhancement is controversial and this effect has not been demonstrated in many studies including ours [4, 6]. Similar to our study, an affect of sex and imaging length on the pulmonary arterial enhancement is not found in most studies [4, 6, 18, 19].
CM injection-related factors are another group that may affect pulmonary arterial enhancement [5, 19] The level of enhancement is determined by the number of iodine molecules given to the patient in a second, indicated by the flow rate rather than the iodine concentration of CM [16, 18, 19]. In general, researchers found that a flow rate of 4 ml/s (3–5 ml/s) was sufficient for CTPA [4, 6, 14, 18, 22–24]. Increasing the flow rate shortens the time required to reach optimal enhancement in the pulmonary arteries [5, 16]. This requires the CTPA to be performed faster. Kim et al. [25] demonstrated that higher peak contrast enhancements in pulmonary arteries are achieved but the contrast enhancement plateau does not last long when using higher flow rates of CM, and capturing this period with the CT is more difficult [16]. However, capturing this short period has become easier with the use of the bolus tracking method in 64-slice CT. Consequently, adequate pulmonary arterial enhancements for CTPA were achieved in each protocol with high flow rate as a result of the imaging in our study.
Multiphasic injection protocols help uniform aortic contrast enhancement in CT angiography [5, 26, 27]. The aim of using the biphasic injection in protocol 3 was to provide homogeneous enhancement in the pulmonary arteries and prolong the injection time, because of the risk of the short injection time of 50 ml compared with the imaging duration. The fact that enhancement of the pulmonary arteries was greatest in protocol 3 can be related to achieving homogeneous enhancement in both central and peripheral pulmonary arteries with biphasic injection.
If the imaging delay time is not suitable, no matter at what flow rate CM was administered, it will either cause the CT to start before or after adequate CM reaches the pulmonary arteries. As a result, it is concluded that determining this time individually would be more suitable, as the time differs according to the CT technology, CM flow rate, and from person to person [5, 16, 18, 28]. The bolus-tracking technique is one of the methods developed to determine this time [16, 17, 29]. The bolus trigger level varies, depending on the CT used for CTPA, such as 100 HU in four-slice CT [19], 100–150 HU in 16-slice CT, and 150 HU in 64-slice CT [4]. The reason for raising the trigger level as CT technology advances is to allow the imaging to start later in order to achieve maximal enhancement time because of the reduction in the imaging duration of the CT [4, 28]. However, pulmonary arterial attenuation levels well over 250 HU were achieved with lower trigger levels (120, 90, and 75 HU) in our study. We believe these areas of high enhancement were achieved by using a combination of low trigger levels and high flow rates in the CTAPs performed with 64-slice CT. Therefore, the CT data acquisition is often complete before most of the administered CM has left the pulmonary system and passes into the systemic circulation with this technique in our study. The fact that the mean ascending aorta attenuation was lower than the pulmonary artery attenuation in each of the three protocols supports this theory.
The CM volume used for CTPA has been reported to be 80–150 ml [4, 6, 14]. When we consider that the imaging duration drops below 5 s in examinations performed with 64-slice CT, reducing the CM volume is essential in CTPA. Therefore, a decrease of 50–60% was achieved in the iodine dose in our study. Performing a saline flush following the CM pushes the CM located in the upper arm and SVC towards the heart and a more effective use of the CM is, therefore, achieved with this method [5, 14, 30–32]. In our study, saline flush was performed following the CM injection in all protocols. Moreover, high density levels in the subclavian vein in all three groups indicate that CM passage continues in the subclavian vein during the CTPA. The CM volume can be reduced even more, therefore, when using 64-slice CT for CTPA.
Various authors have assumed different values, such as 200 HU, 250 HU and 300 HU, for optimal enhancement levels for CTPA to be an adequate examination [4–6, 18]. All three protocols produced adequate results when the optimal pulmonary artery enhancement limit was 250 HU. Protocols 2 and 3 were more successful in achieving optimal enhancements in pulmonary arteries compared with protocol 1. This result indicates that preferring protocols 2 or 3 in CTPA performed with 64-slice CT will increase the success of the imaging regarding pulmonary artery enhancement. In parallel to this finding, the subjective image qualities of protocols 2 and 3 were better than that of protocol 1.
Saline injection helps not only in reducing the CM volume but also decreases the perivenous artefact around the SVC. We detected no difference among the three protocols in terms of the artefacts observed around the SVC. This finding, which is slightly higher than in other similar studies, may be related to the short imaging delay time [5, 30–33].
Conclusion
In conclusion, in our experience the use of proper injection-related factors during CTPA with 64-slice CT, such as a low trigger level (90 HU or 75 HU) and a high flow rate (5 ml/s, or 5 ml/s and 4 ml/s) with 20-ml saline injection following a decreased CM volume (55 ml or 50 ml) will enable adequate pulmonary artery contrast enhancement in the central and peripheral arteries. Using these two protocols for CTPA examination helps reduce CM side effects, such as allergic reactions and renal toxicity, and the costs of the examination.
References
Raptopoulos V, Boiselle PM (2001) Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. Radiology 221:606–613
Schoepf UJ, Holzknecht N, Helmberger TK et al (2002) Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology 222:483–490
Hogg K, Brown G, Dunning J et al (2006) Diagnosis of pulmonary embolism with CT pulmonary angiography: a systematic review. Emerg Med J 23:172–178
Bae KT, Tao C, Gürel S et al (2007) Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology 242:582–589
Lee CH, Goo JM, Lee HJ, Kim KG, Im J, Bae KT (2007) Determination of optimal timing window for pulmonary artery MDCT angiography. AJR Am J Roentgenol 188:313–317
Schoellnast H, Deutschmann HA, Berghold A, Fritz GA, Schaffer GJ, Tillich M (2006) MDCT angiography of the pulmonary arteries: influence of body weight, body mass index, and scan length on arterial enhancement at different iodine flow rate. AJR Am J Roentgenol 187:1074–1078
Yankelevitz DF, Shaham D, Shah A, Rademacker J, Henschke CI (1998) Optimization of contrast delivery for pulmonary CT angiography. Clin Imaging 22:398–403
Washington L, Gulsun M (2003) CT for thromboembolic disease. Curr Probl Diagn Radiol 32:105–126
Schoepf UJ, Costello P (2004) CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology 230:329–337
Qanadli SD, Hajjam ME, Mesurolle B et al (2000) Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 217:447–455
Ghaye B, Szapiro D, Mastora I et al (2001) Peripheral pulmonary arteries: how far in the lung does multi-detector row spiral CT allow analysis? Radiology 219:629–636
Remy-Jardin M, Tillie-Leblond I, Szapiro D et al (2002) CT angiography of pulmonary embolism in patients with underlying respiratory disease: impact of multislice CT on image quality and negative predictive value. Eur Radiol 12:1971–1978
Tilie-Leblond I, Mastora I, Radenne F et al (2002) Risk of pulmonary embolism after a negative spiral CT angiogram in patients with pulmonary disease: 1-year clinical follow-up study. Radiology 223:461–467
Kubo S, Tadamura E, Yamamuro M et al (2006) Thoracoabdominal-aortoiliac MDCT angiography using reduced dose of contrast material. AJR Am J Roentgenol 187:548–554
Andreou AK, Curtin JJ, Wilde A, Clark A (2008) Does pregnancy affect vascular enhancement in patients undergoing CT pulmonary angiography? Eur Radiol 18:2716–2722
Schaefer-Prokop C, Prokop M (2008) CTPA for the diagnosis of acute pulmonary embolism during pregnancy. Eur Radiol 18:2705–2708
U-King-Im JM, Freeman SJ, Boylan T, Cheow HK (2008) Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur Radiol 18:2709–2715
Roggenland D, Peters SA, Lemburg SP, Holland-Letz T, Nicolas V, Heyer CM (2008) CT angiography in suspected pulmonary embolism: impact of patient characteristics and different venous lines on vessel enhancement and image quality. AJR Am J Roentgenol 190:W351–W359
Arakawa H, Kohno T, Hiki T, Kaji Y (2007) CT pulmonary angiography and CT venography: factors associated with vessel enhancement. AJR Am J Roentgenol 189:156–161
Hartmann IJ, Lo RT, Bakker J, de Monye W, van Waes PF, Pattynama PM (2002) Optimal scan delay in spiral CT for the diagnosis of acute pulmonary embolism. J Comput Assist Tomogr 26:21–25
Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic contrast medium enhancement at CT. Part 1. Prediction with a computer model. Radiology 207:647–655
Eyer BA, Goodman LR, Washington L (2005) Clinicians’ response to radiologists’ reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. AJR Am J Roentgenol 184:623–628
Revel MP, Petrover D, Hernigou A, Lefort C, Meyer G, Frija G (2005) Diagnosis pulmonary embolism with four-detector row helical CT: prospective evaluation of 216 outpatients and inpatients. Radiology 234:265–273
Schoep UJ, Holzknechnt N, Helmberger TK et al (2002) Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. Radiology 222:483–490
Kim T, Murakami T, Takahashi S et al (1998) Effects of injection rates of contrast material on arterial phase hepatic CT. AJR Am J Roentgenol 171:429–432
Fleischmann D, Rubin GD, Bankier AA, Hittmair K (2000) Improved uniformity of aortic enhancement with customized contrast medium injection protocols at CT angiography. Radiology 214:363–371
Bae KT, Tran HQ, Heiken JP (2000) Multiphasic injection method for uniform prolonged vascular enhancement at CT angiography: pharmacokinetic analysis and experimental porcine model. Radiology 216:872–880
Remy-Jardin M, Mastora I, Remy J (2003) Pulmonary embolus imaging with multislice CT. Radiol Clin North Am 41:507–519
Cademartiri F, Nieman K, van der Lugt A et al (2004) Intravenous contrast material administration at 16–detector row helical CT coronary angiography: test bolus versus bolus-tracking technique. Radiology 233:817–823
Haage P, Schmitz-Rode T, Hubner D, Piroth W, Gunther RW (2000) Reduction of contrast material dose and artifacts by a saline flush using a double power injector in helical CT of the thorax. AJR Am J Roentgenol 174:1049–1053
Hopper KD, Mosher TJ, Kasales CJ, TenHave TR, Tully DA, Weaver JS (1997) Thoracic spiral CT: delivery of contrast material pushed with injectable saline solution in a power injector. Radiology 205:269–271
Schoellnast H, Tillich M, Deutschmann MJ, Deutschmann HA, Schaffler GJ, Portugaller HR (2004) Aortoiliac enhancement during computed tomography angiography with reduced contrast material dose and saline solution flush: influence on magnitude and uniformity of the contrast column. Invest Radiol 39:20–26
Schoellnast H, Deutschmann HA, Fritz GA, Stessel U, Schaffler GJ, Tillich M (2005) MDCT angiography of the pulmonary arteries: influence of iodine flow concentration on vessel attenuation and visualization. AJR Am J Roentgenol 184:1935–1939
Acknowledgements
This study was presented as a scientific exhibit at ECR 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uysal Ramadan, S., Kosar, P., Sonmez, I. et al. Optimisation of contrast medium volume and injection-related factors in CT pulmonary angiography: 64-slice CT study. Eur Radiol 20, 2100–2107 (2010). https://doi.org/10.1007/s00330-010-1782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1782-y