Abstract
Objectives
To evaluate dose reduction and image quality of 80-kV CT pulmonary angiography (CTPA) reconstructed with knowledge model–based iterative reconstruction (IMR), and compared with 100-kV CTPA with hybrid iterative reconstruction (iDose4).
Materials and methods
One hundred and fifty-one patients were prospectively investigated for pulmonary embolism; a study group of 76 patients underwent low-kV setting (80 kV, automated mAs) CTPA study, while a control group of 75 patients underwent standard CTPA protocol (100 kV; automated mAs); all patients were examined on 256 MDCT scanner (Philips iCTelite). Study group images were reconstructed using IMR while the control group ones with iDose4. CTDIvol, DLP, and ED were evaluated. Region of interests placed in the main pulmonary vessels evaluated vascular enhancement (HU); signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated.
Results
Compared to iDose4-CTPA, low-kV IMR-CTPA presented lower CTDIvol (6.41 ± 0.84 vs 9.68 ± 3.5 mGy) and DLP (248.24 ± 3.2 vs 352.4 ± 3.59 mGy × cm), with ED of 3.48 ± 1.2 vs 4.93 ± 1.8 mSv. Moreover, IMR-CTPA showed higher values of attenuation (670.91 ± 9.09 HU vs 292.61 ± 15.5 HU) and a significantly higher SNR (p < 0.0001) and CNR (p < 0.0001).The subjective image quality of low-kV IMR-CTPA was also higher compared with iDose4-CTPA (p < 0.0001).
Conclusions
Low-dose CTPA (80 kV and automated mAs modulation) reconstructed with IMR represents a feasible protocol for the diagnosis of pulmonary embolism in the emergency setting, achieving high image quality with low noise, and a significant dose reduction within adequate reconstruction times(≤ 120 s).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a potentially life-threatening condition and is responsible for significant morbidity and mortality in adults. Prompt diagnosis is crucial to avoid rapid hemodynamic compromise and death in some patients. The clinical presentation in patients with acute PE varies considerably, and therefore, the diagnosis relies heavily upon clinical suspicion and imaging findings [1] Figs. 1, 2, 3 and 4.
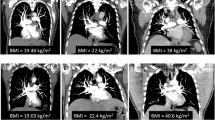
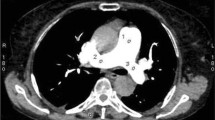
55-Year-old man (a) and a 76-year-old female (b), both underwent CT pulmonary angiography study for suspected pulmonary embolism. A, Axial images acquired on a 256-slice low-kV CTPA and reconstructed with IMR show a mean attenuation value of 1062 HU in the main trunk of pulmonary artery; B, standard-dose 256-slice CT scan reconstructed with hybrid iterative reconstruction (iDose4, Philips Healthcare, Cleveland, OH, USA) shows a mean attenuation value of 497 HU in the main trunk of the pulmonary artery. The image reconstructed with IMR shows better delineation of vascular structure and more homogenous opacification than iDose4; the image quality is expressed by evaluation of HU standard deviation demonstrating a lower value on IMR (18.7 HU) in comparison with iDose4 (28.9)
62-Year-old man with known advanced rectal cancer and massive pulmonary embolism and multiple metastatic lung lesions. a–b Axial images acquired on a 256-slice low-kV CTPA and reconstructed with IMR show a mean attenuation value of 490 ± 10.7 HU in the common pulmonary artery, with multiple emboli in both pulmonary arteries. c Coronal reconstruction of the same patient shows an extensive filling defect in the right pulmonary artery
65-Year-old man suspected for pulmonary embolism that underwent CT pulmonary angiography. a Axial CTPA scan of the thorax reconstructed with IMR shows a mean attenuation value in the main pulmonary trunk of 579.2 ± 8.4 HU. No filling defects are evident. b Axial image with lung windowing shows the high details of IMR algorithm in the assessment of lung parenchyma, demonstrating emphysematous alterations along both lungs. c Coronal reconstruction of the same patient does not show any filling defect
Boxplots in which boundary of boxes closest to zero indicates 25th percentile, line within boxes marks median, and boundary of boxes farthest from zero indicates 75th percentile. Error bars below and above boxes indicate minimum and maximum values, respectively. These boxplots show the comparison of the mean attenuation values in the common pulmonary artery (a) and mean background noise (b) for low-kV CT reconstructed with IMR and standard-dose reconstructed with iDose4
CT pulmonary angiography (CTPA) is currently the first-line imaging procedure for patients suspected of having pulmonary embolism (PE), enabling with thin-section reconstructions accurate analysis of peripheral pulmonary arteries and optimized vessel enhancement by using bolus tracking; these practices have led to a constant increase in the diagnostic accuracy of pulmonary CT angiography in the detection of PE.
As the use of CT angiography has increased in both routine and emergency care, patient exposure to ionizing radiation has increased dramatically [2,3,4]; therefore, various dose-reduction strategies have been implemented in CTPA examinations so far. Apart from the use of noise filters [5] and a higher pitch [6], radiation dose savings could be achieved by a reduction in either kilovoltage (kV) or milliampere second (mAs) settings. Previous studies reported the feasibility of scan protocols with lowered tube current times or a decrease in tube voltage to 100 or 80 kV without losing diagnostic accuracy in PE evaluation [7, 8].
However, reductions in radiation dose are hindered by increased image noise and degraded image quality, mainly as a result of limitations of the standard filtered back-projection (FBP) reconstruction algorithm currently used on most CT systems. For these reasons, in last years, new ways to reduce the dose were applied in clinical practice, mainly represented by iterative reconstruction algorithm (IR).
Unlike conventional FBP, which is based on simpler mathematical assumptions of the tomographic imaging system, IR generates a set of synthesized projections by accurately modeling the data collection process in CT. Adaptive statistical iterative reconstruction (ASIR) and Hybrid Iterative Reconstruction (HIR) are examples of this approach [9,10,11,12,13,14,15,16,17,18].
To date, new reconstruction algorithm was introduced in order to further reduce the radiation dose maintaining the same diagnostic quality images.
The recently developed model-based iterative reconstruction is a much more complex and advanced IR technique than ASIR and HIR [19, 20]. Model-based iterative reconstruction algorithm (IMR) is a particularly computationally intensive algorithm that generates images from raw data and omits back-projection routines entirely. Whereas FBP and most available variations of IR make several ideal assumptions during the reconstruction process such as a point-like focal spot at the anode, a pencil beam, an ideal detector, and a perfect noise sample, IMR adapts the realistic x-ray source dimensions, beam characteristics, detector characteristics, and noise variations, resulting in substantially reduced noise profiles. This modeling may also improve other aspects of image quality such as spatial resolution, low contrast detectability, and edge sharpness [21,22,23,24].
The purpose of the study was to determine whether a model-based iterative reconstruction (IMR) technique maintains high diagnostic image quality, with reduction of the radiation dose delivered to the patient, compared with hybrid iterative reconstruction algorithm (iDose4) in patients undergoing computed tomography pulmonary angiography, using a 256-row multidetector CT respectively at 80 kVp and 100 kVp.
Materials and methods
Study population
This prospective clinical study was performed in a single university center and approved by the Ethical Committee of our Institutional Review Board and for each participant, a written consent was obtained. From February to April 2017, a total of 151 consecutive patients, investigated for pulmonary embolism by the emergency medicine department of our hospital, underwent a 256-MDCT CTPA study. We randomly divided patients in two groups, 76 patients (study group; 40 males; mean age 66.87 ± 12.65 years, range 41–89 years, mean BMI 23.2 ± 1.3) were evaluated using a 256-MDCT (Philips iCT Elite, 80 kV). A control group of 75 patients (38 males; mean age 67.68 ± 11.38 years, range 34–80 years, mean BMI 22.7 ± 1.2) was evaluated using a different 256-MDCT scanner (Philips Brilliance iCT), with different kV settings (100 kV). In the study group, a model-based iterative reconstruction algorithm (IMR, Philips Healthcare, Cleveland, OH, USA) was used to process raw images data, instead of the control group for whom we used a hybrid iterative reconstruction (iDose4, Philips Healthcare, Cleveland, OH, USA) algorithm. Due to the emergency nature of the examination, the only exclusion criteria for contrast-enhanced CT evaluation was a severe renal failure (eGFR < 30 mL/min/1.73m2) or other contraindications for iodinated contrast material, such as previous allergic reaction.
CT angiography protocol
Study group patients underwent a 256-MDCT scan examination of the thorax (iCT Elite, Philips Medical Systems, Best, Netherlands) using a low-dose protocol with the following scan parameters: thickness 0.8 mm, increment 0.4 mm, collimation 128 × 0.625, pitch 0.17, rotation time 0.27 s, FOV 350, matrix 512 × 512, mean reconstruction time 1.5 min, tube voltage 80 kV, and automated tube current modulation (Table 1). Control group patients were analyzed with a different 256-MDCT scan examination of the same anatomic region (Brilliance iCT, Philips Medical Systems, Best, Netherlands) with the following scan parameters: thickness 1 mm, increment 0.5 mm, collimation 64 × 0.625, pitch 0.765, rotation time 0.33 s, FOV 350 mm, matrix 512 × 512, mean reconstruction time 1 min, tube voltage 100 kV, and automated tube current modulation (Table 1). In each patient, an 18-gauge intravenous catheter was placed in an antecubital vein in the upper limb, and contrast media (CM) were injected using a double-syringe injector (Medrad Stellant, Pittsburgh, PA, USA). In order to obtain an optimal intra-luminal contrast enhancement, the start of scanning in both groups was individually obtained for each patient by using bolus tracking (B-T) technique, with a trigger level of 120 HU and a delay time between 4 to 6 s. The trigger area was manually placed at the main pulmonary artery. All scans were obtained in a single breath-hold. In each patient of both groups, low contrast media volume (50 mL; Xenetix 350, 350 mgI/mL, Guerbet Aulnay, France) was administered, with a flow rate of 3.5 mL/s followed by saline flushing (50 mL; flow rate 3.5 mL/s).
Image analysis
Images were analyzed on a dedicated workstation (Brilliance Workspace, Philips Healthcare, Cleveland, OH, USA) by two radiologists with 2 and 10 years’ experience in CTPA who performed qualitative evaluations, in a blinded manner, and measured the vessel contrast enhancement (mean attenuation value, HU) in the axial images, by manually placing a circular region of interest (ROI) in the center of vascular lumen at three different levels along z-axis in the thoracic study as follows: (1) main pulmonary artery, (2) right pulmonary artery, (3) left pulmonary artery. The ROI’s size was as large as possible depending on artery size.
Three ROIs were also drawn in each side of the air surrounding the patient (outside the patient) to assess background noise. These ROIs were sized around 3 cm2 to cover as much air as possible, without including external objects surrounding the patient. The mean values of the three measurements served as background noise (mean background noise).
Two more ROIs (2 cm2) were drawn in the paraspinal and subscapular muscle, in order to evaluate the muscle attenuation (mean muscle attenuation).
The image quality and the visualization of each segment of the major pulmonary branches were evaluated using a 4 points subjective scale [7]: 4 was regarded as “Excellent” (no artifacts, no motion blur, and excellent visualization of boundary and segmental branches); 3 as “good”(good visualization of the boundary, minor artifacts especially in small segmental branches, and good visualization of lobar branches); 2 as “acceptable” (delineation of the boundary equivocal but within an acceptable range, minor artifacts, and sufficient visualization of segmental branches); 1 as “low” (major artifacts, high noise, and unsatisfactory delineation of the boundary or poor visualization of lobar and segmental branches). The mean HU values (mean artery attenuation) of the three ROIs placed in the pulmonary vessels and in the two muscles served to calculate the signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR):
Evaluation of segmental and subsegmental branches was based on axial images only. We used a CT angiography window setting (width, 450 HU; level, 100 HU) and a lung window setting (width, 1500 HU; level - 500 HU) for this analysis [25]. We used the standard nomenclature outlined by Boyden [26] and Jackson and Huber [27] to identify segmental arteries. This nomenclature assigns ten segmental arteries on the right and nine segmental arteries on the left. The medial and anterior segments of the left lower lobe originating from a common trunk were considered as one segmental branch, and the apical and the posterior segmental arteries of the left upper lobe were considered as two different branches. Thus, a total of 19 segmental arteries could be expected per patient if no anatomic variant was present. For the subsegmental arteries, we refrained from using the standard nomenclature because anatomic variations are quite common at this level [27]. Instead, we determined the number of subsegmental arteries individually for each segment. To be rated as analyzable, an artery had to be displayed in the CT angiography window setting with a level of contrast enhancement that was considered high enough to enable identification or ruling out of a pulmonary embolus. Segmental or subsegmental vessels that contained emboli according to the criteria defined by Remy-Jardin and coauthors [28] were thus rated as analyzable. Segmental and subsegmental vessels that showed no intravascular filling defects were only counted as analyzable if they could be depicted from their origin to the next dichotomous branching. To analyze the radiation dose exposure, the CT dose-length product (DLP, mGy × cm) and the CT dose index (CTDIvol, mGy) were recorded for all scans. The effective dose (ED) was also calculated using a DLP conversion factor of 0.014 mSv∕mGy × cm. This value is applicable for chest examinations and is the average between male and female models, as reported in the literature [29].
Statistical analysis
All statistical analysis was performed using commercially available software (Med Calc, Med Calc Software 14.8.1, Mariakerke, Belgium). The Mann-Whitney U test was used to evaluate differences between the study group (80 kV, 50 mL, IMR) and control group (100 kV, 50 mL, iDose4) in terms of age, mean attenuation values of the pulmonary artery (HU), SNR, and CNR. Using the same test, we also compared the radiation dose exposure (CTDI, DLP, and ED) and image quality scores, between the two groups. A p value < 0.05 was considered statistically significant. The inter-observer agreement of mean attenuation values, SNR, and CNR was calculated using Intraclass Correlation Coefficient (ICC). Additionally, the inter-observer agreement of image quality scores was determined by reliability statistics using ICC based on the Alpha (Cronbach) model. Agreement less than 0.61 was considered moderate, between 0.61 and 0.81 good and more than 0.81 excellent, respectively.
Results
Patient demographics
The study group included 40 men and 36 women with a mean age of 66.87 ± 12.85 years and a mean BMI of 23.2 ± 1.3. The control group included 38 men and 37 women with a mean age of 67.68 ± 11.38 years and a mean BMI of 22.7 ± 1.2. No significant differences in terms of age range and BMI were found between the two groups.
Radiation dose
In the study group, we obtained a significantly lower CTDIvol (6.41 ± 0.84 mGy vs 9.68 ± 3.54 mGy) and DLP (248.24 ± 3.2 mGy × cm vs 352.48 ± 3.59 mGy × cm) than that of control group protocols (Table 2) with a 51.1% decrease in CTDIvol and a 41.9% decrease in DLP with low-dose CT.
The estimated effective dose calculated in the study group (3.48 ± 1.2 mSv) shows a 41.6% radiation dose reduction compared with the one of the control group (4.93 ± 1.8 mSv), p < 0.0001 (Table 2).
Image analysis
Low-kV IMR-CTPA showed a mean attenuation value of 670.91 ± 9.09 HU in the common pulmonary trunk, while a mean value of 292.61 ± 15.5 HU was found using iDose4-CTPA (Table 3). Furthermore, image noise was significantly less using IMR algorithm (p < 0.0001), with a lower mean background noise level of 6 ± 1 HU compared to the iDose4 technique (10 ± 4).
Accordingly, CNR was significantly higher for IMR compared with iDose4 (p < 0.0001) with an average CNR nearly6.5 times higher using IMR (CNR = 58.9 ± 18.13) than iDose4 (CNR = 9.19 ± 4.34), and so was the SNR (mean value of 61.6 ± 15.1 vs 18.73 ± 9.84) (Table 3).
The subjective image quality of all patients was considered by both readers as diagnostic, with a mean value of 3.28 ± 0.79 using IMR compared to iDose4 (2.62 ± 0.72).
The inter-observer agreement was excellent for both routine iDose4-CTPA and low-dose IMR-CTPA, with kappa values of 0.921 and 0.865, respectively.
Analysis of segmental and subsegmental arteries
A total of 2812 segmental arteries (study group: 1425; control group: 1387) were analyzed.
One patient in the study group had undergone right lobectomy. In the control group, one patient had undergone left upper lobe resection and one patient had undergone left pneumonectomy.
The percentage of segmental arteries that were considered to have sufficient quality for assessment of PE did not significantly differ between the study group (mean, 93% ± 13; median, 95%; with 50% within the range of 90–100%) and the control group (mean, 86% ± 11; median, 95%; with 50% within the range of 83–94%) (p = 13, Mann-Whitney U test).
A total of 5438 subsegmental arteries were evaluated (2813 in the study group and 2625 in the control group). The number of evaluable subsegmental pulmonary arteries in the study group (mean, 71% ± 17; median, 72%; with 50% within the range of 59–84%) was significantly higher (p < 0.001, Mann-Whitney U test) than in the control group (mean, 55% ± 15; median, 53%; with 50% within the range of 43–70%).
Discussion
In the last decade, several strategies have been proposed for effectively decreasing radiation exposure during chest examinations, including modulation of tube current (mAs), kilovoltage (kV), scan distance, shielding, while sophisticated technical devices, including modulation of tube current relative to the geometry and attenuation of the scanned object, have been introduced into clinical practice.
Indeed, current strategies mainly focus on optimizing tube current and kV by adapting it to the patient’s weight [30, 31].
In addition, IR algorithms have led to increased SNR, CNR, and subjective improvements in image quality while significantly reducing radiation dose. Model-based iterative reconstruction (IMR) is a model-based fully iterative algorithm with additional edge-preserving regularization to improve contrast resolution, and permits a pronounced noise reduction and increase in CNR, overcoming that of HIR (4). Its recent introduction has led to some great achievements in obtaining virtually “noise-free” images [32, 33].
In this prospective study of 151 patients, image quality characteristics of 80-kV chest CT reconstructed with IMR and 100-kV chest CT reconstructed with iDose4 were compared.
The most important drawback of the low-tube-voltage technique is the increased image noise when keeping the tube current at similar levels as in high-tube-voltage protocols. Actually, we observed significant improvements in image noise and streak artifacts reduction using IMR (mean background noise: 6 ± 1) instead of iDose4 (mean background noise: 10 ± 4), with a better overall image quality (3.28 ± 0.79 vs 2.62 ± 0.72).
Moreover, the combination of 80 kVp and IMR allowed an improvement in iodine signal, with a mean attenuation value in the common pulmonary trunk of 670.91 ± 9.09 HU vs 292.61 ± 15.5 HU using iDose4 algorithm at 100 kVp. Significantly higher attenuation values were also found in the left pulmonary artery (595.4 ± 9.44 HU vs 290.17 ± 17.5 HU) and in the right pulmonary artery (589 ± 10.25 HU vs 294.77 ± 21.33 HU), using low-dose IMR-CTPA.
SNR and CNR were significantly higher on the low-kV IMR-CTPA (SNR: 61.6 ± 15.1 CNR: 58.9 ± 18.13) than on the iDose4-CTPA protocol (SNR: 18.73 ± 9.84; CNR: 9.19 ± 4.34).
Improved visualization of small peripheral pulmonary arteries was achieved even though we used a low-dose protocol that reduced radiation exposure to the patient. Although we found no significant impact of the kilovoltage on the visualization of the segmental arteries (93% with 80 kV vs 86% with 100 kV), a significant improvement was seen for the delineation of the subsegmental arteries, in fact with 100 kV, only 55% of subsegmental arteries were rated as analyzable, versus 71% with the 80-kV protocol.
Furthermore, we observed that IMR algorithm permits to decrease the tube voltage and tube current in comparison with iDose4, enabling a significant reduction of radiation exposure in terms of CTDIvol (6.41 ± 0.84 vs 9.68 ± 3.5 mGy), DLP (248.24 ± 3.2 vs 352.4 ± 3.59 mGy × cm), and ED (3.48 ± 1.2 vs 4.93 ± 1.8 mSv) while providing CTPA images with a higher diagnostic quality.
Compared with iDose4-CTPA, there was a 51.1% decrease in CTDIvol and a 41.9% decrease in DLP with low-dose CT.
In addition, reconstruction time less than 3 min for the majority of the reference protocols, and applicability to non-gated and gated acquisitions, enables routine clinical use of IMR across a broad range of patients.
In the series from Zamboni et al. [34], they observed a significant increase in vascular enhancement and in noise values in the vessels comparing a low-dose kV CTPA (80 kV) with a standard 120-kV CTPA, with a mean vascular enhancement value (HU) in the main pulmonary trunk of 571.63 ± 239.33 HU vs 345.12 ± 95.72 HU.
They also measured a significantly higher mean noise value (HU) in the main pulmonary trunk using the low-kV CTPA protocol (36.61 ± 13.41 HU), compared with the standard-dose CTPA (21.63 ± 8.76 HU) with a slightly better image quality in the 120 kV scan compared with the low-dose scan, due to the lower noise level.
No significant differences were found in vessel CNR values between the two groups.
Compared to this low-tube voltage CTPA study, our scan protocol showed a further reduction in radiation dose and contrast media agent while providing a better image quality in terms of vascular enhancement (HU), SNR, CNR, and noise level.
Suntharalingam et al. [35] compared an 80-kV standard pitch protocol using 25 mL of contrast agent with a standard CTPA acquired with 100 kV using 60 mL contrast media administration. They reported that the low-tube voltage CTPA protocol allowed a significant radiation dose saving (0.7 mSv vs 2.4 mSv) while maintaining a comparable objective image quality in terms of SNR and CNR. Compared to this study, our scan protocol delivered a higher radiation dose to the patient in terms of mSv (mean 3.48 ± 1.2 mSv vs 0.7 mSv) due to the different CT scanners used (Philips 256-row iCT Elite vs Siemens Somatom Definition FLASH Dual-Source CT), different scanning protocols data (pitch: 0.17 vs 1.2 and contrast media amount: 50 mL vs 25 mL), and to the emergency setting in which our CTPA scans were performed that required us to not further lower the radiation dose in order to obtain a diagnostic and feasible CTPA protocol for the emergency department. Indeed, our low-kV protocol obtained a significantly better image quality in terms of vascular contrast enhancement (e.g., in the main pulmonary trunk 670.91 ± 9.09 HU vs 201 HU), SNR (61.6 ± 15.1 vs 12), and CNR (5.9 ± 18.13 vs 10.2).
Klingerman et al. [36] compared diagnostic confidence and detection of pulmonary embolism using a 100-kV protocol on a 256-slice CT using 75 mL of iodinated contrast agent, reconstructed with FBP, HIR, and MBIR algorithm; they stated that image noise was significantly less using HIR compared to FBP (p < 0.0001) and using MBIR compared to HIR (p < 0.0001) but the attenuation values were not significantly different between the different reconstruction algorithms.
They also reported that CNR was significantly higher using both MBIR and HIR compared to FBP (p < 0.0001 for both), and at the level of segmental PA, the average CNR was 2.9 times higher using MBIR than FBP and two times higher using MBIR than HIR.
In addition, sensitivity for PE detection was stated 76%, 78.6%, and 82.5% using FBP, HIR, and MBIR, respectively.
Compared to this study, we obtained higher values of attenuation in the main pulmonary arteries using model-based algorithm (average of 618.4 HU vs 357.9 HU) and hybrid algorithm (average of 292.2 HU vs 357.4 HU), a lower noise level using model-based approach (6 vs 17) and hybrid iterative approach (10 vs 29.6), and also a significant difference between attenuation values obtained using IMR and iDose4 (attenuation in the main pulmonary trunk of 670.91 HU vs 292.6 HU), reducing at the same time the amount of contrast media agent (50 mL vs 75 mL) and the radiation dose delivered to the patient in terms of CTDI on both 80-kV model–based CTPA (6.41 vs 10.5 mGy) and 100 kV hybrid iterative-CTPA (9.68 vs 10.5 mGy).
The limitations of this study include the fact that two different cohorts of patients were investigated by using two different CT scanner, both with 256 rows, but one equipped with new knowledge model–based algorithm and the other one with hybrid iterative algorithm and therefore a direct comparison in the same patients between low-kV IMR-CTPA and iDose4-CTPA was not possible. This random categorization may introduce a confounding factor, but, due to ethical concerns, we could not examine each patient twice during the same imaging session. Moreover, we did not evaluate and compare the results of our series in terms of radiation dose, CNR, and SNR, with any reference phantom study to demonstrate the baseline differences between the two scanners and technical parameters, but this could be a starting point for further investigations.
There are only 23 PE in the low-kV IMR-CTPA protocols, which is clearly not enough to draw conclusions on the clinical efficacy of this new low-dose protocol. Nevertheless, the primary objective of this study was to establish the possibility of acquiring low-kV IMR-CTPA and to assess its image quality.
In addition, we used only one level of the IMR (IMR1) and one level of the iDose4 (iDose level 4). It is uncertain whether our results would be similar if other levels had been used.
Although the studies were randomized, and the readers were blinded to the type of reconstruction, the differing appearance of the reconstructions may allow for recognition of the type of reconstruction in many instances.
Conclusions
In conclusion, this prospective study showed that low-kV IMR-CTPA allows a significant dose reduction while improving attenuation values, SNR, and CNR in the pulmonary vessels, as compared with iDose4-CTPA, in routinely practice. IMR shows greater potential than iDose4 for providing diagnostically acceptable low-dose CT images without compromising image quality and has been proven to be helpful for dose reduction, particularly in certain patients and settings, such as vascular imaging in the emergency department or in the clinical routine.
Abbreviations
- CT:
-
Computed tomography
- CTPA:
-
Computed tomography pulmonary angiography
- IMR:
-
Knowledge model–based iterative reconstruction
- iDose4 :
-
Hybrid iterative reconstruction
- MDCT:
-
Multidetector computed tomography
- HU:
-
Hounsfield unit
- DLP:
-
Dose-length product
- CTDIvol:
-
Computed tomography dose index
- ED:
-
Effective dose
- SNR:
-
Signal-to-noise ratio
- CNR:
-
Contrast-to-noise ratio
- mAs:
-
Milliamperage seconds
- ROI:
-
Region of interest
- mSv:
-
Millisievert
- mGy:
-
Milligray
References
Tapson VF (2008) Acute pulmonary embolism. N Engl J Med 358:1037e1052
Nguyen PK, Wu JC (2011) Radiation exposure from imaging tests: is there an increased cancer risk? Expert Rev Cardiovasc Ther 9:177–183
Suzuki K, Yamashita S (2012) Low-dose radiation exposure and carcinogenesis. Jpn J Clin Oncol 42:563–568
Lauer MS (2009) Elements of danger—the case of medical imaging. N Engl J Med 361:841–843
Kalra MK, Maher MM, Sahani DV et al (2003) Low-dose CT of the abdomen: evaluation of image improvement with use of noise reduction filters pilot study. Radiology 228:251–256
Diel J, Perlmutter S, Venkataramanan N, Mueller R, Lane MJ, Katz DS (2000) Unenhanced helical CT using increased pitch for suspected renal colic: an effective technique for radiation dose reduction? J Comput Assist Tomogr 24:795–801
Heyer CM, Mohr PS, Lemburg SP et al (2007) Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 245:577–583
Henes FO, Groth M, Begemann PG et al (2012) Impact of tube current-time and tube voltage reduction in 64-detector-row computed tomography pulmonary angiography for pulmonary embolism in a porcine model. J Thorac Imaging 27:113–120
Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W (2009) Iterative reconstruction technique for reducing body radiation dose at CT: Feasibility study. AJR Am J Roentgenol 193:764–771
Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD (2010) Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: A pilot study. AJR Am J Roentgenol 195:126–131
Leipsic J, Labounty TM, Heilbron B et al (2010) Adaptive statistical iterative reconstruction: Assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195:649–654
Leipsic J, Nguyen G, Brown J, Sin D, Mayo JR (2010) A prospective evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction. AJR Am J Roentgenol 195:1095–1099
Prakash P, Kalra MK, Digumarthy SR et al (2010) Radiation dose reduction with chest computed tomography using adaptive statistical iterative reconstruction technique: Initial experience. J Comput Assist Tomogr 34:40–45
Prakash P, Kalra MK, Kambadakone AK et al (2010) Reducing abdominal CT radiation dose with adaptive statistical iterative reconstruction technique. Invest Radiol 45:202–210
Sagara Y, Hara AK, Pavlicek W, Silva AC, Paden RG, Wu Q (2010) Abdominal CT: Comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol 195:713–719
Singh S, Kalra MK, Gilman MD et al (2011) Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: A pilot study. Radiology 259:565–573
Singh S, Kalra MK, Hsieh J et al (2010) Abdominal CT: Comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology 257:373–383
Cornfeld D, Israel G, Detroy E, Bokhari J, Mojibian H (2011) Impact of adaptive statistical iterative reconstruction (ASIR) on radiation dose and image quality in aortic dissection studies: A qualitative and quantitative analysis. AJR Am J Roentgenol 196:W336–W340
Thibault JB, Sauer KD, Bouman CA, Hsieh J (2007) A three dimensional statistical approach to improved image quality for multislice helical CT. Med Phys 34:4526–4544
Yu Z, Thibault JB, Bouman CA, Sauer KD, Hsieh J (2011) Fast model-based X-ray CT reconstruction using spatially nonhomogeneous ICD optimization. IEEE Trans Image Process 20:161–175
Willemink MJ, de Jong PA, Leiner T et al (2013) Iterative reconstruction techniques for computed tomography Part 1: technical principles. Eur Radiol 23:1623–1631
Deak Z, Grimm JM, Treitl M et al (2013) Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology 266:197–206 1670 Eur Radiol (2015) 25:1665–1671
Katsura M, Matsuda I, Akahane M et al (2013) Model-based iterative reconstruction technique for ultralow-dose chest CT: comparison of pulmonary nodule detectability with the adaptive statistical iterative reconstruction technique. Invest Radiol 48:206–212
Pickhardt PJ, Lubner MG, Kim DH et al (2012) Abdominal CTwith model-based iterative reconstruction (MBIR): initial results of a prospective trial comparing ultralow-dose with standard-dose imaging. AJR Am J Roentgenol 199:1266–1274
Raptopoulos V, Boiselle PM (2001) Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. Radiology 221:606–613
Boyden EA (1995) Segmental anatomy of the lungs. McGraw-Hill, New York, NY
Jackson CL, Huber JF (1943) Correlated applied anatomy of the bronchial tree and lungs with a system of nomenclature. Dis Chest 9:319–326
Remy-Jardin M, Remy J, Artraud D, Deschildre F, Duhamel A (1997) Peripheral pulmonary arteries: optimization of the acquisition protocol. Radiology 204:157–163
Mc Collough (2008) “The measurement,reporting, and management of radiation dose in CT”, Report 96, American Association of Phyicist in Medicine, Report of AAPM Task Group 23, pp. 1–28
Litmanovich D, Boiselle PM, Bankier AA et al (2009) Dose reduction in computed tomographic angiography of pregnant patients with suspected acute pulmonary embolism. J Comput Assist Tomogr 33:961–966
MacKenzie JD, Nazario-Larrieu J, Cai T et al (2007) Reduced-dose CT: effect on reader evaluation in detection of pulmonary embolism. AJR Am J Roentgenol 189:1371–1379
Yuki H, Utsunomiya D, Funama Y et al (2014) Value of knowledge-based iterative model reconstruction in low-kV 256-slice coronary CT angiography. J Cardiovasc ComputTomogr 8:115
Uchá D, Willemink MJ, de Jong PA et al (2014) The impact of a new model-based iterative reconstruction algorithm on prosthetic heart valve related artifacts at reduced radiation dose MDCT. Int J Cardiovasc Imaging 30:785
Zamboni GA, Guariglia S, Bonfante A et al (2012) Low voltage CTPA for patients with suspected pulmonary embolism. Eur J Radiol 81:e580–e584
Suntharalingam S, Mikat C, Stenzel E, Erfanian Y et al (2017) Submillisievert standard-pitch CT pulmonary angiography with ultra-low dose contrast media administration: A comparison to standard CT imaging. PLoS One 12(10):e0186694. https://doi.org/10.1371/journal.pone.0186694 eCollection 2017
Klingerman et al (2015) Detection of pulmonary embolism on computer tomography: improvement using a model-based iterative reconstruction algorithm compared with filtered back projection and iterative reconstruction algorithm. J Thorac Imaging. 30(1):60–68
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Every patient gave his informed consent, as required by our Institution.
Disclosure
The authors have nothing to disclose.
Additional information
Keypoints
• Knowledge Model–based iterative reconstruction (IMR) creates high-quality low-dose CT images.
• IMR significantly reduces image noise and artifacts over adaptive statistical iterative techniques.
• IMR shows greater potential than iDose4 for diagnostically acceptable low-dose CTPA.
Rights and permissions
About this article
Cite this article
Ippolito, D., De Vito, A., Franzesi, C.T. et al. Evaluation of image quality and radiation dose saving comparing knowledge model–based iterative reconstruction on 80-kV CT pulmonary angiography (CTPA) with hybrid iterative reconstruction on 100-kV CT. Emerg Radiol 26, 145–153 (2019). https://doi.org/10.1007/s10140-018-1653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-018-1653-4