Abstract
Objectives
Abusive head trauma (AHT) in infants is usually diagnosed using a multi-disciplinary approach by investigating the circumstances and identifying morphological indicators, for example, subdural hematomas (SDHs), subdural hygromas (SDHys), retinal haemorrhages and encephalopathy. The present morphological study investigates the incidence, radiological characteristics and non-radiological co-factors of bridging vein thrombosis (BVT) in infants with AHT.
Methods
From 2002 to 2013, computed tomography (CT) and magnetic resonance imaging (MRI) material of 628 infants aged 0-2 years were analysed retrospectively. If available, medicolegal expert opinions were additionally considered. Cases with SDHs and/or SDHys were identified and systematically evaluated as to the presence and characteristics of BVT.
Results
SDHs and/or SDHys were present in 29 of the 81 cases exhibiting morphological abnormalities in the initial CT. Among these, 11 cases (40 %) had BVT (mean age = 5.0 months). BVT could be best depicted in the T1-weighted spin echo and T2*/susceptibility-weighted MRI. In one case, BVT could be depicted indirectly using time-of-flight MR venography. The predominant (73 %) BVT shape was found to be tadpole-like (“Tadpole Sign”).
Conclusions
In the absence of appropriate accidental trauma, BVT appears to be a strong indicator of AHT. Therefore, the BVT/Tadpole Sign represents compelling cause to search for other signs of AHT.
Key points
• BVT is an excellent indicator of AHT in SDH/SDHy cases.
• Accidental trauma must be ruled out before diagnosing AHT.
• The Tadpole Sign appears to be the most characteristic shape of BVT.
• BVT can be depicted using CT, MRI and MR venography.
• The Tadpole Sign suggests searching for other signs of AHT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abusive head trauma (AHT) caused by violent shaking (“shaken baby syndrome,” SBS) or direct blunt force to an infant’s head is the leading cause of death in child abuse fatalities [1, 2]. The incidence for children under one year of age ranges between 14 and 28 per 100,000 live births [3–8]. In addition to encephalopathy/diffuse axonal injury and retinal haemorrhage (RH), another morphological sign raising the suspicion of AHT/SBS is the presence of subdural collections, especially subdural hematomas (SDHs) and/or subdural hygromas (SDHys) [9, 10].
It is generally assumed that during violent shaking, SDHs, and possibly consequential SDHys [11], can result from tearing one or more of the 15 to 20 bridging veins (BVs). These cortical veins span bridge-like from the surface of the cerebral cortex through the pia mater and the subarachnoid space to the venous sinuses within the Dura mater [12–15].
Venous blood from injured BVs opens an artificial subdural space, which appears to be a cleaving of the so-called dural border cell layer and, strictly speaking, should, therefore, actually be referred to as an “intradural space.” In addition to the resulting SDH, the venous blood may also lead to clot formation within or around the BVs. Such a bridging vein thrombosis (BVT) can be regarded as a strong indicator of the traumatic nature of SDHs and SDHys in the context of AHT.
The anatomy and traumatology of BVs were occasionally described in experimental post mortem settings [14, 16–23]. However, standard autopsy procedures frequently cause artificial post mortem injuries of the BVs [17, 24] and, therefore, retrospective investigation of vital BV injuries and BVT, e.g., by means of standard autopsy protocols, is very difficult.
In living infants, imaging data about BV ruptures and BVT are likewise sparse. A gold standard for the radiological depiction of BVT does not exist. In 1999, Barlow et al. were able to show BVT by means of T2-weighted Gradient-Echo (T2 GRE) imaging in 4 of 14 AHT cases with SDHs [25]. In 2012, Adamsbaum and Rambaud associated BVT with hypodense/hypointense subdural collections using computed tomography (CT) and magnetic resonance imaging (MRI) in three AHT cases [26]. The authors proposed considering BVTs as “markers of acutely disrupted veins” with “crucial diagnostic value,” arguing for the presence of AHT [26]. Recently, Yilmaz et al. demonstrated BVT in two cases with an AHT-associated SDH by means of susceptibility-weighted imaging (SWI) in MRI [27].
Systematic studies investigating the morphology of BVT by means of modern cross-sectional imaging methods are missing. Therefore, the main objective of the present study is evaluation of the incidence, radiological characteristics and non-radiological co-factors of BVT in living infants with an SDH and/or SDHy in order to increase certainty for forensic physicians and neuroradiologists in diagnosing AHT.
Material and methods
Data acquisition
With approval of the institution’s ethics committee, the radiological reports of all infants aged between 0 and 2 years who underwent cranial computed tomography (cCT) between September 1, 2002 and August 31, 2013 at our radiological department, which belongs to a tertiary care hospital, were analysed retrospectively (n = 628). This approach was chosen because the acute diagnostics of suspected craniocerebral injury (CCI), including AHT, are usually comprised of an initial cCT.
Of these 628 cases, cases with an SDH and/or SDHy were identified (from now on referred to as “SDH/SDHy cases”). Subsequently, all available image material of the SDH/SDHy cases was extracted from the digital archive. The image material consisted of all initially-performed standard cCT data and, if available, the cranial magnetic resonance imaging (cMRI) data. This included at least one T1-weighted (T1) and several T2-weighted (T2) sequences, such as T2*/susceptibility-weighted imaging (SWI), being more sensitive for blood products than simple T2 imaging. However, post-surgical MRI data were not considered as this image material does not depict the original traumatic conditions of the pathologies investigated in this study. The available image material was viewed using a standard picture archiving and communicating system (PACS) workstation (Centricity PACS, GE Healthcare, Princeton, NJ). In addition, the final medical reports, including descriptions of possible injuries other than AHT, were considered for data supplementation. Thus, all medical co-findings, the patients’ demographic data, and the anamneses, usually containing the story told by the caregivers, were recorded.
In order to classify the infants as either abusive or accidental head trauma, the cases were matched with archives of the in-house Institute of Legal Medicine (ILM). If the ILM is involved in a case, usually requiring a request by the police or the department of public prosecution, the diagnosis of AHT is based not only on radiological reports but on the forensic physicians involved that have access to the complete set of the available data (medicolegal physical examination, medicolegal crime scene investigation, medical records from the hospital, police files and, if deceased, autopsy reports). Furthermore, forensic physicians in Germany are trained and experienced in evaluating paediatric injuries not only in dead children during an autopsy but also in living children during medicolegal physical examinations. Medicolegal expert opinions, therefore, represent the most objective and best reference standard for AHT in Germany. If medicolegal expert opinions of the cases existed, these data were analysed further.
Imaging
Non-enhanced cCT (100 kV, 140 mA, slice thickness 3 mm, Kernel J30s) was performed in axial view from the skull base to the vertex using multi-detector CT systems by SIEMENS Healthcare (Erlangen, Germany; SOMATOM Sensation 16/SOMATOM Definition AS/SOMATOM Definition Flash).
Non-enhanced MRI was performed using 1.5 and 3.0 Tesla MR systems by SIEMENS Healthcare (Erlangen, Germany; MAGNETOM Symphony [1.5 T]/MAGNETOM Aera [1.5 T]/MAGNETOM Skyra [3.0 T]). A typical 1.5 Tesla MRI protocol included the following sequences and parameters: An axial T1 spin echo (SE) sequence (slice thickness 5 mm, TR/TE 542/7.7, field of view 115 × 57 mm2, matrix 320 × 158); two T2 turbo spin echo (TSE) sequences (axial and sagittal; slice thickness 2 mm [axial and sagittal], TR/TE 4940/112 [axial] and 4050/113 [sagittal], field of view 230 × 75 mm2 [axial] and 220 × 110 mm2 [sagittal], matrix 512 × 168 [axial] and 512 × 256 [sagittal]); an axial T2 fluid attenuated inversion recovery (FLAIR) sequence (slice thickness 5 mm, TR/TE 9000/105, field of view 115 × 75 mm2, matrix 256 × 168); an axial T2 diffusion-weighted imaging (DWI) sequence (slice thickness 5 mm, TR/TE 4000/84, field of view 230 × 230 mm2, matrix 128 × 128); and an axial T2 susceptibility-weighted imaging (SWI) sequence (slice thickness 2 mm, TR/TE 49/40, field of view 230 × 106 mm2, matrix 320 × 147).
Evaluation of image material
The SDH/SDHy cases were then analysed as to the presence, location, distribution, radiological appearance and general morphology of BVT. In particular, careful investigation of the vertex was carried out for assessment of BVs and the search for BVT. In accordance with previous descriptions [25–27], we assessed vessel-shaped structures with clear hyperattenuation in the cCT images, hyper-signal intensity in the T1 sequences, hypo-signal intensity in the T2 sequences, and signal loss in the SWI as radiological evidence for BVT. Evaluations were done in consensus by two radiologists experienced in the field of paediatric neuroradiology. The examiners were blinded to the medical histories and demographic characteristics of the patients. A Fisher’s exact test (2 × 3 contingency table) was used for statistical analysis. A p value < 0.05 was considered to be significant.
Results
Subdural hematomas and hygromas
During the study period, 81 out of 628 cases (13 %) showed morphologically visible abnormalities via initial non-enhanced cCT (Table 1); CCI was already assumed by the clinicians in each of the 81 cases. SDHs and/or SDHys were found in 29 of the 81 cases (36 %; 10 SDH cases, 11 SDHy cases, 8 SDH + SDHy cases). At the time of admittance to hospital, AHT was strongly suspected in each of the 29 cases.
Among the 29 cases there were 21 (72 %) male and 8 (28 %) female patients with a mean age of 6.6 months. SDHs and SDHys were located predominantly in the parietal and/or frontoparietal regions. Additional pre-surgical non-enhanced cMRI after initial cCT was carried out in 12 of the 29 patients (41 %). The authorities ordered medicolegal expert opinions in 11 of the 29 cases (40 %). In only one of these cases was the infant’s injuries classified as being consistent with an accidental cause (fall from loft bed). AHT was strongly suspected in the other cases.
Bridging vein thromboses
In 11 of the 29 SDH/SDHy cases (40 %), we detected radiological findings in accordance with current BVT descriptions (Fig. 1 CT, Fig. 2 MRI). Table 2 summarizes the details of all BVT cases investigated in this study. Among the 11 cases, there were eight (73 %) male and three (27 %) female patients with a mean age of 5.0 months. The occurrence of BVT showed no significant association with the type of subdural fluid collection (SDH, SDHy, or SDH + SDHy; p = 0.1751).
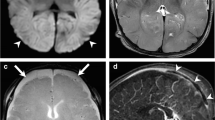
Bridging vein thrombosis in CT. A Incomplete Tadpole sign: Round- to oval-shaped blood clot in the right parietal region (“body of the tadpole,” arrow) directly connected to an expanded BV (“tail of the tadpole”) that can only be seen in this case by further scrolling in the caudal direction; B Tubular-shaped BVT in the left frontal region (arrow)
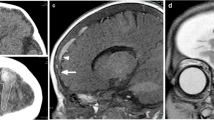
Bridging vein thrombosis in MRI (Tadpole Sign). A Coronal T1-weighted spin echo MR image shows tadpole-like hyper-intense signal in the left frontal region. The round-shaped thrombotic material (“body of the tadpole,” arrow head) corresponds to the clot derived from the injured BV now expanded by clotted blood (“tail of the tadpole,” arrow); B Axial T2*/SWI MR image again visualizing another tadpole-like BVT formation in another case; C TOF MR venogramme of an infant’s head with an SDH. BVT is indirectly demonstrated by interruption of the venous flow signal at the position of the assumed BV rupture (arrow)
Medicolegal expert opinions were available in five of the 11 cases (45 %) which confirmed the diagnosis of AHT without exception. In the remaining six cases, the additional presence of RH (three cases), multiple fractures (three cases), and scalding (one case) likewise strongly suggest SBS/AHT. Moreover, the caregivers denied any adequate trauma in all cases, whereas signs of severe trauma were definitely present. Thus, there was convincing evidence that all 11 infants with BVT were very likely to have been exposed to SBS/AHT.
BVT was exclusively observed in the parasagittal regions at the convexities of the frontal and/or parietal lobes with about the same frequency on each brain side. Cranial MRI was assessable in four cases. While BVT can be easily overseen in the standard axial view, the coronal view appeared to be more sensitive for locating and evaluating BVT. RH were diagnosed in seven of the 11 BVT cases (64 %). Skeletal injuries were found in six cases (55 %).
In eight of the 11 cases (73 %), the BVT strongly resembled a tadpole, with an oval- to round-shaped “body” representing thrombotic material within the subarachnoid rather than in the subdural space, and a bent “tail” reflecting a torn BV expanded by clotted blood. Figure 2A and B demonstrate this tadpole-shaped BVT in different perspectives. Accordingly, we termed this shape as the “Tadpole Sign”. Figure 1A presents an incomplete Tadpole Sign. In one case, time-of-flight (TOF) MR venography was available and showed interruption of venous fluid signals at the commencement of the BVT (Fig. 2C). In addition to the tadpole-like shape, three cases displayed a simple tubular shape (Fig. 1B). In this BVT type the aforementioned “body of the tadpole” is missing such that only the injured BV, apparently expanded by clotted blood, can be recognized.
In four of the 11 BVT cases (36 %), the time interval between commencement of clinical symptoms (according to the parents) and performance of the initial cCT was more than one day (5, 7, 14 and 15 days). All four cases showed SDHys alone or in combination with an SDH. The tadpole-like shape was the only BVT shape observed in these four cases.
Discussion
Child abuse has multiple faces [28–31]. SBS/AHT, as a manifestation of abuse, represents a situation in which missing the diagnosis can have serious and life-threatening consequences for the child because repeated maltreatment is likely. For this reason, more neuroradiological indicators of abuse are desirable to increase diagnostic certainty. Classically, SDHs and SDHys have been widely accepted as such indicators. However, an infantile SDH can also be a result of non-traumatic causes [32, 33]. BV injuries are considered a major traumatic origin of SDHs since BVs vary in their wall thickness. In particular, vessel segments that penetrate the dural border cell layer can have a thickness of only 10 μm, whereas the wall thickness of the subarachnoid portion range between 50 and 200 μm [12]. Thus, increased fragility of the dural segment of a BV is assumed. This might explain why SDHs are more common than subarachnoid haemorrhages in AHT cases.
In contrast to SDHs, the pathogenesis of SDHys in the context of AHT is probably attributed to different mechanisms [11]. On one hand, SDHys can be a delayed consequence of SDHs [probably by dilution of the SDH with water and/or cerebrospinal fluid (CSF)], and on the other hand, SDHys can also appear rapidly (probably by traumatically-induced arachnoid tears and consecutive CSF influx). Thus, BV injuries would be expected more likely in the former situation.
Since the presence of BVT strongly indicates the presence of BV injury, some light has been shed on this relatively new radiological criterion, thereby indicating a high diagnostic potential [25–27]. In the present study, comprising an 11-year period with 628 infants aged between 0 and 2 years, we found 29 cases with an SDH and/or SDHy, of which 11 cases showed BVT. The male sex was overrepresented for both SDH/SDHy and BVT, a phenomenon possibly due to the presumed greater fragility of meningeal structures in males [34]. Furthermore, the mean ages of both groups (SDH/SDHy and BVT) range within the child development phase associated with an increased age-related incidence of SBS/AHT. This is possibly attributable to the increased vulnerability of infants at that age and the increased challenges of child-care (e.g., managing crying) [35].
There was convincing evidence that all 11 infants were very likely to have been exposed to SBS/AHT. Among the SDH/SDHy cases, BVT appears therefore to be an excellent indicator of head trauma. Of course, it must be assumed that BVT also occurs in accidental head trauma. However, our study design did not allow for the identification of a sufficient number of unambiguous accidental head trauma cases that occurred among witnesses in public spaces. For this reason, we believe that this aspect should be surveyed in a case-control study comparing accidental with abusive head trauma cases. Hence, based on the observations of the present study, BVT can be considered as an additional marker of AHT, but only if accidental trauma has clearly been ruled out. In cases with several other signs of child abuse, the presence of BVT strongly supports the diagnosis of AHT.
Regarding the radiological diagnostic process, we propose to search for tadpole-like structures within the frontoparietal region of axial CT or, even better, coronal MR images because we found this shape to be the most characteristic shape of BVT in our cohort. We termed this phenomenon as the Tadpole Sign. It represents a striking shape of BVT in infants and is relatively easy to identify. The locations of BVT are consistent with the supposed trauma mechanism (tearing of BVs caused by acceleration-deceleration and rotational forces during violent shaking of the infant) [1] and with previous case reports [25–27].
One may speculate whether the Tadpole Sign can be interpreted as an acute or early sub-acute sign of BV injury since the round-shaped thrombus (“body of the tadpole”) located adjacent to an enlarged and thrombosed BV (“tail of the tadpole”) always appeared hyper- to isodense in CT, hyperintense in T1-weighted MRI, and iso- to hypointense in T2-weighted MRI. However, the CT- and MR-morphologic changes of blood products within an SDH over time [36–39] may not be directly transferable to clot formations within or associated with BVs as they rely upon other pathophysiological conditions.
In addition to T2 GRE [25] and SWI [27], another promising technique for demonstrating BVT or a BV rupture might be TOF MR venography (Fig. 2C). This technique can depict thrombosed veins by signal loss in the vessels or discontinuance in the course of the vessel. On the other hand, flow artefacts and normal variants can be confusing. Perhaps additional contrast-enhanced imaging might support the evaluation. What needs to be considered, however, is that any administration of a contrast to infants should be restricted to indicated situations. To date, no systematic data investigating the potential of this technique for the purpose of diagnosing AHT are available.
A significant limitation of our study must be seen in the retrospective study design. The spectrum of neuroradiological investigations requested for infants suspected of suffering from AHT was found to be very heterogeneous and strongly varies in the course of time due to changing protocols and guidelines. Moreover, some information was not acquirable in this setting, e.g., the outcome of all patients. MRI was not performed in each case, which, in our opinion, should be a standard procedure in all cases suspecting AHT.
To conclude, careful investigation of the parasagittal frontoparietal region in cranial CT and MRI of living infants is very useful for uncovering BVT, which can be recognized by its tadpole-like appearance in many cases. The presence of BVT, as indicated by the predominantly observed Tadpole Sign or, of course, in tubular shape, always represents compelling cause to search for other signs of SBS/AHT, such as RH or fractures. Further studies are needed to increase the diagnostic value of BVT.
Abbreviations
- AHT:
-
Abusive head trauma
- SBS:
-
Shaken baby syndrome
- SDH:
-
Subdural hematoma
- SDHy:
-
Subdural hygroma
- BV:
-
Bridging vein
- BVT:
-
Bridging vein thrombosis
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- RH:
-
Retinal haemorrhages
- CCI:
-
Craniocerebral injury
References
American Academy of Pediatrics: Committee on Child Abuse and Neglect (2001) Shaken baby syndrome: rotational cranial injuries-technical report. Pediatrics 108:206–210
Duhaime AC, Christian CW, Rorke LB, Zimmerman RA (1998) Nonaccidental head injury in infants–the "shaken-baby syndrome". N Engl J Med 338:1822–1829
Jayawant S, Rawlinson A, Gibbon F et al (1998) Subdural haemorrhages in infants: population based study. BMJ 317:1558–1561
Barlow KM, Minns RA (2000) Annual incidence of shaken impact syndrome in young children. Lancet 356:1571–1572
Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH (2003) A population-based study of inflicted traumatic brain injury in young children. JAMA 290:621–626
Hobbs C, Childs AM, Wynne J, Livingston J, Seal A (2005) Subdural haematoma and effusion in infancy: an epidemiological study. Arch Dis Child 90:952–955
Talvik I, Metsvaht T, Leito K et al (2006) Inflicted traumatic brain injury (ITBI) or shaken baby syndrome (SBS) in Estonia. Acta Paediatr 95:799–804
Fanconi M, Lips U (2010) Shaken baby syndrome in Switzerland: results of a prospective follow-up study, 2002-2007. Eur J Pediatr 169:1023–1028
Matschke J, Herrmann B, Sperhake J, Körber F, Bajanowski T, Glatzel M (2009) Shaken baby syndrome: a common variant of non-accidental head injury in infants. Dtsch Arztebl Int 106:211–217
Christian CW, Block R (2009) Committee on Child Abuse and Neglect; American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics 123:1409–1411
Wittschieber D, Karger B, Niederstadt T, Pfeiffer H, Hahnemann ML (2014) Subdural hygromas in abusive head trauma: pathogenesis, diagnosis and forensic implications. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3989
Yamashima T, Friede RL (1984) Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry 47:121–127
Morrison CN, Minns RA (2005) The biomechanics of shaking. In: Minns RA, Brown JK (eds) Shaking and other non-accidental head injuries in children. Mac Keith Press, London, pp 106–146
Nierenberger M, Wolfram-Gabel R, Decock-Catrin S et al (2013) Investigation of the human bridging veins structure using optical microscopy. Surg Radiol Anat 35:331–337
Minns RA (2014) Non-accidental head injury in children. In: Madea B (ed) Handbook of forensic medicine. Wiley Blackwell, Chichester, pp 702–724
Norman MG, Smialek JE, Newman DE, Horembala EJ (1984) The postmortem examination on the abused child. Pathological, radiographic, and legal aspects. Perspect Pediatr Pathol 8:313–343
Maxeiner H (1997) Detection of ruptured cerebral bridging veins at autopsy. Forensic Sci Int 89:103–110
Maxeiner H (2001) Demonstration and interpretation of bridging vein ruptures in cases of infantile subdural bleedings. J Forensic Sci 46:85–93
Ehrlich E, Maxeiner H, Lange J (2003) Postmortem radiological investigation of bridging vein ruptures. Legal Med (Tokyo) 5:225–227
Stein KM, Ruf K, Ganten MK, Mattern R (2006) Representation of cerebral bridging veins in infants by postmortem computed tomography. Forensic Sci Int 163:93–101
Depreitere B, Van Lierde C, Sloten JV et al (2006) Mechanics of acute subdural hematomas resulting from bridging vein rupture. J Neurosurg 104:950–956
Han H, Tao W, Zhang M (2007) The dural entrance of cerebral bridging veins into the superior sagittal sinus: an anatomical comparison between cadavers and digital subtraction angiography. Neuroradiology 49:169–175
Monea AG, Baeck K, Verbeken E et al (2014) The biomechanical behaviour of the bridging vein-superior sagittal sinus complex with implications for the mechanopathology of acute subdural haematoma. J Mech Behav Biomed Mater 32:155–165
Squier W, Mack J (2009) The neuropathology of infant subdural haemorrhage. Forensic Sci Int 187:6–13
Barlow KM, Gibson RJ, McPhillips M, Minns RA (1999) Magnetic resonance imaging in acute non-accidental head injury. Acta Paediatr 88:734–740
Adamsbaum C, Rambaud C (2012) Abusive head trauma: don't overlook bridging vein thrombosis. Pediatr Radiol 42:1298–1300
Yilmaz U, Körner H, Meyer S, Reith W (2014) Multifocal signal loss at bridging veins on susceptibility-weighted imaging in abusive head trauma. Clin Neuroradiol Med. doi:10.1007/s00062-014-0283-9
Trübner K, Schubries M, Beintker M, Bajanowski T (2013) Genital findings in boys suspected for sexual abuse. Int J Legal Med 127:967–970
Schulte B, Rothschild MA, Vennemann M, Banaschak S (2013) Examination of (suspected) neonaticides in Germany: a critical report on a comparative study. Int J Legal Med 127:621–625
Herrmann B (2002) Körperliche Misshandlung von Kindern. Monatsschr Kinderheilkd 150:1324–1338
Friedrich K, Becker K, Rothschild MA, Banaschak S (2013) Child abuse inflicted by small children. Int J Legal Med 127:627–630
Stray-Pedersen A, Omland S, Nedregaard B, Klevberg S, Rognum TO (2011) An infant with subdural hematoma and retinal hemorrhages: does von Willebrand disease explain the findings? Forensic Sci Med Pathol 7:37–41
Drigo P, Burlina AB, Battistella PA (1993) Subdural hematoma and glutaric aciduria type 1. Brain Dev 15:460–461
Vinchon M, Delestret I, DeFoort-Dhellemmes S, Desurmont M, Noulé N (2010) Subdural hematoma in infants: can it occur spontaneously? Data from a prospective series and critical review of the literature. Childs Nerv Syst 26:1195–1205
Leventhal JM, Martin KD, Asnes AG (2010) Fractures and traumatic brain injuries: abuse versus accidents in a US database of hospitalized children. Pediatrics 126:e104–e115
Bradley WG Jr (1993) MR appearance of hemorrhage in the brain. Radiology 189:15–26
Barnes PD, Krasnokutsky M (2007) Imaging of the central nervous system in suspected or alleged nonaccidental injury, including the mimics. Top Magn Reson Imaging 18:53–74
Vezina G (2009) Assessment of the nature and age of subdural collections in nonaccidental head injury with CT and MRI. Pediatr Radiol 39:586–590
Schwartz ES, Barkovich AJ (2012) Brain and spine injuries in infancy and childhood. In: Barkovich AJ, Raybaud C (eds) Pediatric neuroimaging. Lippincott Williams & Wilkins, Philadelphia, pp 240–366
Acknowledgments
The scientific guarantor of this publication is Daniel Wittschieber, MD. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Approval from the institutional animal care committee was not required because the study did not use any animals. No study subjects or cohorts have been reported previously. Methodology: retrospective, diagnostic study / observational study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hahnemann, M.L., Kinner, S., Schweiger, B. et al. Imaging of bridging vein thrombosis in infants with abusive head trauma: the “Tadpole Sign”. . Eur Radiol 25, 299–305 (2015). https://doi.org/10.1007/s00330-014-3443-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3443-z