Abstract
Background
Abusive head trauma (AHT) is an important cause of serious brain injury in infants and young children who have characteristic clinical and imaging findings that are discordant with the clinical history provided. Recent attention has focused on abnormalities of the cranial venous sinuses and cortical veins, both on MRI and at autopsy. Although many have interpreted these to be secondary to the AHT, some have recently argued that these venous abnormalities represent primary cortical sinus and venous thrombosis that leads secondarily to subdural hemorrhage and secondary brain injury. Direct trauma to the veins and sinuses has been reported at autopsy in AHT, but there has been no systematic study of venous abnormalities in cases of AHT.
Objective
The purpose of this study was to define the incidence and characteristics of venous and sinus abnormalities in AHT.
Materials and methods
We included all children <36 months of age who were diagnosed with abusive head trauma between 2001 and 2012 and who had MRI and magnetic resonance (MR) venography as part of their diagnostic workup. We analyzed age, gender and clinical findings. MRI and MR venography were analyzed independently by two neuroradiologists with a focus on abnormalities involving the intracranial veins and venous sinuses.
Results
A total of 45 children were included. The median age was 3 months (range 15 days to 31 months) and 28 were boys (62%). Clinical findings included retinal hemorrhage in 71% and extracranial fractures in 55%. CT or MRI demonstrated subdural hemorrhage in 41 (91%); none had subdural effusions. In 31 cases (69%) MR venography demonstrated mass effect on the venous sinuses or cortical draining veins, with either displacement or partial or complete effacement of the venous structures from an adjacent subdural hematoma or brain swelling. We also describe the lollipop sign, which represents direct trauma to the cortical bridging veins and was present in 20/45 (44%) children.
Conclusion
Evidence of displacement or compression of cortical veins and sinuses from subdural hemorrhage or edema on MR venography was present in the majority of children with abusive head trauma. Evidence of direct trauma to the veins (lollipop sign) was identified in nearly half of cases. It is important to understand the superimposed effects of subdural hematoma and brain swelling on the veins and sinuses to differentiate it from cortical sinus and venous thrombosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abusive head trauma (AHT) is the most frequent cause of traumatic brain injury in infants, with a mortality of 15–35% and significant neurological morbidity in the majority of survivors [1]. Subdural hemorrhage and retinal hemorrhages are present, on average, in 80% of cases; these hemorrhages are often associated with traumatic or hypoxic–ischemic brain injury, cervical spine injury and extracranial injuries such as rib and long-bone fractures such as classic metaphyseal fractures. The diagnosis of AHT is considered when the clinical and imaging findings considered in toto are discordant with the clinical history provided. Traumatic, hypoxic–ischemic or metabolically induced neuronal injury; cervicomedullary, upper spinal cord and upper cervical nerve root trauma; and most germane to the present study, tearing of cortical bridging veins, have all been invoked as pathophysiological mechanisms in AHT [2].
A number of other well-described clinical conditions can, on occasion, mimic AHT [3], although most of these have additional clinical features that readily distinguish them from AHT. Recently, the concept of intracranial cortical sinus or venous thrombosis has been introduced in legal proceedings as a potential mimic of AHT. Identification of cortical sinus and venous thrombosis can be a challenge because of the anatomical variations in venous anatomy and limitations of noninvasive neuroimaging techniques such as MR venography and CT venography. However, several previous studies have defined the clinical and neuroimaging findings in children with cortical sinus and venous thrombosis [4–8].
Several pre-existing conditions have been associated with cortical sinus and venous thrombosis including infection, dehydration, sepsis, thrombophilia, malignancy, chemotherapy (most commonly with asparaginase) and trauma. These pre-existing causes are usually readily identifiable; moreover, the types and patterns of cranial injury in cortical sinus and venous thrombosis have been well-defined and are usually distinctive and involve edema and hemorrhage localized to the area drained by the involved venous system. On rare occasions subdural effusions have been reported. However, outside of the perinatal period, during which both subdural hemorrhage and retinal hemorrhage have been described as a consequence of normal childbirth, neither acute SDH nor retinal hemorrhage has ever been identified as a consequence of cortical sinus and venous thrombosis. Moreover, because bridging vein trauma has been documented at surgery and at autopsy in cases of AHT [9–13], it is not surprising that cortical venous abnormalities would be present on neuroimaging studies. In evaluating cases of alleged AHT it is therefore important to distinguish whether any identified sinus or cortical venous abnormalities are secondary to trauma or primary and caused by intravenous thrombosis. There has been no systematic study of cranial venous abnormalities in cases of AHT. The purpose of this study was therefore to define the incidence and characteristics of venous and sinus abnormalities in AHT.
Materials and methods
Following approval from the Human Subjects Protection Office of Pennsylvania State University, we retrospectively identified all children <36 months who presented between 2000 and 2012 with AHT and had MRI and MR venography. Our institute maintains a child abuse registry, and the abusive head trauma cases were manually identified from that registry. Children presenting with acute or chronic intracranial injuries that were inconsistent with the history or developmental age of the child and not caused by another medical condition were assessed by a multidisciplinary clinical team that included pediatric neurosurgeons, pediatric ophthalmologists, pediatric intensivists, pediatric neuroradiologists and child abuse pediatricians. Cases were identified as abusive head trauma if, after a diagnostic workup, there was consensus that the findings most likely represented abusive head trauma or there was a perpetrator confession or judicial ruling of abusive head trauma. For all cases, clinical data were abstracted from the medical records to determine patient demographics, clinical findings and outcome. All neuroimaging studies including cranial CT, MRI and MR venography were independently reviewed by two experienced neuroradiologists (A.K.C. and K.T., with more than 8 years’ and 6 years’ experience, respectively) blinded to the clinical history and diagnosis; any interobserver differences were resolved by consensus. Skeletal surveys were reviewed by an experienced pediatric radiologist (D.K.B.B, with more than 30 years’ experience).
We analyzed intracranial abnormalities that were identified on any neuroimaging study. Specific abnormalities sought on the MR venograms included direct vascular trauma, intravascular thrombosis, and compression or displacement from adjacent subdural hemorrhage or parenchymal edema or swelling. Thrombosis was defined as absence of veins or sinuses on 3-D phase-contrast MR venography (both source and reformatted images) with velocity encoding (VENC) set to 15 cm/s corroborated by indisputable evidence of thrombus on other sequences including T1, T2, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI) and susceptibility-weighted imaging (SWI)/T2* gradient recalled echo (GRE) sequences. Compression was identified when a cortical vein or sinus narrowed abnormally, particularly if associated with displacement by an adjacent subdural hemorrhage. We termed the “‘lollipop sign” a specific venous pattern that reflected parasagittal bridging veins that abruptly terminated in the region of an overlying subdural hemorrhage and hypothesized that this represents a bridging vein tear and subsequent post-traumatic thrombosis at the torn terminal end. This sign was best identified on conventional MRI sequences, particularly GRE. The subdural hemorrhage was classified as small if there was no mass effect upon the brain parenchyma, moderate if there was some evidence of mass effect upon the underlying brain parenchyma, and large if there was significant mass effect upon the underlying brain parenchyma with midline shift. Interobserver agreement was calculated using kappa statistical measures, with k values of 0–0.2, 0.21–0.40, 0.41–0.60, 0.61–0.80, 0.81–0.99 and 1.00 values representing slight, fair, moderate, substantial, excellent and absolute agreement, respectively. All statistical measures were calculated using SPSS software (version 18.0; IBM Corp., Armonk, NY).
Results
Patients
A total of 235 children with abusive head trauma (AHT) <36 months old were identified from the 2000–2012 time period, of whom 90 (38.3%) had MRI scans. Of this group, 45 children (representing 50% of those with MRI scans and 19.1% of the total) had both MRI and MR venography and were therefore included in the study. There were 28 boys (62%) and 17 girls (38%). The median age was 3 months, with a range of 15 days to 31 months. All children had undergone a multi-disciplinary review at our institution and were thought to be victims of AHT. All of these children were reported to the Pennsylvania Office of Children, Youth and Families and were documented as substantiated cases of AHT in Child Line, a central statewide registry of child maltreatment maintained by the Pennsylvania Department of Public Welfare.
Thirty-two of the 45 children (71%) had retinal hemorrhages. Twenty-five (55%) had evidence of extracranial injuries. Nine (20%) had a documented history of trauma including being thrown to ground by two men, being beaten by father, being shaken repeatedly, falling, and sustaining injury to the face (six cases).
Radiologic findings are chronicled in Table 1. Forty-one children (91%) had subdural hemorrhage. Altogether, 30 children (67%) had additional traumatic injuries at the time of presentation. We identified four patterns of venous and sinus abnormalities. These were termed lollipop sign, lack of lollipop sign, positive compression sign (Fig. 1), and isolated non-terminal long-segment venous thrombosis without any vascular disruption (Figs. 2, 3, 4, 5 and 6). On MR venography, 14 children (31%) showed no evidence of venous compression, including 10 with and 4 without subdural hemorrhage. The remaining 31 children (69%) had imaging evidence of cortical vein or sinus compression. Venous compression was most commonly bilateral, in 14 of these children (45.2%), with the remainder equally distributed on the right side (8 cases, 25.8%) and left side (9 cases, 29.0%). In 17 of these 31 children (54.8%) only the cortical veins were compressed, whereas in 11 (35.5%) both cortical veins and sinuses were compressed. In three children (9.7%) only the sinus was compressed.
Patterns of venous and sinus abnormalities. Graphic demonstrates: a Positive lollipop sign. The bridging vein (short arrow) terminates in a subarachnoid blood clot (long arrow) and does not drain into the sinus. b Lack of lollipop sign. The bridging vein (short arrow) goes through the blood clot (long arrow) and subdural hemorrhage and drains into the sinus. c Positive compression sign. The bridging vein (short arrow) terminates abruptly without any associated blood clot at its termination
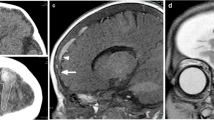
Lollipop sign in a 31-month-old boy who presented with tibial metaphyseal fracture, multiple vertebral compression fractures, bilateral subdural hemorrhage, retinal hemorrhage and diffuse hypoxic–ischemic injury of the brain. Brain MRI demonstrates lollipop sign (arrows) overlying the left cerebral convexity, which is (a) hypointense with blooming on axial T2* gradient recalled echo sequence. b, c CT venogram confirms terminal hyperattenuation on (b) unenhanced image and lack of intraluminal contrast on (c) post-contrast CT in the cortical bridging vein in question, which terminates abruptly
Cortical bridging vein in a 7-month-old boy. a Coronal MP-RAGE MR sequence demonstrates large mixed-signal-intensity subdural hematoma bilaterally with cortical bridging vein extending through the subdural hematoma and draining into the superior sagittal sinus on the left side (arrow). b Three-dimensional phase-contrast MR venography demonstrates the cortical bridging vein draining into the superior sagittal sinus. There is mild stenosis of the vein where it crosses the dura (arrow). Displacement of the cortical vein is also well demonstrated on the left side (arrowheads). MP-RAGE magnetization-prepared rapid gradient echo imaging
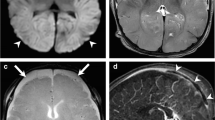
Compression sign in a 5-month-old girl with multiple rib and extremity fractures, retinal hemorrhage, cervical spine ligamentous injuries and spinal subdural hematoma. a Coronal MP-RAGE MR sequence of the brain demonstrates left-side subdural hematoma hypointense to gray matter overlying the convexity (arrow). b Three-dimensional phase-contrast MR venography shows paucity and abrupt termination of cortical bridging veins draining into the superior sagittal sinus superiorly on the left side (arrow), consistent with absent flow distally resulting from positive compression sign. No evidence of lollipop sign or intraluminal thrombosis was seen on other sequences. It is important to not mistake this appearance of paucity of cortical veins on MR venography as widespread thrombosis. MP-RAGE magnetization-prepared rapid gradient echo imaging
Lollipop and compression signs in a 2-month-old boy with multiple extremity and rib fractures. a Coronal MP-RAGE MR sequence demonstrates large subdural hemorrhage bilaterally (arrows). b Axial T2* gradient recalled echo sequence shows lollipop sign with a torn bridging vein terminating in a clot with terminal thrombosis, demonstrating blooming (arrow) with bilateral subdural hemorrhage (arrowheads). c Reconstructed MR venography sequence demonstrates paucity of cortical bridging veins draining into the superior sagittal sinus superiorly, consistent with compression. MP-RAGE magnetization-prepared rapid gradient echo imaging
MR imaging in 20 children (44%) demonstrated one or more lollipop signs. All children showing lollipop sign also showed evidence of venous compression of other cortical veins from an overlying subdural hemorrhage (Fig. 1). The lollipop sign was seen in children with only vein compression (70.6%), with only sinus compression (100%) and with both (54.5%).
Among 22 children with a small-volume subdural hemorrhage, 15 (68.2%) had evidence of venous compression and 10 (45.4%) had a lollipop sign. Among 19 cases of moderate or large subdural hemorrhage, 16 (84.2%) had evidence of venous compression and 10 (52.6%) had a lollipop sign (Table 1). The four remaining children without subdural hemorrhage had neither venous compression nor lollipop sign.
One child had isolated non-terminal long-segment venous thrombosis without any vascular disruption (Fig. 7). This case, a 3-month-old boy, presented with unusual cry, stiffening of his extremities and pallor. No history of trauma was provided but he had facial bruising and a right parietal fracture, both indicating impact trauma, and retinal hemorrhages. Neuroimaging demonstrated bilateral supratentorial and posterior fossa subdural hemorrhage, with thrombosis of the left vein of Labbé. We interpreted the venous thrombosis to be post-traumatic because (1) there was other evidence of cranial trauma and (2) bilateral supratentorial and infratentorial subdural hemorrhage is incompatible with a unilateral venous thrombosis.
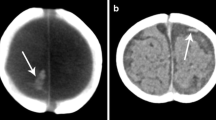
Thrombosis in a 3-month-old boy who presented with unusual cry, stiffening of extremities and pallor. No history of trauma was provided, but facial bruising, right parietal skull fracture and retinal hemorrhage were present. a Coronal head CT demonstrates bilateral supratentorial and posterior fossa subdural collection (white arrows) with hyperattenuation in the region of the left vein of Labbé (black arrow). b Axial T2 and (c) axial T2* gradient recalled echo MR sequences show thrombosis of the vein of Labbé (arrow). d MR venography demonstrates absence of vein of Labbé, consistent with thrombosis (circle). The arrow represents superior sagittal sinus and the circle represents the expected location of the left vein of Labbé
Statistical analysis
Interobserver agreement for assessment of presence or absence of intracranial venous abnormalities including thrombosis was excellent at 0.95 for lollipop sign and 1.0 for compression sign (Table 2).
Discussion
Bridging veins have two components, the subarachnoid segment and dural segment [14, 15]. Violent shaking of the infant can result in bridging vein rupture, either in the subarachnoid or subdural segment. A recent study postulated that rupture of bridging vein in subarachnoid space leads to minimal blood loss because the pressure inside and outside the vein equalizes. The torn ends then thrombose terminally [15]. On the other hand, an intradural tear of the bridging vein results in a larger subdural bleed and is likely caused by a positive feedback mechanism that leads the central venous pressure to increase as the intracranial pressure is increased [15]. In a biomechanical study of bridging vein–superior sagittal sinus complex at intermediate and high strain rates, the failure occurred in most of the cases immediately below or along the bridging vein dural cuff, suggestive of relative weakness in this region [14].
This is a systematic review of the intracranial venous system using MRI and MR venography in children with abusive head trauma (AHT). This study suggests that nearly 70% of children with AHT have some abnormality — compression, displacement, intrinsic abnormality or thrombosis — of the intracranial venous system. We have interpreted these findings to be secondary to trauma, but with increasing frequency, these types of venous abnormalities have been interpreted in court as primary cortical sinus and venous thrombosis with resultant subdural hemorrhage and retinal hemorrhage — even though there is no evidentiary support in the literature for a relationship between cortical sinus and venous thrombosis and either subdural hemorrhage or retinal hemorrhage. Finally, we would point out that the venous abnormalities are not multifocal or widespread, but cortical, not deep system, and are localized to the areas of a clot. If this were primary venous sinus thrombosis we would expect more widespread changes involving both the superficial and deep venous systems, and thrombosis of vessels in areas where there is no subdural hemorrhage. It is also worth noting that the four children without subdural hemorrhage had no evidence of lollipop sign or compression sign. It is important to identify and characterize the venous abnormalities that are seen in AHT in order to properly differentiate these cases from primary cortical sinus and venous thrombosis.
Imaging of the intracranial venous anatomy can be accomplished using a variety of techniques including formal angiography, digital subtraction angiography, CT venography and MR venography. Each of these techniques has strengths and weaknesses; MRI and MR venography are the most frequently used because of their noninvasive nature, absence of radiation exposure, and three-dimensional imaging characteristics. In addition to the standard MRI sequences, specific MRI techniques for visualizing flowing blood within the intracranial vasculature include time-of-flight and phase-contrast sequences as well as newer techniques such as 3-D elliptic-centric contrast-enhanced and post-contrast 3-D MP-RAGE (magnetization-prepared rapid gradient echo imaging) [16]. To properly interpret these images and avoid misdiagnosis, one must understand several inherent limitations of MR venography imaging, including (1) technical aspects and inherent artifacts of MR venography, (2) anatomical variations in venous anatomy, (3) variations in the appearance of intraluminal thrombus and (4) additional imaging peculiarities specific to young children.
Technical limitations and inherent artifacts of magnetic resonance venography
A number of technical artifacts on MR venography have been well described and include in-plane saturation of spins and physiological variations in both anatomy and flow (slow or alternating flow, sinus hypoplasia, complex blood flow patterns, and enhancement of late-stage thrombus mimicking blood flow on contrast-enhanced MR venography [16, 17]. Post-processing can generate images that underestimate the size of vessels and overemphasize the degree of vessel stenosis and flow gaps [17].
Finally it is important to remember that MR venography images measure flow only through the larger veins; occlusion of smaller cortical veins, below the threshold for imaging, is difficult to identify. Dynamic imaging such as digital subtraction or formal angiography might be required to demonstrate collateral venous flow, tortuous veins or delayed venous drainage [18]. Although interobserver agreement is good to excellent for sinus occlusion, greater discordance has been demonstrated in identifying isolated cortical vein thrombosis [19].
Variations in venous anatomy
A number of anatomical variants can mimic sinovenous occlusion, including sinus atresia and hypoplasia, asymmetrical and variant venous drainage patterns such as occipital sinus and sinus duplications, and normal sinus filling defects such as arachnoid granulations or septae within the sinus [20, 21]. Superficial cortical veins emptying into the dural sinuses are variable in size, morphology and location [4]. Although these veins may be of nearly equal size, more commonly there are one or two dominant veins. Three veins in particular are large and consistent enough to be named — the superficial middle cerebral vein and the veins of Trolard and Labbé [22]. There may also be significant right–left asymmetries in the size of the cortical bridging veins [23]. Four anastomotic venous patterns have been described, with varying dominance of the draining veins [23]. A variant venous drainage pattern with a paucity of cortical veins should not be construed as thrombosis with collateral drainage pattern on MR venography [22].
Variations in the appearance of venous thrombus
Direct MRI findings indicative of cortical sinus and venous thrombosis include an absence of flow void as well as the presence of altered signal intensity indicative of intraluminal clot [4]. A hyperintense vein sign has been reported as an additional direct indication of cortical vein thrombosis [4, 24]. However, there are a number of pitfalls in identifying intraluminal thrombus. Intraluminal clot in the acute stage (0–5 days) contains intracellular deoxyhemoglobin within red blood cells and therefore is predominantly isointense on T1- and hypointense on T2-weighted images, mimicking a flow void and leading to diagnostic error [4]. Similarly, beyond 15 days a partly recanalized thrombus might be iso- or hyperintense on T2- and isointense on T1-weighted images and appear similar to slowly moving oxygenated blood. The thrombus is easier to diagnose during the subacute stage (6–15 days) because then it is predominantly hyperintense on both T1- and T2-weighted images [4]. Post-contrast MR venography can also be challenging in more chronic stages because there is sometimes enhancement of a thrombus as well as slow flow in dural and intrathrombus collateral channels. Restricted diffusion is observed in 41% of patients with cortical sinus and venous thrombosis [25] and might be identified as blooming on susceptibility-weighted or gradient echo sequences. However, it is crucial to differentiate blood within the vessel from blood outside the vessel and within the adjacent subarachnoid or subdural spaces [26], making the interpretation of intraluminal thrombosis particularly challenging in the setting of alleged abusive head trauma.
Imaging peculiarities in children
There are a number of special considerations in imaging infants and young children. The venous sinuses are smaller and blood flow is slower in infants [27], which can lead to increased turbulence, intraluminal heterogeneity, voxel dephasing and artifactual flow gaps in the infant venous sinuses, particularly the superior sagittal sinus on MR venography. Focal narrowing of the superior sagittal sinus has been demonstrated in infants at the junction of the sagittal and lambdoid sutures (lambda) in the supine position because of inbending of the adjacent occipital bone [27, 28]. Additional changes in superior sagittal sinus blood flow can be induced in infants from both positional changes and occipital pressure [29]. These effects have been thought to predispose infants to cortical sinus and venous thrombosis [30]. Changes in intracranial pressure (such as idiopathic intracranial hypertension as well as spontaneous intracranial hypotension) are known to alter the caliber of the dural venous sinuses. Globally reduced cerebral blood flow can also produce flow gaps in the sinuses and veins.
Primary versus secondary thrombosis
The primary diagnostic consideration in the setting of venous abnormalities is primary cortical sinus and venous thrombosis, a rare disorder with an incidence of approximately 2–7 cases per million people. It is estimated that a typical tertiary care referral center could therefore see 5–8 cases per year [4]. In children, predisposing factors are present in up to 95% [3, 31, 32]. Cortical sinus and venous thrombosis is usually multifocal; among 510 cases of cortical sinus and venous thrombosis reported in the literature between 1995 and 2003, cortical sinus and venous thrombosis was multifocal in 90% of cases — 63% involved the superior sagittal sinus and cortical veins, 57% involved the transverse sinus, and 15% involved the sigmoid sinus. Isolated cortical vein thrombosis was present in only 6% of cases [4].
Most cases of cortical sinus and venous thrombosis are associated with some identifiable precipitating factor. Outside of the perinatal period, predisposing factors include dehydration, an underlying malignancy or chemotherapy (primarily with asparaginase), iron deficiency anemia, infection or sepsis, or thrombophilia from, for example, protein C or protein S deficiency, MTHFR gene mutations or oral contraceptive use [4, 31]. Trauma is an important precipitating factor of cortical sinus and venous thrombosis and can be caused by direct trauma (laceration) to a sinus or vein; sinus compression (leading to slowing or cessation of flow) by a depressed skull fracture, adjacent hematoma or cerebral edema or swelling [33]; and intramural hemorrhage caused by rupture of small sinusoids, injury to the endothelial lining, or extension of thrombus from injured emissary veins [34]. Traumatic dural venous sinus thrombosis can be found in 40% of patients with blunt head trauma who had skull fracture extending to a dural venous sinus or jugular bulb [35]. Our case of thrombosis of the vein of Labbé probably represents a similar etiology of secondary thrombosis.
The clinical presentation of primary cortical sinus and venous thrombosis is well described in children and is almost universally one of a progressive, subacute decline, often over several days, usually in the context of another identifiable illness or process. This stands in stark contrast to the majority of infants with AHT, who suffer an acute, significant and prolonged neurological collapse. Seizures can occur in both conditions, causing an abrupt change in neurological function with subsequent recovery. However abrupt collapse (within seconds or minutes) with prolonged coma in a previously healthy child has not been described in primary cortical sinus and venous thrombosis to our knowledge. Although retinal hemorrhage has been described in retinal vein thrombosis, these hemorrhages are readily distinguished from those that occur in AHT, and multiple, multilayered retinal hemorrhages have not been described in any child with primary cortical sinus and venous thrombosis. In contrast, retinal hemorrhages are present in approximately 80% of children with AHT, the number, pattern and distribution of which are quite distinctive in most cases, and their presence has been highly correlated with AHT in multiple studies [36].
The neuroimaging features of cortical sinus and venous thrombosis have also been described in multiple studies [4–8]. An obvious feature is absence of the normal veins or sinus on MRI or MR venography, but equally important is the demonstration of a thrombus within the vessel lumen on MRI. Other important secondary findings of primary cortical sinus and venous thrombosis include parenchymal edema, hemorrhagic infarction and increased flow through collateral veins. Subarachnoid hemorrhage is relatively common and there are rare reports of associated subdural effusions that, compared with cerebrospinal fluid, are isodense on CT and isointense on MRI. Among 160 children with cortical sinus and venous thrombosis described by deVeber et al. [31], 14 (9%) had “extra-axial collections,” but neither the location (epidural, subdural or subarachnoid) nor nature (hemorrhage, effusion) of these collections was described. Multiple studies have documented the absence of acute subdural hemorrhage among children with cortical sinus and venous thrombosis [5, 37–39]; in fact, we cannot find a single reported example of acute subdural hemorrhage in the setting of isolated or primary pediatric cortical sinus and venous thrombosis.
The routine use of MR venography since 2008 in suspected AHT at our institute has revealed a number of previously unreported abnormalities of the intracranial venous system. The presence of cortical vein displacement and narrowing in areas with adjacent subdural hemorrhage we interpret to be caused by primary trauma to the parasagittal bridging veins. In this series we sometimes found intact cortical veins running through the subdural hematoma with no evidence of thrombosis (Fig. 4), but in nearly half of cases we found at least one vein that was abruptly cut off with adjacent blood clot and no demonstrated flow distal to the point of disruption, a radiographic finding that we have called the lollipop sign. We interpret this to be a sign of bridging vein rupture, usually over the convexity near its attachment to the superior sagittal sinus. These torn and thrombosed bridging vessels have also been recently described at autopsy [6]. Although extrinsic compression and vasospasm are considerations, these disrupted vessels did not reappear on delayed imaging when it was performed. Direct evidence of non-terminal long-segment intraluminal thrombus was seen in only one of our cases, and we determined this to be secondary to traumatic injury of the vessel.
The abnormalities described in this series may superficially mimic primary cortical sinus and venous thrombosis; in fact, trauma is an identified predisposing factor in primary cortical sinus and venous thrombosis so it is not surprising that others have (we think erroneously) interpreted these neuroradiologic findings as primary cortical sinus and venous thrombosis, and the question arises as to which came first. Our interpretation of these as a consequence of trauma is supported by other evidence of both cranial and extracranial trauma in two-thirds of these children, the abrupt onset of symptoms in these children, and the presence of subdural hemorrhage and retinal hemorrhage that, to our knowledge, have not been reported in non-traumatic cortical sinus and venous thrombosis. Additionally, it is illogical to conclude that isolated cortical vein thrombosis leads to diffuse parenchymal injury, four-compartment subdural hemorrhage, or retinal hemorrhage.
It is also important to review all of the MRI sequences and the source images from the MR venography for both primary and secondary evidence of cortical sinus and venous thrombosis. In particular, differentiating intraluminal thrombus from extraluminal blood in the presence of extra-axial hemorrhage should be undertaken with caution because this can be misleading. This study confirms that true intraluminal thrombus is uncommonly identified in abusive head trauma. Contrast-enhanced MR venography, dynamic MR venography and post-contrast MP-RAGE, CT venogram and even digital subtraction angiography or angiography might provide additional information, although we think the total clinical and neuroimaging features are sufficiently distinctive that such studies are rarely needed.
It has also been suggested by some that a thrombophilia workup be undertaken in all cases of suspected AHT to exclude primary cortical sinus and venous thrombosis as a mimic of AHT [40]. A normal screening MR venography will almost certainly exclude cortical sinus and venous thrombosis; conversely, additional studies are warranted if the clinical and neuroimaging features suggest cortical sinus and venous thrombosis as a diagnostic probability. Second, like clotting factors, norms of thrombophilic factors are expressed as quantitative ranges and some individuals may have only mildly abnormal values that are of dubious clinical significance; the mere presence of an abnormal value is not synonymous with a clinical thrombotic condition [41, 42]. Third, some of the thrombophilic factors such as proteins C and S are nonspecifically altered in the setting of acute conditions [41, 43, 44]. Fourth, norms for infants have not been firmly established [41, 42, 45]. Last, the mere presence of such a marker does not exclude AHT. A finding of an altered thrombophilic marker must be interpreted in the context of the entire case.
This study has several limitations. First it is retrospective and selective. Second, in the interest of full disclosure, there is one case for which another “expert witness” testified to the presence of cortical sinus and venous thrombosis and the perpetrator was found not guilty. For all of the reasons cited above, we respectfully disagree with the conclusions drawn by this other individual. Third, the issue of circular reasoning has been raised with respect to all research concerning AHT, the accusation being that the determination of AHT is subjective and the inclusion of cases identified in this manner results in a pre-ordained outcome. Because the primary diagnostic consideration in the present study is cortical sinus and venous thrombosis, the presence of other indicia of trauma in two-thirds of the cases and the similarities among those without other such indicia reduce the potential for this type of bias.
Another limitation of our retrospective study is that prior to 2008 some children had MR venography and some did not, quite possibly depending upon the source of referral and also the severity of abusive head trauma. Subsequently, as we learned more about the venous injury seen in abusive head trauma, we started obtaining MR venography routinely for all infants suspected of having abusive head trauma. Therefore, our findings cannot be generalized to all infants with abusive head trauma, but only to those selected for our study by having the necessary imaging.
This study suggests that MR venography adds information about the nature and pathophysiology of AHT, but the modality might also create confusion when studies are interpreted by a radiologist who is not well-versed in the technical limitations, pediatric-specific issues, anatomical variants, and other limitations of such studies. It is hazardous to establish a diagnosis of intraluminal thrombosis based solely on MR venography without consideration of all of the other MRI sequences and MR venography source images. We recommend that all such images be evaluated by an experienced pediatric neuroradiologist and that consideration be given to multi-reviewer interpretations of difficult or challenging cases. Spinal subdural hemorrhage and cervical spine ligamentous injuries have been seen in a higher proportion of abusive head trauma cases as compared to accidental trauma cases, and the mechanism of injury has been postulated as a possible differentiating factor [46, 47]. Although more research is needed, the presence of the lollipop sign in conjunction with spinal subdural hemorrhage and cervical spine ligamentous injuries might reflect a mechanism of shaking and abusive head trauma as causative etiology.
Conclusion
This is a comprehensive systematic review of the neuroimaging abnormalities involving the intracranial venous system in cases of abusive head trauma (AHT) and adds to our understanding of the imaging findings and pathophysiology of subdural hemorrhage in AHT. There are frequent abnormalities of the venous system in AHT. Although signs of bridging vein injury such as subdural hemorrhage, cortical vein compression or displacement, and the lollipop sign are common, it is rare to directly demonstrate intraluminal clot. Trauma is a well-described predisposing factor in cortical sinus and venous thrombosis and establishing trauma as the etiology of the venous abnormality is usually not difficult if the entire clinical and neuroimaging findings are considered. The routine use of MR venography might address the legal challenge of thrombophilia and cortical sinus and venous thrombosis as a mimic of AHT, but abnormal findings should be interpreted by an experienced pediatric neuroradiologist with knowledge about the limitations and pitfalls of MR venography in order to make the appropriate diagnosis.
References
Makaroff KL, Putnam FW (2003) Outcomes of infants and children with inflicted traumatic brain injury. Dev Med Child Neurol 45:497–502
Christian CW, Block R, Committee on Child Abuse and Neglect, Pediatrics American Academy of Pediatrics (2009) Abusive head trauma in infants and children. Pediatrics 123:1409–1411
Hedlund GL, Frasier LD (2009) Neuroimaging of abusive head trauma. Forensic Sci Med Pathol 5:280–290
Leach JL, Fortuna RB, Jones BV et al (2006) Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 26:S19–S41
McLean LA, Frasier LD, Hedlund GL (2012) Does intracranial venous thrombosis cause subdural subdural hemorrhage in the pediatric population? AJNR Am J Neuroradiol 33:1281–1284
Bracken J, Barnacle A, Ditchfield M (2013) Potential pitfalls in imaging of paediatric cerebral sinovenous thrombosis. Pediatr Radiol 43:219–231
Hedlund GL (2013) Cerebral sinovenous thrombosis in pediatric practice. Pediatr Radiol 43:173–188
Eichler F, Krishnamoorthy K, Grant PE (2007) Magnetic resonance imaging evaluation of possible neonatal sinovenous thrombosis. Pediatr Neurol 37:317–323
Adamsbaum C, Rambaud C (2012) Abusive head trauma: don’t overlook bridging vein thrombosis. Pediatr Radiol 42:1298–1300
Barlow KM, Gibson RJ, McPhillips M et al (1999) Magnetic resonance imaging in acute non-accidental head injury. Acta Paediatr 88:734–740
Maxeiner H (2001) Demonstration and interpretation of bridging vein ruptures in cases of infantile subdural bleedings. J Forensic Sci 46:85–93
Morris MW, Smith S, Cressman J et al (2000) Evaluation of infants with subdural hematoma who lack external evidence of abuse. Pediatrics 105:549–553
Stein KM, Ruf K, Ganten MK et al (2006) Representation of cerebral bridging veins in infants by postmortem computed tomography. Forensic Sci Int 163:93–101
Monea AG, Baeck K, Verbeken E et al (2014) The biomechanical behaviour of the bridging vein–superior sagittal sinus complex with implications for the mechanopathology of acute subdural haematoma. J Mech Behav Biomed Mater 32:155–165
Miller JD, Nader R (2014) Acute subdural hematoma from bridging vein rupture: a potential mechanism for growth. J Neurosurg 120:1378–1384
Klingebiel R, Bauknecht HC, Bohner G et al (2007) Comparative evaluation of 2D time-of-flight and 3D elliptic centric contrast-enhanced MR venography in patients with presumptive cerebral venous and sinus thrombosis. Eur J Neurol 14:139–143
Ayanzen RH, Bird CR, Keller PJ et al (2000) Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol 21:74–78
Boukobza M, Crassard I, Bousser MG et al (2009) MR imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. AJNR Am J Neuroradiol 30:344–348
Ferro JM, Morgado C, Sousa R et al (2007) Interobserver agreement in the magnetic resonance location of cerebral vein and dural sinus thrombosis. Eur J Neurol 14:353–356
Widjaja E, Griffiths PD (2004) Intracranial MR venography in children: normal anatomy and variations. AJNR Am J Neuroradiol 25:1557–1562
Curé JK, Van Tassel P, Smith MT (1994) Normal and variant anatomy of the dural venous sinuses. Semin Ultrasound CT MR 15:499–519
Osborn AG (1994) Normal vascular anatomy. In: Osborn AG (ed) Diagnostic neuroradiology, 1st edn. Mosby, Maryland Heights, pp 145–152
Rhoton AL (2002) The cerebral veins. Neurosurgery 51:S159–S205
Thamburaj K, Choudhary A (2009) Hyperintense vessel sign: isolated cortical venous thrombosis after L-asparaginase therapy. Pediatr Radiol 39:757
Favrole P, Guichard JP, Crassard I et al (2004) Diffusion-weighted imaging of intravascular clots in cerebral venous thrombosis. Stroke 35:99–103
Leach JL, Strub WM, Gaskill-Shipley MF (2007) Cerebral venous thrombus signal intensity and susceptibility effects on gradient recalled-echo MR imaging. AJNR Am J Neuroradiol 28:940–945
Widjaja E, Shroff M, Blaser S et al (2006) 2D time-of-flight MR venography in neonates: anatomy and pitfalls. AJNR Am J Neuroradiol 27:1913–1918
Newton TH, Gooding CA (1975) Compression of superior sagittal sinus by neonatal calvarial molding. Radiology 115:635–640
Cowan F, Thoresen M (1985) Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Pediatrics 75:1038–1047
Tan M, Deveber G, Shroff M et al (2011) Sagittal sinus compression is associated with neonatal cerebral sinovenous thrombosis. Pediatrics 128:e429–e435
DeVeber G, Andrew M, Adams C et al (2001) Cerebral sinovenous thrombosis in children. N Engl J Med 345:417–423
Dlamini N, Billinghurst L, Kirkham FJ (2010) Cerebral venous sinus (sinovenous) thrombosis in children. Neurosurg Clin N Am 21:511–527
Steinborn M, Schäffeler C, Kabs C et al (2010) CT and MR imaging of primary cerebrovascular complications in pediatric head trauma. Emerg Radiol 17:309–315
Huisman TA, Holzmann D, Martin E et al (2001) Cerebral venous thrombosis in childhood. Eur Radiol 11:1760–1765
Delgado Almandoz JE, Kelly HR, Schaefer PW et al (2010) Prevalence of traumatic dural venous sinus thrombosis in high-risk acute blunt head trauma patients evaluated with multidetector CT venography. Radiology 255:570–577
Binenbaum G, Forbes BJ (2014) The eye in child abuse: key points on retinal hemorrhages and abusive head trauma. Pediatr Radiol 44:S571–S577
Sébire G, Tabarki B, Saunders DE et al (2005) Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain 128:477–489
Lafitte F, Boukobza M, Guichard JP et al (1999) Deep cerebral venous thrombosis: imaging in eight cases. Neuroradiology 41:410–418
Teksam M, Moharir M, Deveber G et al (2008) Frequency and topographic distribution of brain lesions in pediatric cerebral venous thrombosis. AJNR Am J Neuroradiol 29:1961–1965
Barnes PD (2011) Imaging of nonaccidental injury and the mimics: issues and controversies in the era of evidence-based medicine. Radiol Clin N Am 49:205–229
Goldenberg NA, Manco-Johnson MJ (2008) Protein C deficiency. Haemophilia 14:1214–1221
Monagle P, Ignjatovic V, Savoia H (2010) Hemostasis in neonates and children: pitfalls and dilemmas. Blood Rev 24:63–68
Bauer KA (2001) The thrombophilias: well-defined risk factors with uncertain therapeutic implications. Ann Intern Med 135:367–373
D’Angelo A, Vigano-D’Angelo S, Esmon CT et al (1988) Acquired deficiencies of protein S. Protein S activity during oral anticoagulation, in liver disease, and in disseminated intravascular coagulation. J Clin Invest 81:1445–1454
Lippi G, Salvagno GL, Rugolotto S et al (2007) Routine coagulation tests in newborn and young infants. J Thromb Thrombolysis 24:153–155
Choudhary AK, Bradford RK, Dias MS et al (2012) Spinal subdural hemorrhage in abusive head trauma: a retrospective study. Radiology 262:216–223
Choudhary AK, Ishak R, Zacharia TT et al (2014) Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol 44:1130–1140
Conflicts of interest
Dr. M. Dias provides expert witness testimony in child abuse cases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choudhary, A.K., Bradford, R., Dias, M.S. et al. Venous injury in abusive head trauma. Pediatr Radiol 45, 1803–1813 (2015). https://doi.org/10.1007/s00247-015-3399-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3399-4