Abstract
Objective
To evaluate the effect of a newly developed connecting tube, which generates a spiral flow of saline, on aortic and hepatic contrast enhancement during hepatic-arterial phase (HAP) and portal venous phase (PVP) computed tomography (CT).
Methods
Eighty patients were randomly assigned to one of two protocols: with a new or a conventional tube. The contrast material (600 mgI/kg) was delivered over 30 s; this was followed by the administration of 25 ml saline solution delivered at the same injection rate as the contrast material. Unenhanced and contrast-enhanced CT images of the upper abdomen were obtained. We calculated the changes in the CT number (∆HU) for the aorta during HAP and PVP, and for the liver during PVP. We compared ∆HU between protocols.
Results
The mean ∆HU for the abdominal aorta during HAP was significantly higher with the new tube protocol than with the conventional tube protocol (322 ± 53 vs. 290 ± 53, P < 0.01). There were no significant differences in the mean ∆HU for the abdominal aorta and liver during PVP between the two protocols (P > 0.05).
Conclusion
The new connecting tube increased the effect of a saline chaser and significantly improved aortic enhancement during HAP.
Key Points
• Optimal administration of intravenous contrast material is essential for optimal CT quality.
• A new connecting tube can generate spiral flow, which improves intravenous administration.
• The new connecting tube improved aortic contrast enhancement during the hepatic-arterial phase.
• The new connecting tube increased the effect of a saline chaser.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A saline chaser is routinely used in modern multidetector computed tomography (MDCT) injection protocols, especially for CT angiography (CTA) [1–5]. The saline pushes the contrast medium bolus to the central blood volume and causes late peak enhancement at the end of the injection. The consequence is increased intravascular contrast enhancement due to higher efficiency of the injected contrast medium [6–10].
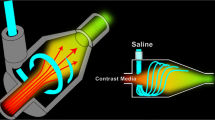
To increase the transport efficiency of contrast medium to the central blood, previous studies analysed the effect of the injection rate and volume of a saline chaser [1, 6, 11–13]. However, to our knowledge, the effects of the type of liquid motion have not been analysed. For pneumatic transportation in a pipeline, previous reports suggested that spiral flow has a higher carrying capacity than laminar flow [14, 15]. Therefore, we hypothesised that a saline chaser with spiral flow might push the contrast medium bolus to the central blood volume more efficiently and increase vessel enhancement. Recently, we developed a new connecting tube for the saline chaser, which generates a spiral flow of saline, to confirm this hypothesis.
The purpose of this study was to evaluate the effect of different connecting tubes on aortic and hepatic contrast enhancement during hepatic-arterial phase (HAP) CT and portal venous phase (PVP) CT.
Materials and methods
This prospective study received institutional review board approval; prior informed consent to participate was obtained from all patients.
Connecting tubes
The conventional connecting tube included a T-shaped joint (Fig. 1). The capacity of the conventional connecting tube was 2.2 ml and its length was 60 cm.
The conventional connecting tube (the bi-directional type extension tube for dual injector). The saline inflow opening (red arrow) and the contrast material inflow opening (black arrow). The conventional connecting tube included a T-shaped joint. Both contrast agent injection and saline solution flushing can be handled with one tube
The new connecting tube includes a chamber for generating a spiral flow (Fig. 2a). The spiral flow-generating chamber has a curved inner surface, which is a circumferential inner surface forming a columnar space. The saline inflow opening extends in the tangential direction of the circumference of the curved inner surface (Fig. 2b). The capacity of the new connecting tube was 3.3 ml and its length was 57 cm. In clinical study, the connecting tube was connected to the extension tube (the capacity was 1.0 ml).
The new connecting tube. a The saline inflow opening (red arrow) is formed on an end surface in front of the spiral flow-generating chamber (yellow arrow), and the contrast material inflow opening (black arrow) is formed on a side surface of the spiral flow-generating chamber. b The spiral flow-generating chamber (yellow arrow) has a curved inner surface, which is a circumferential inner surface forming a columnar space. The saline inflow opening (red arrow) extends in the tangential direction of the circumference of the curved inner surface. This causes the generation of a spiral flow of the saline
Movie 1 (Electronic Supplementary Material) was an image obtained by actually imaging the pushed state of the contrast agent using the new connecting tube. The portion appearing black in Movie 1 was the flow of the contrast agent. As understood from Movie 1, the new connecting tube was able to generate the spiral flow.
Phantom study
An outline of our flow phantom that can simulate the circulatory system of a human weighing 60 kg was presented in the previous study [16]. The flow rate of the pump was set at 5.2 l/min, a rate equivalent to cardiac output flow rate in a human weighing about 60 kg. The contrast material used in the phantom study was iohexol with an iodine concentration of 350 mg/ml; it was administered with a double-headed power injector (Dual Shot; Nemoto-Kyorindo, Tokyo, Japan). The phantom was completely flushed with water after each experiment. The new and the conventional tubes were used during injection protocols 1 and 2. As shown in Table 1, during injection protocol 1 we administered doses of 30 ml at injection rates of 3.0 ml/s over a period of 10 s. During injection protocol 2 we administered doses of 40 ml at injection rates of 4.0 ml/s over a period of 10 s. During the two injection protocols contrast injections were followed by the administration of 20 ml saline solution delivered at the same injection rate as the contrast material. All protocols were repeated two times. We used a 16-detector CT system (Optima CT 580 W; GE Medical Systems, Milwaukee, USA). Unenhanced axial CT images were obtained to provide a baseline CT value for the phantom aorta. Then, single-level serial CT datasets of the simulated aorta were acquired at 2-s intervals from 2 to 100 s after the start of contrast medium delivery. Unenhanced and single-level serial CT datasets were also obtained; the CT parameters were 1.0-s rotation time, 5.0-mm image thickness, 15-cm field of view (FOV), 50 mA, and 120 kVp.
To measure the attenuation values of the simulated aorta, we used a circular region-of-interest cursor on all images of the single-level serial CT dataset. Attempts were made to maintain a constant ROI area of approximately 5 cm2. Enhancement of the simulated aorta was calculated as the absolute difference in the attenuation value (in Hounsfield units) between the unenhanced image and each single-level serial CT image. For each injection protocol we calculated the mean aortic enhancement value in each of two repeat experiments and constructed time-enhancement curves by connecting all time points. We compared maximum CT values of the new and the conventional tube groups.
Clinical study
Between May and June 2012, 84 consecutive patients were considered for participation in this prospective study. We recorded body weight to tailor the amount of contrast medium used.
Our inclusion criteria were a suspicion of metastatic liver tumour and patients who had undergone HAP and PVP contrast-enhanced CT at 120 kVp.
Patients (n = 4) with renal failure (eGFR less than 30 ml/min/1.73 m2), a history of allergic reactions to iodinated contrast media and proven or suspected pregnancy were excluded; they were three patients with an eGFR of less than 30 ml/min/1.73 m2 and one patient with a history of previous reactions to iodinated contrast media. Consequently, 80 patients were enrolled in this study; they were 46 men and 34 women, ranging in age from 26 to 86 years (mean 63.9 years).
CT and contrast infusion protocols
All patients underwent CT using 256-row MDCT equipment (Brilliance iCT; Philips Healthcare, Cleveland, USA). Of the 80 enrolled patients, 40 patients were randomly assigned to the new tube protocol using a random table. The other 40 patients were assigned to the conventional tube protocol.
In all examinations, Iohexol-300 (Omnipaque-300; Daiichi-Sankyo, Tokyo, Japan) was delivered via a 20-gauge catheter inserted into the antecubital vein; a power injector (DUAL SHOT; Nemoto-Kyorindo, Tokyo, Japan) was used. All patients were examined in accordance with the hospital’s routine protocol. The contrast material (600 mg iodine per kilogram of body weight) was delivered over 30 s; this was followed by the administration of 25 ml saline solution delivered at the same injection rate as the contrast material.
CT data acquisition for all helical CT studies was started at the top of the liver and proceeded in a cephalocaudal direction; unenhanced and HAP and PVP contrast-enhanced helical CT datasets were obtained. The anatomical range varied from patient to patient. An automatic bolus-tracking program (Bolus Pro Ultra; Philips Medical Systems, Cleveland, USA) was used to time the start of CT data acquisition for each phase after contrast injection. HAP and PVP CT were started 18 and 55 s after triggering. The patients were instructed to hold their breath with tidal inspiration during CT data acquisition. Image reconstruction was performed in a 25- to 35-cm display FOV, depending on the patient’s physique.
Quantitative image analysis
Mean CT numbers in Hounsfield units for the abdominal aorta at L2 and liver were measured in all patients with a CT console monitor by placement of a circular region-of-interest cursor, which ranged in diameter from 10 to 30 mm. CT numbers in the aorta were measured on unenhanced, HAP and PVP images. CT numbers in hepatic parenchyma were measured on unenhanced and PVP images. Focal hepatic lesions, blood vessels, bile ducts, calcifications and artefacts were carefully excluded from regions of interest. The degree of contrast enhancement is expressed as the change in CT number (∆HU), which was calculated by subtracting CT numbers on unenhanced images from those on contrast-enhanced images. We compared ∆HU for the abdominal aorta during HAP and for the abdominal aorta and liver during PVP between the two protocols.
Statistical analysis
To compare maximum CT value measured in the phantom study, ∆HU and the contrast medium administration parameters between the two protocols, we used two-tailed Student’s t-test. Differences of P < 0.05 were considered statistically significant. Statistical analyses were performed with the free statistical software, R (version 2.6.1; The R Project for Statistical Computing; http://www.r-project.org/).
Results
Phantom study
Figure 3 shows the time-enhancement curves for injection protocols 1 and 2. The maximum CT value in injection protocol 1 was: new tube, 295.6 ± 1.3 HU; conventional tube, 254.5 ± 1.4 HU (P < 0.01 [95 % confidence interval 35.3, 46.9). The maximum CT value in injection protocol 2 was: new tube, 374.1 ± 8.8 HU; conventional tube, 300.7 ± 4.0 HU (P < 0.01 [95 % confidence interval 43.8, 102.8]) (Table 2). In the same tube group, the magnitude of aortic peak enhancement increased with increasing the contrast material dose.
a The time-enhancement curve for protocol 1. The maximum CT value in flow rate 3.0 ml/s was: conventional tube, 254.5 ± 1.4HU; new tube, 295.6 ± 1.3HU (P < 0.01). b The time-enhancement curve for protocol 2. The maximum CT value in flow rate 4.0 ml/s was: conventional tube, 300.7 ± 4.0HU; new tube, 374.1 ± 8.8HU (P < 0.01)
Clinical study
The baseline characteristics of our study subjects are shown in Table 3. There was no significant difference between the patients subjected to the two protocols with respect to sex, body weight and age. There were no significant differences in contrast medium injection rates and volumes between the two protocols (Table 3).
The mean ∆HU for the abdominal aorta during HAP was significantly higher with the new tube protocol than with the conventional tube protocol (abdominal aorta, 322 ± 53 vs 290 ± 53, P < 0.01 [95 % confidence interval 8.5, 55.6]) (Fig. 4 and Table 4).
Results of the quantitative analysis. The mean ∆HU for the abdominal aorta during HAP was significantly higher with the new tube protocol than with the conventional tube protocol (abdominal aorta, 322 ± 53 vs 290 ± 53, P < 0.01). There was no significant difference in the mean ∆HU for the abdominal aorta and liver during PVP between the new and conventional tube protocols (abdominal aorta, 112 ± 18 vs 109 ± 14, P = 0.41; hepatic parenchyma, 55 ± 10 vs 54 ± 9, P = 0.87)
There was no significant difference in the mean ∆HU for the abdominal aorta and liver during PVP between the new and conventional tube protocols (abdominal aorta, 112 ± 18 vs 109 ± 14, P = 0.41 [95 % confidence interval −4.3, 10.4]; hepatic parenchyma, 55 ± 10 vs 54 ± 9, P = 0.87 [95 % confidence interval −3.9, 4.6]) (Fig. 4 and Table 4).
Discussion
The results of phantom study supported the effectiveness of the new connecting tube. In the phantom study, the maximum CT value was significantly higher with the new tube group than with the conventional tube group regardless of the injection rate. Furthermore, in the phantom study, when the injection duration was constant, the magnitude of aortic peak enhancement increased with increasing the contrast material dose in the same tube group. This finding was consistent with the results actually observed in humans in previous studies [17].
Previous studies reported that a saline chaser improves intravascular contrast enhancement because a saline chaser has a transportation role for contrast medium; that is, the saline pushes the contrast medium from the peripheral vein to the central vessels [4, 6]. To increase the transport efficiency of contrast medium to the central blood, previous studies analysed the effect of the injection rate and volume of a saline chaser [10]. However, to our knowledge, the effects of spiral flow of saline chaser have not been analysed in contrast agent injection protocols.
Our clinical study demonstrated that the new connecting tube significantly improved aortic contrast enhancement during HAP; however, there was no statistically significant difference in enhancement of aorta and liver during PVP. A possible explanation for this finding is that the tail end of the administered contrast medium bolus is pushed forward more efficiently into the venous system when the saline solution is injected at spiral flow. Thereby, the volume of iodine delivery into the arterial system might increase, resulting in a greater magnitude of aortic enhancement. This phenomenon was not reported in the study about saline chaser; however, previous reports suggested that spiral flow has a higher carrying capacity than laminar flow for pneumatic transportation in a pipeline [14, 15]. Other characteristics of spiral flow were reported as follows. (1) The spiral flow technique has a large kinetic energy to accelerate particles at the initial conveying stage. (2) Critical air velocities of spiral flow pneumatic conveying are lower than those of axial flow pneumatic conveying. (3) The power consumption is decreased by a spiral flow pneumatic conveying system. On the basis of our results, the spiral flow of saline chaser might also increase the transportation efficiency of the contrast medium.
The new connecting tube may increase intravascular contrast enhancement due to higher efficiency of the injected contrast medium in CTA; in addition, the new connecting tube may allow contrast medium dose saving. We believe further clinical studies might be needed to determine the usefulness of the new connecting tube.
There are limitations to our study. The major limitation of our study was that we did not directly prove the transfer of the spiral flow from the chamber over the venous access of the patient into the blood vessels. We need to observe the motion of liquid ejected from the 20-gauge venous catheter in a small blood vessel phantom (we also need to produce the slow flow in blood vessel phantom). Unfortunately, in our institution, we were not able to use such a phantom system or perform a phantom study. However, we believe our study supported that the spiral flow was transferred from the chamber over the venous access of the patient into the blood vessels. Secondly, we did not evaluate the differences between the cardiovascular statuses of the two patient groups. This could have contributed to the differences in enhancement between the protocols [18]. However, we think that random assignment nearly equalised the influence of the cardiovascular status.
In conclusion, our study demonstrated that the new connecting tube increased the effect of saline chaser and significantly improved aortic contrast enhancement during HAP.
References
Kim DJ, Kim TH, Kim SJ et al (2008) Saline flush effect for enhancement of aorta and coronary arteries at multidetector CT coronary angiography. Radiology 246:110–115
Schoellnast H, Tillich M, Deutschmann HA et al (2004) Improvement of parenchymal and vascular enhancement using saline flush and power injection for multiple-detector-row abdominal CT. Eur Radiol 14:659–664
Schoellnast H, Tillich M, Deutschmann MJ, Deutschmann HA, Schaffler GJ, Portugaller HR (2004) Aortoiliac enhancement during computed tomography angiography with reduced contrast material dose and saline solution flush: influence on magnitude and uniformity of the contrast column. Invest Radiol 39:20–26
Haage P, Schmitz-Rode T, Hubner D, Piroth W, Gunther RW (2000) Reduction of contrast material dose and artifacts by a saline flush using a double power injector in helical CT of the thorax. AJR Am J Roentgenol 174:1049–1053
Irie T, Kajitani M, Yamaguchi M, Itai Y (2002) Contrast-enhanced CT with saline flush technique using two automated injectors: how much contrast medium does it save? J Comput Assist Tomogr 26:287–291
Behrendt FF, Bruners P, Keil S et al (2010) Effect of different saline chaser volumes and flow rates on intravascular contrast enhancement in CT using a circulation phantom. Eur J Radiol 73:688–693
Lee K, Kishimoto M, Shimizu J, Iwasaki T, Miyake Y, Yamada K (2010) Effect of a saline chaser for contrast enhancement of computed tomographic angiography in cattle. Vet Rec 166:137–139
Yoon DY, You SY, Choi CS et al (2006) Multi-detector row CT of the head and neck: comparison of different volumes of contrast material with and without a saline chaser. Neuroradiology 48:935–942
de Monye C, Cademartiri F, de Weert TT, Siepman DA, Dippel DW, van Der Lugt A (2005) Sixteen-detector row CT angiography of carotid arteries: comparison of different volumes of contrast material with and without a bolus chaser. Radiology 237:555–562
Behrendt FF, Jost G, Pietsch H et al (2011) Computed tomography angiography: the effect of different chaser flow rates, volumes, and fluids on contrast enhancement. Invest Radiol 46:271–276
Schindera ST, Nelson RC, Howle L, Nichols E, DeLong DM, Merkle EM (2008) Effect of varying injection rates of a saline chaser on aortic enhancement in CT angiography: phantom study. Eur Radiol 18:1683–1689
Marin D, Nelson RC, Guerrisi A et al (2011) 64-section multidetector CT of the upper abdomen: optimization of a saline chaser injection protocol for improved vascular and parenchymal contrast enhancement. Eur Radiol 21:1938–1947
Lee CH, Goo JM, Bae KT et al (2007) CTA contrast enhancement of the aorta and pulmonary artery: the effect of saline chase injected at two different rates in a canine experimental model. Invest Radiol 42:486–490
Li H, Tomita Y (1998) An experimental study of swirling flow pneumatic conveying system in a vertical pipeline. J Fluid Eng 120:200–203
Li H, Tomita Y (1996) An experimental study of swirling flow pneumatic conveying system in a horizontal pipeline. J Fluid Eng 118:526–530
Awai K, Hatcho A, Nakayama Y et al (2006) Simulation of aortic peak enhancement on MDCT using a contrast material flow phantom: feasibility study. AJR Am J Roentgenol 186:379–385
Awai K, Hiraishi K, Hori S (2004) Effect of contrast material injection duration and rate on aortic peak time and peak enhancement at dynamic CT involving injection protocol with dose tailored to patient weight. Radiology 230:142–150
Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256:32–61
Acknowledgements
There was no direct financial support from industry for this study. This study received the connecting tube from Nemoto-Kyorindo, Tokyo, Japan, the manufacturer of the connecting tube used in this study. K.Y. is an employee of Nemoto-Kyorindo. Y.P. is chief executive officer of EBM, Tokyo, Japan. Y.P. and T.Y have pending and/or awarded patents for the subject matter described in the manuscript and receive royalty income for a patent license from Nemoto-Kyorindo. The authors (M.K, T.N, K.A, T.K, K.H, Y.Y) who were not employees of Nemoto-Kyorindo or EBM had control of data and information that might have presented a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
An image obtained by actually imaging the pushed state of the contrast agent using the new connecting tube. A black ink was mixed to the contrast agent. The saline flow rate was 4 ml/s. The pushed state was imaged by 500 frames per second using a high-speed camera. The portion appearing black in Movie 1 was the flow of the contrast agent. As understood from Movie 1, the new connecting tube was able to generate the spiral flow (MPG 2948 kb)
Rights and permissions
About this article
Cite this article
Kidoh, M., Nakaura, T., Awai, K. et al. Novel connecting tube for saline chaser in contrast-enhanced CT: the effect of spiral flow of saline on contrast enhancement. Eur Radiol 23, 3213–3218 (2013). https://doi.org/10.1007/s00330-013-2923-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2923-x