Abstract
Objectives
To compare 3D-TOF magnetic resonance angiography (MRA) and contrast-enhanced MRA (CE-MRA) sequences at 3T in the follow-up of coiled aneurysms with digital subtracted angiography (DSA) as the gold standard.
Methods
DSA, 3D-TOF and CE-MRA were performed in a prospective series of 126 aneurysms in 96 patients (57 female, 39 male; age: 25–75 years, mean: 51.3 ± 11.3 years). The quality of aneurysm occlusion was assessed independently and anonymously by a core laboratory.
Results
Using DSA (gold standard technique), total occlusion was depicted in 57 aneurysms (45.2%), neck remnant in 34 aneurysms (27.0%) and aneurysm remnant in 35 aneurysms (27.8%). Sensitivity, specificity, positive predictive value and negative predictive value were very similar with 3D-TOF and CE-MRA. Visibility of coils was much better with 3D-TOF (95.2%) than with CE-MRA (23.0%) (P < 0.001). Also, substantial artefacts were less frequent with 3D-TOF (4.0%) than with CE-MRA (11.9%; P = 0.012).
Conclusions
In this large prospective series of patients with coiled aneurysms, at 3T 3D-TOF MRA was equivalent to CE-MRA for the evaluation of aneurysm occlusion, but coil visibility was superior at 3D-TOF. Thus the use of 3D-TOF at 3T is recommended for the follow-up of coiled intracranial aneurysms.
Key Points
• Different Magnetic Resonance (MR) imaging techniques are used to evaluate intracranial aneurysms.
• At 3T MR, 3D-TOF and CE-MRA appear equivalent for evaluating coiled aneurysms..
• Coils are better visualised on 3D-TOF than on CE-MRA.
• Combined analysis of 3D-TOF and CE-MRA does not seem helpful.
• At 3T, 3D-TOF techniques are recommended for monitoring patients with coiled aneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment with coils is now widely used in the management of ruptured and unruptured intracranial aneurysms [1, 2]. One drawback of aneurysm coiling is the potential risk of aneurysm recanalisation observed in approximately 20% of cases with retreatment in around 10% of cases [3]. Thus, a regular anatomical follow-up is needed to evaluate the quality of aneurysm occlusion in the mid- and long term. Intra-arterial digital subtraction angiography (DSA) has been used for a long time as the standard follow-up investigation, but the technique has several disadvantages: it is invasive, can be associated in a small number of cases with clinical complications and exposes patients to ionising radiation [4].
The use of magnetic resonance angiography (MRA) at both 1.5T and 3T has been widely evaluated showing a moderate to high diagnostic performance for both time-of-flight (TOF) MRA and contrast-enhanced (CE) MRA [5–13]. Most series that compare MRA at 1.5T and 3T have shown the higher diagnostic performance of 3T MRA [7, 10, 11, 14, 15]. Both 3D-TOF MRA and CE-MRA were also evaluated at 3T showing good diagnostic performance, but no clear conclusion was provided regarding the most useful sequence, probably because of the small size of the series [9, 11–15].
In order to clarify this question, a prospective, consecutive, monocentric study was conducted in patients having follow-up examinations including DSA for coiled intracranial aneurysms. In this group of patients, 3D-TOF MRA and CE-MRA were performed at 3T. Using a strong methodology and DSA as the gold standard, the diagnostic accuracy of 3D-TOF and CE-MRA at 3T were compared.
Materials and methods
Study population
Institutional review board approval of Reims hospital was obtained. Written informed consent was obtained for all patients.
All patients with ruptured or unruptured intracranial aneurysm(s) treated with coils in our hospital are monitored using MRA and DSA. Usually MRA is performed 3 months after the endovascular treatment. At 1 year, the quality of aneurysm occlusion is evaluated by both MRA and DSA. Further follow-up is tailored according to the specific situation of each patient, mostly based on MRA. Another follow-up DSA is performed when a retreatment has to be discussed or if a neck or the aneurysm remnant is changing over time.
From April 2006 to September 2008, all patients eligible for follow-up DSA were prospectively included except if they had a contraindication for MRI, if they were claustrophobic, or if they refused. Patients younger than 18 years were not included. As the goal was to compare 3D-TOF and CE-MRA at 3T in patients with coiled aneurysms, aneurysms treated by parent vessel occlusion or with stenting were excluded from the series. In patients with multiple aneurysms, aneurysms treated with clips or stenting were also excluded from the series. Within the inclusion period, some patients were evaluated twice after undergoing repeat coiling, coiling of another aneurysm or if repeated DSA was judged useful to evaluate an evolution of a remnant.
Digital subtraction angiography and 3T MRA were performed during a short hospital stay of less than 24 h.
Imaging technique for intra-arterial DSA
Intra-arterial DSA was performed with a biplane angiographic system (Axiom Artis, Siemens Medical Systems, Erlangen, Germany). Using transfemoral catheterisation, selective injections of the internal carotid artery (ICA) or vertebral artery (VA) were performed according to aneurysm location. The following standard projections were obtained: anteroposterior view, lateral view and working view (optimal projection used at coil embolisation). For ICA, 8 mL of non-ionic contrast agent (iodixanol, Visipaque; GE Healthcare, Oslo, Norway) was injected at a velocity of 4 mL/s. For VA, 8–10 mL was injected at a velocity of 4–5 mL/s.
Imaging technique for MRA
Magnetic resonance angiography examinations were performed on a 3T Philips (Achieva, Philips, Best, the Netherlands); the parameters are shown in Table 1. Contrast-enhanced MRA (CE-MRA) randomly sampled the central k-space during venous injection of a gadolinium-based contrast agent (meglumine gadoterate, Dotarem, Guerbet, Aulnay-sous-Bois, France). A bolus of 20 mL was used followed by 30 mL saline with a scopic-based detection of the bolus (phase contrast survey).
Data collection
Clinical and anatomical data regarding patient (gender, age), aneurysm (number, location, size of aneurysm) and method of treatment (standard coiling technique, remodelling technique, stenting, or parent artery occlusion) were collected. The time interval between aneurysm treatment and anatomical evaluation was also collected (in months).
Aneurysm location was classified into four groups: anterior cerebral artery/anterior communicating artery aneurysms (ACA/Acom), internal carotid artery (ICA), middle cerebral artery (MCA), and vertebrobasilar system (VB). Aneurysm size was classified into three groups: ≤5 mm, >5 mm and <10 mm, ≥10 mm.
Data analysis
All examinations (DSA, 3D-TOF and CE-MRA) were made anonymously with a different number randomly assigned by series. All images were independently evaluated in a random order by two experienced interventional neuroradiologists (A.B. and J.Y.G.), and in the case of disagreement a consensus was established by a third interventional neuroradiologist (L.P.).
3D-TOF, CE-MRA and DSA were evaluated separately without knowledge of the other MRA or DSA examinations. The pre-treatment DSA was not available, but the location of the aneurysm(s) to be evaluated was provided to the readers. For both 3T MRA series, source images and maximum intensity projection (MIP) reconstructions were analysed.
Aneurysm occlusion was evaluated using the three-grade Montreal scale: total occlusion, neck remnant, aneurysm remnant [16]. Two two-grade scales derived from the Montreal scale were used for statistical analysis: adequate occlusion (total occlusion or neck remnant)/aneurysm remnant and total occlusion/remnant (neck or aneurysm).
Artefacts produced by the coils were evaluated using a two-grade scale: 0 = no artefacts or moderate artefacts with feasible evaluation of aneurysm occlusion, 1 = important artefacts making evaluation of aneurysm occlusion difficult.
The visibility of the coils was also evaluated using a two-grade scale: 0 = coils not visible, 1 = coils visible.
To evaluate the potential value of the combination of 3D-TOF and CE-MRA in the follow-up of coiled aneurysms, a combined reading of 3D-TOF and CE-MRA was performed when the results of both techniques were different (L.P.).
Statistical analysis
Quantitative variables are reported as means ± standard deviation and qualitative variables as number and percentage. Artefacts and visibility of coils observed on 3D-TOF and CE-MRA were compared as dichotomous variables using paired Student’s t-test. A P value <0.05 was considered statistically significant.
As the degree of aneurysm occlusion was defined using the Jean Raymond simplified three-point classification scale (total occlusion, neck remnant and aneurysm remnant), weighted kappa statistics were used to obtain interobserver and intertechnique agreement for each investigation. According to Landis and Koch, the interpretation of k was as follows: k < 0 indicated no agreement; k = 0–0.19, poor agreement; k = 0.20–0.39, fair agreement; k = 0.40–0.59, moderate agreement; k = 0.60–0.79, substantial agreement; and k = 0.80–1.00, almost perfect agreement [17]. Using consensual intra-arterial DSA as a reference test to evaluate the degree of aneurysm occlusion, sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were calculated for MR angiography with corresponding 95% confidence intervals (CI). Two-point classification scales were also used: adequate occlusion (total occlusion or neck remnant) and aneurysm remnant or total occlusion versus remnant. All analysis was performed using SPSS software (version 17.0; SPSS, Chicago, IL, USA).
Results
Study population
During the follow-up period, 100 patients with coiled aneurysms were evaluated by DSA and 3T MRA. Four patients were excluded: one was treated by parent vessel occlusion and two were treated with coiling and stenting. In one case, 3T MRA was performed but the patient refused gadolinium injection.
The final population was 96 patients (age: 25–75 years, mean: 51.3 ± 11.3 years, median: 52 years) including 57 female (59.4%) and 39 male patients (40.6%). Seventy-three patients had one aneurysm, 17 patients had two aneurysms, and 6 patients had three aneurysms. Eight patients were evaluated twice (five had one aneurysm and three had two aneurysms).
A total of 136 aneurysms were to be evaluated. In 10 patients with multiple aneurysms, one aneurysm was treated with clips (9 patients) or a stent (1 patient). These 10 aneurysms were excluded from the final population.
Finally 126 aneurysms were evaluated in 96 patients. Eighty-two aneurysms were ruptured (65.1%) and 44 unruptured (34.9%). Aneurysm location was ACA/Acom in 48 aneurysms (38.1%), ICA in 39 aneurysms (31.0%), MCA in 19 aneurysms (15.1%) and VB in 20 cases (15.9%). Pre-treatment aneurysm size was between 2 and 24 mm (mean: 6.8 ± 3.5 mm). Fifty-two aneurysms measured ≤5 mm (41.3%), 55 between 5 and 10 mm (43.7%), and 19 measured ≥10 mm (15.1%).
The interval time between aneurysm treatments was 3–215 months (mean: 22.7 months ± 32.6 months, median: 12 months). Interval time was <11 months in 9 aneurysms (7.1%), between 11 and 13 months in 63 aneurysms (50.0%), and >13 months in 54 aneurysms (42.9%).
Interobserver agreement
Evaluating aneurysm occlusion using the three-grade Montreal scale, k was 0.50 for DSA, 0.61 for 3D-TOF, and 0.49 for CE-MRA.
Using the two-grade scale adequate occlusion/aneurysm remnant, k was 0.61 for DSA, 0.77 for 3D-TOF and 0.67 for CE-MRA.
Finally using the two-grade scale total occlusion/remnant, k was 0.61 for DSA, 0.60 for 3D-TOF and 0.56 for CE-MRA.
Aneurysm occlusion
Using DSA, total occlusion was depicted in 57 aneurysms (45.2%), neck remnant in 34 aneurysms (27.0%) and aneurysm remnant in 35 aneurysms (27.8%). Adequate occlusion was seen in 91 cases (72.2%). A remnant (aneurysm or neck) was depicted in 69 cases (54.8%).
Using 3D-TOF MRA, total occlusion was depicted in 65 aneurysms (51.6%), neck remnant in 29 aneurysms (23.0%) and aneurysm remnant in 32 aneurysms (25.4%). Adequate occlusion was seen in 94 cases (74.6%). A remnant (aneurysm or neck) was depicted in 61 cases (48.4%).
Using CE-MRA, total occlusion was depicted in 68 aneurysms (54.0%), neck remnant in 22 aneurysms (17.5%) and aneurysm remnant in 36 aneurysms (28.6%). Adequate occlusion was seen in 90 cases (71.4%). A remnant (aneurysm or neck) was depicted in 58 cases (46.0%).
Anatomical results (adequate occlusion/aneurysm remnant) evaluated by 3D-TOF and CE-MRA are compared with DSA results in Table 2.
Intermodality agreement
Evaluating aneurysm occlusion using the three-grade Montreal scale, intertechnique agreement was 0.52 for 3D-TOF/DSA, 0.53 for CE-MRA/DSA and 0.60 for 3D-TOF + CE-MRA/DSA. Using the two-grade scale (adequate occlusion/aneurysm remnant), intertechnique agreement was 0.70 for 3D-TOF/DSA, 0.71 for CE-MRA/DSA and 0.71 for 3D-TOF + CE-MRA/DSA. Using the two-grade scale (total occlusion/remnant), intertechnique agreement was 0.56 for 3D-TOF/DSA, 0.57 for CE-MRA/DSA and 0.65 for 3D-TOF + CE-MRA/DSA.
Sensitivity, specificity, positive predictive value, negative predictive value
These values are shown in Table 3 for the dichotomisation adequate occlusion/aneurysm occlusion and in Table 4 for the dichotomisation total occlusion/neck or aneurysm remnants.
Visibility of the coils, artefacts
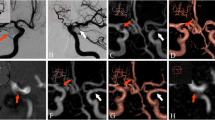
At 3D-TOF, coils were visible in 120 cases (95.2%) and not visible in 6 cases (4.8%). At CE-MRA, coils were visible in 29 cases (23.0%) and not visible in 97 cases (77.0%). The difference was highly statistically significant (P < 0.001; Figs. 1, 2).
At 3D-TOF, no or moderate artefacts were observed in 121 aneurysms (96.0%) and substantial artefacts in 5 cases (4.0%). Substantial artefacts were related to the patient’s movements in three cases and to arterial flow in two cases. At CE-MRA, no or moderate artefacts were observed in 111 aneurysms (88.1%) and substantial artefacts in 15 cases (11.9%). Substantial artefacts were related to venous opacification in six cases (Fig. 3), coil artefacts in six cases, and other causes in three cases. The difference between 3D-TOF and CE-MRA was statistically significant (P = 0.012).
Discussion
The methodological quality of the studies dealing with MRA for evaluation of coiled aneurysm occlusion is often moderate [7]. Our study was built using Kwee and Kwee criteria in order to obtain a high methodological quality. It was conducted from April 2006 to September 2008. During this period, the use of stents in our department was very limited and flow diverters were not available. As the performance of MRA is probably not the same in aneurysms treated only with coils and with coiling and stenting, the decision was to exclude the three aneurysms treated with stents to compare the efficacy of both MRA techniques in a homogeneous population of patients with coiled aneurysms [18, 19]. Three scales were used to measure the quality of aneurysm occlusion: the Montreal scale and two two-grade scales derived from the Montreal scale: adequate occlusion (total occlusion or neck remnant)/aneurysm remnant and total occlusion/remnant of any type (neck or aneurysm). From the literature, it seems that what is significant from a clinical point of view is the presence or absence of an aneurysm remnant [20, 21]. Accordingly the first two-grade scale (adequate occlusion/aneurysm remnant) is probably the most important.
Our large, prospective series demonstrates very good diagnostic performance of 3D-TOF and CE-MRA for the detection of aneurysm remnants. The sensitivity of CE-MRA is a little higher than 3D-TOF (respectively, 0.80 and 0.74) with a high number of aneurysm remnants detected by both techniques (28 for CE-MRA and 26 for 3D-TOF out of 35 detected by DSA). Also the NPV is quite similar with both techniques (0.90 for 3D-TOF and 0.92 for CE-MRA) with nine and seven aneurysm remnants respectively falsely detected as adequate occlusion by 3D-TOF and CE-MRA. Similar results were obtained for the detection of any type of remnant (neck or aneurysm). Intertechnique agreement (3D-TOF/DSA and CE-MRA/DSA) was also quite similar whatever the scale used, but interobserver agreement was better with 3D-TOF than with CE-MRA.
3D-TOF was superior to CE-MRA for coil visualisation: coils were visible in 120 cases (95.2%) with 3D-TOF and in 29 cases with CE-MRA (23.0%; P < 0.001). For the readers, the visibility of the coils was an advantage as it permitted the relation between coil mesh and residual flow to be analysed precisely. Thus analysis of 3D-TOF sequences was based on both native images and MIP reconstructions. For the analysis of CE-MRA, native images were less useful as coils were in most cases not clearly visible.
Important artefacts were also less frequent with 3D-TOF than with CE-MRA (respectively, 4.0 and 11.9%, P = 0.012) with CE-MRA artefacts mostly related to venous opacification or coils and 3D-TOF artefacts mostly related to patient’s movements.
The results of previous 3T studies were relatively heterogeneous. In a short series of 37 patients with 41 aneurysms, 3D-TOF showed better agreement with DSA than with CE-MRA [11]. In another small series of 52 patients with 54 aneurysms, 3D-TOF and CE-MRA were similarly efficacious for the visualisation of residual aneurysm patency in 43 aneurysms, but CE-MRA was better in 11 cases [9]. Artefacts were also less frequent with CE-MRA than 3D-TOF contrary to what was observed in our series. In a series comparing 3D-TOF and CE-MRA in 72 aneurysms, sensitivity, specificity, PPV and NPV were similar in both groups [13]. From the experience of 58 patients with 63 coiled aneurysms, using a four-grade scale, CE-MRA seemed more likely than TOF-MRA to classify neck and aneurysm remnants [14].
In our series and numerous other series in the literature, 3D-TOF and CE-MRA have been found to be equivalent in the detection of aneurysm remnants with a better visibility of coils and fewer artefacts with 3D-TOF. Contrast medium administration also has several disadvantages, including risk of renal damage, risk of allergic reactions and patient discomfort [22]. Moreover the use of gadolinium is associated with a higher cost. Contrary to what was proposed by some authors, our analyses showed no value of adding 3D-TOF and CE-MRA sequences for the follow-up of coiled aneurysms [14]. Intertechnique agreement (with DSA) was not modified or slightly increased by combining the two techniques. Also for the detection of aneurysm remnants, the combination of the two techniques was not associated with higher sensitivity, specificity, or negative and positive predictive values. Finally 3D-TOF at 3T is currently the MRA sequence of choice for the follow-up of coiled aneurysms.
Digital subtraction angiography is considered the standard of reference for evaluating aneurysm occlusion after coiling. However this method has several disadvantages: (1) Aneurysm assessment can be limited by superimposition of arteries and (2) aneurysm or neck remnants can sometimes be partially or totally masked by the “helmet effect” [23]. The coils that surround the aneurysmal remnant create an impenetrable radiodense helmet around the remnant because of a lack of photon penetration. (3) Contrary to MRA where native images (singularly for the 3D-TOF sequence, see above) and multiple MIP projections can be analysed, DSA often offers a limited number of projections (Fig. 4). This limitation will probably be partially overcome by using 3D-DSA (not analysable in our series).
Our study has some limitations. First, a potential limitation was that some aneurysms were evaluated twice. In most cases a long period of time elapsed between the two evaluations, and the second evaluation was related to the treatment of another aneurysm (in the case of multiple aneurysms) or to a change in the quality of aneurysm occlusion. Second, aneurysms treated with stents of flow diverters were not included in the present series. However, preliminary series have shown that evaluation of aneurysm occlusion with MRA in aneurysms treated with coils and stents was difficult owing to stent artefacts [18]. Thus, it was logical to compare the two MRA sequences in a homogeneous population only treated with coiling.
In this large prospective series of patients, 3D-TOF and CE-MRA at 3T were equivalent for the evaluation of coiled intracranial aneurysms with better visibility of coils and fewer artefacts with 3D-TOF. As 3D-TOF is not associated with the disadvantages of gadolinium injection, 3D-TOF at 3T should be used for the follow-up of coiled intracranial aneurysms.
Abbreviations
- ACA:
-
Anterior cerebral artery
- Acom:
-
Anterior communicating artery
- CE-MRA:
-
Contrast-enhanced magnetic resonance angiography
- DSA:
-
Digital subtraction angiography
- ICA:
-
Internal carotid artery
- MCA:
-
Middle cerebral artery
- MIP:
-
Maximum intensity projection
- MRA:
-
Magnetic resonance angiography
- TOF:
-
Time-of-flight
References
Molyneux AJ, Kerr RSC, Stratton I et al (2002) International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360:1262–1263
Pierot L, Spelle L, Vitry F, ATENA Investigators (2008) Clinical outcome of patients harbouring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA trial. Stroke 39:2497–2504
Ferns SP, Sprengers MES, von Rooij WJ et al (2009) Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 40:e523–e529
Kaufmann TJ, Huston J, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF (2007) Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 243:812–819
Boulin A, Pierot L (2001) Follow-up of intracranial aneurysms treated with detachable coils: comparison of gadolinium 3D time-of-flight MR angiography and digital subtraction angiography. Radiology 219:108–113
Pierot L, Delcourt C, Bouquigny F et al (2006) Follow-up of intracranial aneurysms selectively treated with coils: prospective evaluation of contrast-enhanced MR angiography. AJNR Am J Neuroradiol 27:744–749
Kwee TC, Kwee RM (2007) MR angiography in the follow-up of intracranial aneurysms treated with Guglielmi detachable coils: systematic review and meta-analysis. Neuroradiology 49:703–713
Urbach H, Dorenbeck U, von Falken hausen M et al (2008) Three-dimensional time-of-flight MR angiography at 3T compared to digital subtraction angiography in the follow-up of ruptured and coiled aneurysms. Neuroradiology 50:383–389
Anzalone N, Scomazzoni F, Cirillo M et al (2008) Follow-up of coiled aneurysms at 3T: comparison of 3D time-of-flight MR angiography and contrast-enhanced MR angiography. AJNR Am J Neuroradiol 29:1530–1536
Buhk JH, Kallenberg K, Mohr A, Dechent P, Knauth M (2008) No advantage of time-of-flight magnetic resonance angiography at 3 Tesla compared to 1.5T in the follow-up after endovascular treatment of cerebral aneurysms. Neuroradiology 50:855–861
Ramgren B, Siemund R, Cronqvist M et al (2008) Follow-up of intracranial aneurysms treated with detachable coils: comparison of 3D inflow MRA at 3T and 1.5T and contrast-enhanced MRA at 3T with DSA. Neuroradiology 50:947–954
Kau T, Gasser J, Celedin S et al (2009) MR angiographic follow-up of intracranial aneurysms treated with detachable coils: evaluation of a blood-pool contrast medium. AJNR Am J Neuroradiol 30:1524–1530
Sprengers MES, Schaafsma JD, van Rooij WJ et al (2009) Evaluations of the occlusion status of coiled aneurysms with MR angiography at 3T: is contrast enhancement necessary? AJNR Am J Neuroradiol 30:1665–1671
Kaufmann TJ, Huston J, Cloft HJ et al (2010) A prospective trial of 3T and 1.5T time-of-flight and contrast-enhanced MR angiography in the follow-up of coiled intracranial aneurysms. AJNR Am J Neuroradiol 31:912–918
Schaafsma JD, Velthuis BK, Majoie CBLM et al (2010) Intracranial aneurysms treated with coil placement: test characteristics of follow-up MR angiography—multicenter study. Radiology 256:209–218
Raymond J, Guilbert F, Weill A et al (2003) Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 34:1398–1403
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Kovacs A, Möhlenbruch M, Hadizadeh DR et al (2011) Noninvasive imaging after stent-assisted coiling of intracranial aneurysms: comparison of 3-T magnetic resonance imaging and 64-row multidetector computed tomography: a pilot study. J Comput Assist Tomogr 35:573–582
Buhk JH, Kallenberg K, Mohr A, Dechent P, Knauth M (2009) Evaluation of angiographic computed tomography in the follow-up after endovascular treatment of cerebral aneurysms—a comparative study with DSA and TOF-MRA. Eur Radiol 19:430–436
Johnston SC, Dowd CF, Higashida RT et al (2008) Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke 39:120–125
Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC (2005) Late rebleeding of ruptured intracranial aneurysms treated with detachable coils. AJNR Am J Neuroradiol 26:2542–2549
Kuo PH, Kanal E, Abu-Alfa CSE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 27:2118–2120
Agid R, Willinsky RA, Lee SK, TerBrugge KG, Farb RI (2008) Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced MR angiography versus catheter digital subtraction angiography. AJNR Am J Neuroradiol 29:1570–1574
Conflict of interest
The authors declare that they have no conflict of interest in relation to the topic of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pierot, L., Portefaix, C., Boulin, A. et al. Follow-up of coiled intracranial aneurysms: comparison of 3D time-of-flight and contrast-enhanced magnetic resonance angiography at 3T in a large, prospective series. Eur Radiol 22, 2255–2263 (2012). https://doi.org/10.1007/s00330-012-2466-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2466-6