Abstract
The aim of this study was to evaluate whether pregnancy affects contrast enhancement within the pulmonary arteries during computed tomography pulmonary angiography (CTPA). This was a retrospective analysis of the CTPA examinations of 16 pregnant and 16 non-pregnant female patients, suspected of having an acute pulmonary embolus (PE), during the same time period. Pulmonary vascular enhancement was evaluated by measuring the CT density within the pulmonary arteries. In a blinded evaluation, subjective grading of contrast enhancement within the pulmonary arteries was also performed. There was a significant difference in arterial enhancement between the two groups, with pregnant patients having a mean pulmonary arterial density 112 HU less than patients in the control group [mean attenuation of 259.79 ± 59.31 HU in pregnant patients versus 371.88 ± 60.63 HU in non-pregnant patients (p < 0.001)]. The mean subjective pulmonary arterial enhancement score in the pregnant group was 8.19 ± 2.51 versus 13.69 ± 3.07 in the control group (p < 0.001). Pregnant women undergoing CTPA have significantly decreased pulmonary arterial enhancement compared to non-pregnant patients, probably due to the increase in cardiac output in pregnancy. We may need to reconsider how we perform CTPA in this group in order to ensure adequate opacification for diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolus (PE) is the leading cause of maternal mortality during pregnancy [1, 2]. Pregnancy increases the risk of PE by a factor of four over that of a non-pregnant woman of similar age, occurring in approximately 1 in 1,500 deliveries [3]. The clinical diagnosis of PE is difficult in the general population, but it is further complicated in pregnancy as some of the clinical symptoms of PE can be regarded as normal symptoms of pregnancy. Because PE is treatable, early and accurate diagnosis is mandatory. Precise diagnosis in pregnancy is also vital to prevent unnecessary diagnosis of PE, as treatment is associated with potential side effects to both the mother and foetus. A diagnosis of pulmonary embolus in a pregnant patient also has other important implications, including the need for long-term anticoagulation, avoidance of breast feeding if on oral anticoagulants, the potential need for prophylaxis during future pregnancies, and concern about future oral contraceptive use [4].
CT pulmonary angiography (CTPA) is now a well-validated investigation with a sensitivity and specificity between 94% and 100% [5, 6]. The negative predictive value of a normal CTPA is over 99%, allowing anticoagulation to be safely withheld if the CTPA is negative [7]. CTPA also allows direct thrombus visualisation and can be used to identify other causes of symptoms if no embolus is present [8–10].
Poor contrast opacification, motion artefacts and technical factors cause 5–10% of CTPA examinations in non-pregnant patients to be non-diagnostic [11]. It was our subjective impression that CTPA vascular enhancement in pregnant patients was suboptimal. This study was therefore performed to assess objectively whether pregnancy affects pulmonary arterial enhancement in patients undergoing CTPA and thus compromise diagnostic accuracy.
Materials and methods
This study was a retrospective analysis of multislice CTPA studies performed at our institution over a 4-year period. As this was an analysis of existing data that had no effect on patient care, the guidelines under which our institution operates did not require review of the study by our ethics and research committees.
Patients
A total of 32 patients suspected of having an acute PE were included in this analysis. The study group was comprised of 16 pregnant patients (mean age 30.00, range 18–39 years). This represents all the pregnant patients who underwent CT pulmonary angiography for suspected acute PE in our institution during the 4-year period. At our institution we perform half-dose isotope perfusion lung studies on pregnant patients during normal working hours, and outside of normal working hours we perform CTPA. This accounts for the small number of CTPAs of pregnant patients over the study period. Two patients in the study group were in their first trimester of pregnancy, and seven patients were in their second and seven in their third trimester.
The control group consisted of 16 non-pregnant female patients (mean age 30.13, range 18–39), who underwent CT pulmonary angiography during the same time period. The 16 control patients were chosen to match the study group for age, concentration and volume of contrast administered, injection rate and mode of image acquisition.
Image acquisition
Multislice CT studies were performed with a four-slice CT scanner (Lightspeed plus, GE Healthcare, Milwaukee WI). All patients were scanned in a cranio-caudal direction from the top of the aortic arch to the level of the left hemi-diaphragm, in a supine position. The images were obtained with a slice thickness and increment of 1.25 mm, a table speed of 7.5 (high speed mode) and pitch of 1.5. Rotation time was 0.5 s. An x-ray tube voltage of 120 KV and a current of 80–400 auto mA were used in all examinations. In the pregnant patients the mean dose length product (DLP) was 324.8 mGY cm (range 172–500.5), and in the control group the mean DLP was 379.61 mGY cm (range 128.3–525).
A CT injection system (MEDRAD EnVision CT™) was used to deliver a bolus of 120 mls of intravenous contrast medium (Omnipaque™ Iohexol) at a flow rate of 4 ml/s, following a fixed delay of 20 s from the start of injection of the intravenous contrast medium, before initiating CT data acquisition. In six patients (three pregnant and three non-pregnant), in whom a volume of 100 mls or less of intravenous contrast was administered, a flow rate of 3 mls/s was used, therefore still ensuring an injection time of at least 30 s. Several studies assessing contrast enhancement in CT pulmonary angiography report no significant differences in image quality between fixed delay and bolus tracking techniques and have found that a fixed delay of 20 s is valid for almost all patients [12–15]. In two patients (one pregnant and one non-pregnant), the delay was estimated using a semiautomatic bolus-tracking system (SmartPrep, GE Healthcare, Milwaukee, WI).
Twenty-four of the 32 patients had 120 mls of Omnipaque 350 (350 mg of iodine per millilitre). In two pregnant and two non-pregnant patients a volume of 95 mls of Omnipaque 350 was used. One pregnant and one non-pregnant patient received 120 mls of Omnipaque 300 (300 mg of iodine per millilitre), and in a further two patients (one pregnant and one non-pregnant) 100 mls of Omnipaque 300 was administered.
Image evaluation
CT images were retrieved from the institution’s picture archiving and communications system (PACS) and were analysed at a personal computer-based PACS diagnostic workstation (GE Centricity™ PACS version 2.1). Vascular enhancement was assessed using quantitative and subjective analyses.
Quantification of vascular enhancement was evaluated by measuring the CT number (in Hounsfield units) at specific sites, using a circular region of interest cursor, which was chosen to be half the diameter of the vessels. Care was taken that the section being measured had the least breathing or motion artefact within the chosen anatomical range. Measurements were taken at the main pulmonary artery, right and left pulmonary arteries, right and left lower lobe arteries just proximal to their segmental divisions and at the ascending aorta.
Subjective evaluation of pulmonary arterial enhancement was assessed by a consultant chest radiologist with 13 years’ experience, who was blinded. A four-point scoring system was used to subjectively assess the enhancement within the main pulmonary arteries, lobar, segmental and sub-segmental pulmonary arteries [16]. Thus, the maximum possible score for each patient was 16. A score of 1 corresponded to poor opacification of the pulmonary arteries insufficient for diagnosis; a score of 2 to fair opacification, borderline for diagnosis; a score of 3 to good opacification, diagnostic quality; and a score of 4 to excellent pulmonary arterial opacification.
Image noise was also assessed in both groups. Image noise was objectively quantified by measuring the standard deviation of CT numbers in a homogeneous region of interest (size >1 cm2; range 1.0–1.7 cm2) that was free of motion artefact and was located in the main pulmonary artery [17]. Image noise was also subjectively assessed by one of the authors, who was blinded, using a two-point scoring system. A score of 1 corresponded to no significant degradation of image quality by noise; a score of 2 corresponded to significant degradation of image quality by noise.
Statistical analysis
A matched t-test was used to test the significance of the differences in the average vascular enhancement, at each of the measured sites, between the two groups. At all sites the differences between the two groups were normally distributed (Shapiro-Wilk test all had p-values >0.05). A matched t-test was also used to assess the differences in image noise between the two groups. A McNemar’s test was used to assess the differences in image noise using the subjective scoring data.
Results
Analysis of vascular enhancement
There was a significant difference in arterial enhancement between the study and control groups at each of the sites measured, with pregnant patients having a lower pulmonary arterial enhancement compared to the control group (p < 0.001), (Table 1) (Figs. 1, 2, and 3). The mean attenuation in the pulmonary arteries was 259.79 ± 59.31 HU in the pregnant patients versus 371.88 ± 60.63 HU in the non-pregnant patients. The average pulmonary arterial density in the pregnant patients was 112 HU less than in the control group.
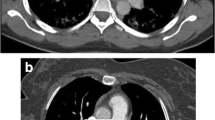
Transverse CT images acquired at the level of the main pulmonary artery, comparing vascular enhancement between the two groups. Both images viewed with a window level of 100 and window width of 700. The image on the left is of a non-pregnant patient showing good opacification of the main pulmonary artery (450 HU; subjective score 4). The image on the right is of a pregnant patient, demonstrating poor enhancement in the main pulmonary artery (194 HU; subjective score 2)
Transverse CT images acquired at the segmental level of the lower lobe pulmonary arteries, comparing vascular enhancement between the two groups. Both images viewed with a window level of 100 and window width of 700. The image on the left is a nonpregnant patient and on the right of a pregnant woman. Enhancement values within the right lower lobe pulmonary artery are 440 HU (subjective score 4) and 176 HU (subjective score 2), respectively, demonstrating the reduction in vascular opacification seen in pregnant patients
Contrast enhancement in the aorta was also significantly lower in the pregnant group (p < 0.001) compared to the non-pregnant group. Mean aortic enhancement was 227.88 HU in the pregnant patients and 314.94 in the non-pregnant patients. In both groups aortic enhancement was consistently lower than mean pulmonary arterial enhancement.
There was also a significant difference in the subjective scoring of vascular opacification between the two groups, with pregnant patients having a lower enhancement score for central, lobar, segmental and sub-segmental pulmonary arteries (Fig. 4). The mean subjective pulmonary arterial enhancement score in the pregnant group was 8.19 ± 2.51 versus 13.69 ± 3.07 in the non-pregnant group. Ten pregnant patients had a score of 2 or less within the central pulmonary arteries compared to only 1 in the control group. A score of 2 or less within the segmental or sub-segmental arteries was seen in 14 out of the 16 pregnant patients and in 5 of the non-pregnant patients.
No association was found between differences in contrast enhancement and the stage of pregnancy; however, the number in this analysis (n = 16) is small. One pregnant patient and three patients in the control group were found to have PE. The sites at which these emboli occurred did not interfere with the regions of interest from which enhancement scores were measured.
Analysis of image noise
Statistical analysis showed no significant difference in image noise between the two groups (p = 0.79, 95% CI -7.06-9.09). Analysis of the subjective assessment of image noise again showed no significant difference (p = 0.32).
Discussion
Our results demonstrate that pregnant women undergoing CTPA have significantly decreased pulmonary arterial enhancement compared to non-pregnant patients. This was supported by both the quantitative and subjective analyses between the control and study groups. A significant reduction in vascular enhancement was seen in both central and peripheral pulmonary arteries. Pulmonary arterial density was on average 112 HU less in the pregnant group. Subjectively, reduced pulmonary arterial opacification graded as insufficient for diagnosis, or borderline for diagnosis, was seen in 10 of the 16 pregnant patients.
Cardiac output increases during pregnancy, initially due to an increase in pulse rate, soon followed by an increase in stroke volume [18, 19]. Cardiac muscle hypertrophy occurs so that the heart chambers enlarge and output increases up to 50% above non-pregnant levels [20, 21]. This occurs rapidly in the first half of pregnancy and steadies off in the second. The reduction in vascular enhancement demonstrated in this study may be explained by the dilution of intravenous contrast medium caused by the physiological increase in cardiac output associated with pregnancy. As cardiac output and thus blood flow rate increases, the bolus of contrast medium administered is diluted within a larger volume of blood reaching the pulmonary arteries.
In a porcine model study on the effects of cardiac output on aortic enhancement, Bae et al. demonstrated that the magnitude of peak aortic enhancement increased substantially and proportionally as cardiac output decreased [22, 23]. Average peak aortic enhancement increased by 60%, with a 50% reduction in cardiac output. From this data one would assume that a similar relationship would apply to pulmonary arterial enhancement and that an increase in cardiac output would result in decreased opacification. Based on Bae et al.’s figures and assuming that unopacified blood has a density of 50 HU, a reduction in attenuation by 112 HU between the pregnant and non-pregnant patients results in a decrease in arterial enhancement by 35%, which correlates to an increase in cardiac output of approximately 35% [22].
Pulmonary arterial attenuation during CTPA is also influenced by patient weight. Studies have suggested that there is a small but statistically significant negative correlation between patient body weight and pulmonary arterial enhancement [24–26]. Increase in weight has also been shown to result in an increase in cardiac output, which may explain the reduction in vascular enhancement seen in the previous studies [27]. Pregnancy is associated with an increase in weight of around 25% of the non-pregnant weight, approximately 12.5 kg in the average woman. This relatively small increase in weight, however, would not be sufficient on its own to account for the large differences in arterial enhancement seen between the two groups in our study [24–26]. However, as in this study patients were not matched for weight, weight may still be in part responsible for some of the differences observed.
Physiological changes in respiration that occur during pregnancy may also contribute to the differences in arterial attenuation between the two groups. During pregnancy there is a 30–40% increase in tidal volume, and inspiratory capacity increases by 5–10%. Deep inspiration before scanning may lead to a large influx of IVC blood that does not contain contrast into the right side of the heart, diluting the contrast bolus, causing poor vascular opacification [28]. However, we routinely tell all patients to take a deep breath in and hold it prior to image acquisition. As vital lung capacity is unchanged in pregnancy, by following this breathing command there should not be any difference in opacification between the two groups.
In this study, aortic enhancement was also lower in the pregnant patients, refuting the possibility that poor contrast enhancement in the pulmonary arteries could be due to increased shunting through a patent foramen ovale [29].
Ventilation-perfusion (V/Q) imaging has historically been the primary screening study for PE during pregnancy [30]. PE can be confidently excluded with a normal V/Q, and in pregnant women lung scintigraphy is associated with a lower incidence of non-diagnostic tests (low and intermediate probability) compared to non-pregnant patients [31]. The estimated foetal dose for V/Q examinations ranges from 100 μGy to 370 μGy, i.e., up to three times greater than for CTPA [32, 33]. Most centres therefore advocate the use of reduced or half-dose perfusion imaging during pregnancy [30]. All quoted radiation doses though are below the thresholds estimated to be associated with any significant risk. CTPA is associated with a lower foetal radiation dose than V/Q imaging during all three trimesters, with doses for CTPA ranging from 3.3 μGy to 130.8 μGy [34]. CTPA though imparts a substantially higher maternal radiation exposure than scintigraphy with breast doses ranging from 10 to 35 mGy [35, 36]
Limitations to our study have been acknowledged. First, the study was a retrospective analysis, and the sample size was small. Prospective studies involving larger patient numbers are necessary to confirm the data. Second, there was no standardisation of injection rate and the concentration and volume contrast medium administered during the CTPA examinations, across the two groups. Patients in both groups were however matched for the concentration and volume of contrast administered and injection rate used, allowing for comparison between the two groups. Finally, the results of our study are based on comparisons between two patient groups without accounting for individual patient variations. In particular, specific parameters characterising the cardiac function of the patients were not documented. Patient weight, which may also affect vascular enhancement, was not recorded. The focus of this study however, was to demonstrate the difference in vascular opacification between pregnant and non-pregnant women, not the mechanisms producing their difference.
In conclusion, the results of this study demonstrate that pulmonary arterial enhancement in CTPA is significantly reduced in pregnant patients. The most likely cause for this is the dilution of intravenous contrast medium due to the increase in cardiac output associated with pregnancy. Accurate diagnosis of PE in pregnant patients is essential and has greater implications compared to a non-pregnant population. As poor vascular enhancement may affect diagnostic accuracy, it may be necessary to adjust the imaging protocols for pregnant patients undergoing CTPA. Arterial attenuation may be increased by raising the contrast flow rate or by using a contrast medium with a high iodine concentration [37, 38].
References
Rochat RW, Koonin LM, Atrash HK, Jewett JF (1988) Maternal mortality in the United States: report from the Maternal Mortality Collaborative. Obstet Gynecol 72:91–97
Atrash HK, Rowley D, Hogue CJR (1992) Maternal and perinatal mortality. Curr Opin Obstet Gynecol 4:61–71
Prevention of venous thrombosis and pulmonary embolism (1986) NIH Consensus Development. JAMA 256:744–749
Toglia MR, Weg JG (1996) Venous thromboembolism during pregnancy. N Engl J Med 335:108–114
Blachere H, Latrabe V, Montaudon M, Valli N, Couffinhal T, Raherisson C, Leccia F, Laurent F (2000) Pulmonary embolism revealed on helical CT angiography: comparison with ventilation-perfusion radionuclide lung scanning. AJR Am J Roentgenol 174:1041–1047
Remy-Jardin M, Remy J, Baghaie F, Fribourg M, Artaud D, Duhamel A (2000) Clinical value in the thin collimation diagnostic workup of pulmonary embolism. AJR Am J Roentgenol 175:407–411
Quiroz R, Kucher N, Zou KH, Kipfmueller F, Costello P, Goldhaber SZ, Schoepf UJ (2005) Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA 293:2012–2017
Bates SM, Ginsberg JS (2002) How we manage venous thromboembolism in pregnancy. Blood 100:3470–3478
Goodman LR (2000) CT diagnosis of pulmonary embolism and deep vein thrombosis. RadioGraphics 1201–1205
Garg K, Welsh CH, Feyerabend AJ, Subber SW, Russ PD, Johnston RJ, Durham JD, Lynch DA (1998) Pulmonary embolism: diagnosis with spiral CT and ventilation-perfusion scanning–correlation with pulmonary angiographic results or clinical outcome. Radiology 208:201–208
Ryu JH, Swensen SJ, Olson EJ, Pellikka PA (2001) Diagnosis of pulmonary embolism with use of computed tomographic angiography. Mayo Clin Proc 76:59–65
Hartmann IJ, Lo RT, Bakker J, de Monye W, van Waes PF, Pattynama PT (2002) Optimal scan delay in spiral CT for the diagnosis of acute pulmonary embolism. J Comput Assist Tomogr 26:21–25
Gotway MB, Patel RA, Webb WR (2000) Helical CT for the evaluation of suspected acute pulmonary embolism: diagnostic pitfalls. J Comput Assist Tomogr 24:267–273
Sahagun O, Wittram C (2005) A population-controlled comparison of fixed delay and Smart-Prep enhancement for CT pulmonary angiography. (abstr) RSNA scientific assembly and annual meeting program. Chicago, IL: RSNA 2005:546
Chang SA, Matthews CC, Kay D (2005) CT pulmonary angiography for the evaluation of pulmonary embolus: fixed delay versus bolus tracking techniques of contrast administration.(abstr) RSNA scientific assembly and annual meeting program. Chicago, IL: RSNA 2005:639
Rubin GD, Lane MJ, Bloch DA, Leung AN, Stark P (1996) Optimization of thoracic spiral CT: effects of iodinated contrast medium concentration. Radiology 201:785–791
Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, Herold CJ, Prokop M (2006) CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. Radiology 241:899–907
Robson SC, Hunter S, Boys RJ, Dunlop W (1989) Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 256:H1060
Capeless EL, Clapp JF (1989) Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol 161:1449
Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL (1994) A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol 170:849–856
Duvekot JJ, Peeters LL (1994) Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv 49(Suppl 12):S1–S14
Bae KT, Heiken JP, Brink JA (1998) Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology 207:657–662
Bae KT (2003) Peak contrast enhancement in CT and MR angiography: When does it occur and why? Pharmacokinetic study in a porcine model. Radiology 227:809–816
Schoellnast H, Deutschmann HA, Berghold A, Fritz GA, Schaffler GJ, Tillich M (2006) MDCT angiography of the pulmonary arteries: influence of body weight, body mass index and scan length on arterial enhancement at different iodine flow rates. AJR Am J Roentgenol 187:1074–1078
Bae KT, Tao C, Gürel S, Hong C, Zhu F, Gebke TA, Milite M, Hildebolt CF (2007) Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. Radiology 242:582–589
Arakawa H, Kohno T, Hiki T, Kaji Y (2007) CT pulmonary angiography and CT venography: factors associated with vessel enhancement. AJR 189:156–161
De Simone G, Daniels SR, Mureddu G, Kimball TR, Witt S (1997) Stroke volume and cardiac output in normotensive children and adults: assessment of relations with body size and impact of overweight. Circulation 95:1837–1843
Kuzo RS, Pooley RA, Crook JE, Heckman MG, Gerber TC (2007) Measurement of caval blood flow with MRI during respiratory maneuvers: implications for vascular contrast opacification on pulmonary CT angiography studies. AJR Am J Roentgenol 188:839–842
Henk CB, Grampp S, Linnau K, Thurnher MM, Czerny C, Herold CJ, Mostbeck GH (2003) Suspected pulmonary embolism: enhancement of pulmonary arteries at deep-inspiration CT angiography – influence of patent foramen ovale and atrial-septal defect. Radiology 226:749–755
Boiselle PM, Reddy SS, Villas PA, Liu A, Seibyl JP (1998) Pulmonary embolus in pregnant patients: survey of ventilation-perfusion imaging policies and practices. Radiology 207:201–206
Chan WS, Ray JG, Murray S, Coady GE, Coates G, Ginsberg JS (2002) Suspected pulmonary embolus in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 162:1170–1175
Ginsberg JS, Hirsh J, Rainbow AJ, Coates G (1989) Risk to the fetus of radiological procedures used in the diagnosis of maternal venous thromboembolic disease. Thromb Haemost 61:189–196
Russell JR, Stabin MG, Sparks RB, Watson E (1997) Radiation absorbed dose to the embryo/fetus from radiopharmaceuticals. Health Phys 73:756–769
Winer-Muram HT, Boone JM, Brown HL, Jennings SG, Mabie WC, Lombardo GT (2002) Pulmonary embolism in pregnant patients: Fetal radiation dose with helical CT. Radiology 224:487–492
Cook JV, Kyriou J (2005) Radiation from CT and perfusion scanning [letter]. BMJ 331:350
Remy-Jardin M, Remy J (1999) Spiral CT angiography of the pulmonary circulation. Radiology 212:615–636
Fleischmann D (2003) Use of high concentration contrast media: principles and rationale. Eur J Radiol 45(supp 1):S88–S93
Schoellnast H, Deutschmann HA, Fritz GA, Stessel U, Schaffler GJ, Tillich M (2005) MDCT angiography of the pulmonary arteries: influence of iodine flow concentration on vessel attenuation and visualisation. AJR Am J Roentgenol 184:1935–1939
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andreou, A.K., Curtin, J.J., Wilde, S. et al. Does pregnancy affect vascular enhancement in patients undergoing CT pulmonary angiography?. Eur Radiol 18, 2716–2722 (2008). https://doi.org/10.1007/s00330-008-1114-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1114-7