Abstract
The purposes of this study were (1) to evaluate the possible identification of trajectories of fibre tracts, (2) to examine the useful of a neuronavigation system for pre-surgical planning, (3) to assess pre- and post-surgery patients’ clinical condition and (4) to evaluate the impact of this information on surgical planning and procedure. Twenty-eight right-handed patients were prospectively and consecutively studied. All the patients were clinically assessed by a neurologist in both pre- and post-surgical phases. Separately the pyramidal tract, optic radiation and arcuate fasciculus were reconstructed. The trajectories were considered suitable for surgical planning if there were no interruptions of any of the layers at the level of the lesion. Dedicated software ‘merged’ the acquired images with the tractographic processing, and the whole dataset was sent to the neuronavigation system. The assessment of the 37 visualised trajectories close to the tumour resulted in a modification of the surgical approach to corticotomy in six patients (21%); the impact on the definition of the resection margins during surgery was 64% (18 cases). The overall impact percentage on the surgical procedure was 82%. In 27 cases, the symptoms had not changed. MR-tractography provides the neurosurgeon with a new anatomical view that has an impact on the surgical resection planning for brain neoplasms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) has a well-established role in the identification, localisation and characterisation of cerebral tumours. Conventional MRI studies, however, do not provide any information regarding the localisation of eloquent cortical functional areas or of white-matter tracts adjacent to a brain mass.
As an alternative, functional MRI (fMRI) can be employed to identify eloquent cortical areas next to a cerebral tumour or, more recently, the use of MRI with diffusion tensor imaging (DTI) has been proposed to evaluate in vivo patterns compatible with cerebral white-matter fibres [1–3].
The combination of these two techniques permits the achievement of better results in the pre-surgical planning for patients with brain tumours, thus allowing a wider extension of the margins to be resected [4]. In addition, it has been recently proven that tractography data can be integrated with a neuronavigational system [5]. The intra-operative localisation of main white-matter tracts is a valuable tool in the prevention of serious functional damage caused by more extensive resections [6] and in the approach to the neoplasia. Although this technique has been used for a few years already, only a small number of studies have systematically analysed the real impact of the data acquired by DTI on surgical planning and, in particular, on the outcome of the procedure [5, 7]; the frequency with which the information provided modifies the neurosurgeon’s procedural approach has not been defined as yet [8].
Our study had the following goals:
-
To evaluate the cases in which the presence of the tumour does not allow the identification of trajectories related to specific myelinated fibres as well as the frequency of such cases
-
To examine the possibility of integrating tractography images within a three-dimensional (3D) anatomical image dataset to be loaded into a neuronavigational system and employed in pre-surgical planning
-
To assess pre- and post-surgery patients’ clinical condition to determine the effective preservation of the myelinated fibres related to the white-matter trajectories involved
-
To evaluate the impact of this information on the surgical planning and procedure
Materials and methods
Patients
Between January and June 2008, a prospective group of patients undergoing neurosurgery for an intra-axial cerebral tumour in the Brain Suite of our Department (which was equipped with an intra-operative MRI device) was performed. In all the cases, an MR-tractography had previously been carried out and tractographic images—after having been imported in the operating theatre—were evaluated and employed in the neurosurgery planning. If needed, MR-tractography was repeated intra-operatively. Twenty-eight right-handed patients (19 men and 9 women, with ages ranging from 38 to 77 years) were included in the present study, 13 of them with glioblastoma multiforme, 7 with single metastases, 3 with grade III and 5 with grade II gliomas. Table 1 reports their characteristics, the histological diagnoses of the lesions and tumour localisations. Informed consent was obtained in all cases. The local ethics committee approved the study.
Clinical evaluation
All the patients were clinically assessed by a neurologist before and after surgery and 1 month later (Table 2) to define those symptoms possibly involving the pyramidal tract, the optic radiation and the arcuate fasciculus in both pre- and post-surgical phases.
The National Institute of Health stroke scale—adjusted for the evaluation of motor, visual or speech deficits—was employed to assess the patients’ medical condition. Motor disorders were measured by considering upper and lower limbs separately and by assigning a score from 0 to 4 (0 = no deficit; 1 = mild limb inequality; 2 = severe limb inequality; 3 = no limb effort against gravity; 4 = lack of movement). For visual field assessment, the scores were as follows: 0 = no deficit; 1 = partial hemianopia; 2 = complete hemianopia. Speech skills were scored: 0 = no aphasia; 1 = mild aphasia; 2 = severe aphasia. The same neurologist evaluated the patients with the same technique 1 month post-surgery, classifying them as worsened (onset of new deficits or deterioration of the previous ones), stable, or improved.
Pre-operative MRI study protocol
At our Department of Neuroradiology, pre-operative MRI study protocol includes the acquisition of sequences aimed at characterising the lesion and highlighting its relationship with the surrounding structures.
All DT-MRI examinations were performed on a 1.5-T magnet (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany). T2-weighted sequences [time to echo (TE)/time to repetition (TR) = 105/2,200 ms; matrix = 157 × 320; field of view (FoV) = 250 mm; slice thickness = 5 mm], fluid-attenuated inversion recovery sequences [TE/TR/inversion time (TI) = 125/10,000/2,005 ms, matrix = 144 × 256, FoV = 250 mm, slice thickness = 5 mm] and isotropic volumetric magnetisation-prepared rapid gradient echo T1-weighted sequences (TR/TE = 4.38/1,780 ms; matrix = 256 × 256; FoV = 250 mm; slice thickness = 1 mm) were all acquired before and after intravenous administration of paramagnetic contrast medium and in six motion-probing gradient directions. The DTI study was carried out in 12 non-collinear directions (b value = 0 and 1,000 s/mm2) with echo-planar sequences [9] (TE/TR = 92/9,400 ms, matrix = 128 × 128, FOV = 230 mm, slice thickness = 1.9 mm, bandwidth = 1,502 Hz/Px, slices = 60, no gap, acquisition time = 6.18 min, 3 NEX).
The tractography processing was carried out by a methodology similar to that suggested by Basser et al., Mori et al. and Stieltjies et al. [10–13] with the employment of the DTI task card software, version 1.6 (Magnetic Resonance Center, Massachusetts General Hospital, Boston, MA, USA), implemented on a dedicated console (BrainLAB SA, Heimstetten, Germany). Colour maps were utilised to define an appropriate region of interest (ROI) for the following tractographic procedure. As suggested by other authors in recent literature [14, 15], the fibre-tracking technique undertakes the 3D reconstruction of white-matter trajectories by employing a fractional anisotropy (FA) threshold of 0.15 and a processing angle above 55°. The positioning of the ROI for the fibre tracking changed according to the trajectories of the fibres to be reconstructed (posterior arm of the internal capsule for the pyramidal tract, geniculate ganglion for the optic radiation and, only in the 17 right-handed patients with lesions on the left side, an ROI encompassing the horizontal fibres lateral to the corona radiata and medial to the cortex of the posterior part of the ventrolateral frontal lobe) [13, 15, 16]. The trajectories were reconstructed on both cerebral hemispheres and transformed into tri-dimensional objects. Compared with the previously reconstructed trajectories, the outer margins of these objects were automatically enlarged by the software 2 mm in every direction (Fig. 1). Dilating the 3D reconstructed trajectories allowed registration errors to be minimised (usually below 3 mm) and a safer margin for surgery to be introduced. To assess the real nature of the tract considered—on the tumour side only, when available—the topographic relationship between the tract and its cortical functional areas was evaluated. The trajectory examined was considered compatible with the white-matter tract if comparable to the anatomical atlases of Wakana et al [17]. Tractographic processing was performed in consensus by two expert neuroradiologists.
The same neuroradiologists also assessed whether a contiguity between the trajectory and the tumour was present (a distance from the enhancement margins or the altered signal margins in the case of grade II gliomas of less than 1 cm). The trajectories were considered suitable for surgical planning if there were no interruptions in any of the layers at the level of the lesion (Fig. 2).
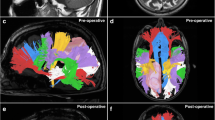
Patient 12. Left temporal lobe glioblastoma. Incomplete depiction of the temporal component of the arcuate fasciculus in the presence of peritumoral oedema that altered signal intensity on FA map (a–c) and T1-weighted images after gadolinium administration (d-f). In g and h, the three-dimensional reconstructions are presented
All tractography results were saved in a file that included x/y/z coordinates for each tract. These data were imported (together with b = 0 diffusion images) by the navigation software (iPlan 1.0, written by U. Mezger, BrainLAB SA, Heimstetten, Germany). After rigid registration of b = 0 images with a volumetric anatomical dataset and after ensuring data consistency (discrepancies ≤3 mm) in the tumour area, trajectory reconstructions can be visualised within anatomical images. The margins of the fibres are then segmented to permit their definition as ‘objects’ within the neuronavigation system and their visualisation during surgery. This processing is usually performed the day before the procedure and takes approximately half an hour.
Pre-operative MRI evaluation in the Brain Suite
To facilitate MRI data registration in the neuronavigation system, a multi-channel head coil—incorporating stereotactic ‘markers’ that are ‘read’ by the navigator video-camera and registered by the navigator itself—was employed. Dedicated software ‘merged’ the acquired images with tractographic processing, and the whole dataset was sent to the neuronavigation system.
In the Brain Suite (equipped with the same MR unit as was used for standard pre-operative imaging) volumetric, isotropic T1-weighted images were acquired with the same characteristics as those previously obtained except without the administration of paramagnetic contrast medium. These sequences represent the ‘baseline’ for the subsequent merging with pre-operative images and are aimed at the identification of the markers.
The total acquisition time of pre-surgery images for operating-theatre planning is therefore very limited: approximately less than 10 min. Should a tractographic reassessment be required during the neurosurgical procedure (i.e. for a re-evaluation of the tumoral margins with respect to specific myelinated fibres), this process is repeated (with the same sequence parameters and identical MRI equipment) and re-sent to the neuronavigation system to update the anatomical parameters. In our cases, this occurred in seven patients only, as they had high-grade tumours and total resection was not the final aim of neurosurgery.
The role of planning in the outcome of neurosurgery
To evaluate the impact that the information provided by DTI had on the procedure planning and execution, the following technique was selected.
During the first prospective phase, two neurosurgeons (LF, GDA) were asked to assess—in a double-blind fashion and with consensus in the case of discrepancy—the kind of surgical approach (the site of corticotomy) established on the basis of a conventional MR examination with intravenous enhancement. They were also asked to assess this on the basis of the same images integrated with tractography (by evaluating the trajectories related to the cortical spinal tract, the optic radiation and the arcuate fasciculus) (Table 3).
During the second retrospective phase, the operator neurosurgeon was asked to describe whether the evaluation of tractographic images acquired pre- and intra-procedurally (imported in the neuronavigation system) contributed to the definition of the lesion resection margins.
The results obtained were arranged into a table as positive/negative criteria for the two parameters (corticotomy and resection margins) (Table 4). If the information provided by tractography had modified at least one of the two parameters, the technique was considered as having influenced and somewhat changed the surgery planning.
Results
The integration of tractographic data into the volumetric dataset for neuronavigation was technically possible in all the cases. In the areas of interest (areas of anatomical relationship between the trajectory and the tumour), there was correspondence between b = 0 and 3D images, with a deviation which never exceeded 3 mm. This deviation had a tendency to increase in more cranial and caudal cerebral portions which, however, did not represent the aim of our study (all our patients, in fact, harboured a deep-seated tumour).
Nine patients did not have symptoms related to an involvement of myelinated fibres (in six of them early symptoms were of the epileptic type, while three patients had headache with disorders of the somatosensory system). The remaining 19 patients presented symptoms involving the pyramidal tract, the arcuate fasciculus or the optic radiation. At 1-month follow-up, one patient who had previously been asymptomatic complained of a speech disorder (sensory aphasia), probably caused by damage to the arcuate fasciculus (in this patient, the depiction of the whole trajectory of these myelinated fibre had not been achieved). In the other 27 cases, the symptoms had not changed, although a tendency towards a certain improvement was noted in 17 of the 19 patients with symptoms involving the mentioned myelinated fibres (Table 2).
The assessment of the 37 visualised trajectories with a relationship to the lesion resulted in an a priori modification of the surgical approach to corticotomy in 6 (21%) patients, without any discrepancy in the opinions of the two neurosurgeons (Fig. 3); the impact on the definition of the resection margins during surgery was 64% (18 cases) (Fig. 4). The overall impact percentage on the surgical procedure was 82% (Table 4).
Patient 26. Right temporal lobe glioblastoma. A priori modification of the surgical approach to corticotomy related to the position of the optical radiation. a-c Three-dimensional reconstructions. d, e T1-weighted images after gadolinium administration with superimposition of tractographic images. In f-h, the post-operative control is represented with tumour resection and preservation of the optic radiation
Patient 2. Left thalamus glioma (III grade). Definition of the resection margins during surgery. In three-dimensional and orthogonal images, the position of the cortico-spinal tract (red) position is evident close to the tumour, and it was used as the limit of the resection margin during the surgical procedure. The arcuate fasciculus (violet) is displaced laterally because of the presence of peritumoural oedema. The optic radiation could not be depicted due to oedema. After resection of the tumour, both the cortico-spinal tract and the arcuate fasciculus (yellow) are regularly represented and shifted posteriorly and anteriorly respectively
In seven cases (five glioblastomas and two grade II tumours) in which the tractography was repeated at the request of the neurosurgeon during the surgical procedure and then employed for updating of neuronavigational data during tumour resection, a trajectory shifting was documented (Fig. 5). The shift ranged from 2 to 8 mm and was in a craniotomy direction in six cases (four involving the pyramidal tract and two involving the optic radiation). All these cases belong to the group of patients in whom the visualisation of the trajectory modified the resection margins. In three cases, the updated tractographic evaluation allowed a further tumour resection; in the remaining patients, the new trajectory did not permit a more extensive resection.
Patient 27. Glioblastoma of the left temporal area. Intra-operative brain shifting following the surgical removal of the cerebral neoformation. As demonstrated, the tumour and the optical radiation (violet)—medially shifted—are very close (a, b). The cortico-spinal tract (green) is medially shift but is far from the tumour (c-d). Following the procedure, the cortical spinal tract (orange), slightly, and the optic radiation (yellow), more so, appear misplaced laterally in the direction of the craniotomy (e, f)
Discussion
The neurosurgical intervention in a cerebral tumour should balance the maximum surgical resection on one side and the maximum preservation of function on the other. Extensive resection of the lesion can, in fact, reduce the possible risk of recurrences (especially in the case of low-grade gliomas) and achieves better results than radio/chemotherapy. On the other hand, the saving of “functionally relevant” brain areas—with the preservation of motor, visual and language functions—improves the quality of life of these patients. The employment of neuroradiological techniques (including fMRI and MR-tractography) was aimed at achieving these purposes; these sequences proved to be potentially useful in the determination of the relationship between the tumour and adjacent areas of cortical function (fMRI) together with the condition of some tracts of white matter (DTI). The awareness of the localisation of some myelinated fibres with respect to a cerebral tumour is of vital importance in neurosurgical planning, for both the identification of the access site and the definition of resection margins.
DTI represents recent important progress in the field of diagnostic imaging. It is, in fact, the only technique able to visualise in vivo white-matter tracts compatible with cerebral myelinated fibres, and it has been proven already that this finding is of great help in pre-operative neurosurgical planning [1].
The main purpose of the present study was the evaluation of the effects of tractography on the approach to corticotomy and the definition of the resection margins. As a first step, the identification of a priori parameters of reliability for tractographic depictions was required. A few authors [7, 14, 18] have recently assessed the positions of the trajectories and their visualisation when these are adjacent to a brain mass. In particular, Stadlbauer and, after him, Bello, analysed how much peritumoral/infiltrate oedema (and, consequently, FA variations) can affect the depiction of white-matter trajectories, also thanks to intra-operative controls following subcortical stimulations. These studies stressed that a trajectory reconstruction performed with a 0.2- or 0.3-FA threshold—as suggested by previous studies to prevent improper inclusions (grey matter, cerebral spine fluid)—can affect their correct visualisation by underestimating their presence and increasing the distance between them and the tumour. If the FA threshold ranges from 0.2 to 0.15, a better compromise between visualisation of the fibres and their distance from the tumour is achieved instead, as has also been confirmed by subcortical stimulations. According to these considerations, we therefore decided to employ an FA threshold of 0.15.
A potential shortcoming of tractography is the anisotropy changes of the white matter adjacent to the tumour that can affect the visualisation of trajectories. The well-known difficulty of defining the anatomical and prognostic meaning of such visual interruption of trajectories (whether caused by infiltrate, oedema, or self-destruction) is not the aim of the present study; our attention focused only on the identification of specific trajectories. By excluding those anatomically far from the lesion, the visualisation of one of the trajectories related to the three myelinated fibres (the aim of the present study) was missed in 24% of cases. Anisotropy changes mainly affected the arcuate fasciculus and, by considering those patients whose tumour was contiguous to this tract, its identification was possible in only 17% of cases. The analysis of the pyramidal tract and the optic radiation achieved better results: the trajectories were not visualised in 11 and 7% of cases respectively. As expected, with regard to small perifocal oedema, anisotropy changes able to affect the depiction of the trajectories in low-grade malignant lesions were never recorded.
Another consideration should evaluate potential errors; registration errors were accurately analysed [5] by taking into account pyramidal tract processing. As mentioned in this paper, in our study registration errors did not exceed 3 mm, within the range of integration between fMRI and neuronavigation data [19]. To define a safety margin, Nimsky proposed 5 mm as a reliable distance for the pyramidal tract. This margin may change however in non-central areas of the brain, in consideration of the typical distortion of echo-planar images near the cranial vault and the skull base. Furthermore, the anatomical modifications induced by the intervention during tumour resection may introduce an additional variable (Fig. 2). The surgical manoeuvre may in fact determine shifts of the cerebral tissue, either in the same or in the opposite direction of craniotomy. It is therefore evident that pre-operative tractography assessment (obtained before the opening of the skull) might lose its significance. Nimsky [6, 20–22] had already considered this aspect by stating that the cortico-spinal tract may undergo some shifts during tumour removal. More recently, the same author stressed that the pyramidal tract shift occurring after the resection of a large part of a cerebral mass may reach 8 mm [5]. This observation was also confirmed by our experience in those cases in which the tractography was repeated during surgery. This finding emphasises the need for repeating tensor image acquisition, at least in more extensive resections. Such information may in fact be of vital importance if a complete tumour resection is required. Nimsky calculated that, in a group of patients with characteristics similar to those of our study group, this may occur in about 30% of cases. This percentage could be even higher in those patients with a low-grade malignant tumour in whom the total resection is a more relevant issue (this kind of tumour represented a small percentage in both our and Nimsky’s study population).
Even when the aforementioned technical difficulties are overcome, the reliability of tractographic images in defining trajectories that are anatomically compatible with a myelinated tract represents an important factor that is not easily manageable. A possible validation of the technique is based on the co-registration with intra-operative neurophysiological mapping. Preliminary studies have however reported data discrepancy on this subject. According to Kinoshita et al. [23], tractography and intra-operative subcortical neurostimulation cannot be easily correlated, while other authors [24] believe in the accordance of the findings of these two techniques. Gambini et al. [25], in particular, have reported correspondence between intra-operative subcortical neurostimulation sites and tractography of 84 and 79% for the motor tract and the speech circuit respectively. It should however be underlined that electrophysiological mapping techniques identify only those subcortical pathways next to the resection margins (2 or 3 mm maximum). This means that the evaluation is possible only when the resection is near or within the myelinated fibre, which may determine, according to some authors, the onset of transient (37%) or permanent (7%) neurological damage. In addition, other authors [26] believe that the identification of a subcortical pathway is impossible in 50% of cases, thus making the comparison between intra-operative tractography and electrophysiological mapping even more complicated. Furthermore, the possible dislocation of cerebral structures as a consequence of surgical resection may represent an additional complication in the comparison of the two techniques.
In our study, as we did not have the possibility of achieving a deep, intra-operative neurophysiological acquisition, we assessed the real preservation of myelinated fibres on the basis of pre- and post-procedural clinical evaluations. In only one patient (4%) of the 25 studied were new post-surgical symptoms recorded, involving the arcuate fasciculus. The course of the trajectory related to this case, however, was not fully visualised, probably because of the changes induced by the (asymptomatic) perilesional oedema. In all the remaining patients, new neurological symptomatology was not recorded; furthermore, an improvement in previous neurological deficits was noted in 80% of cases. These data are comparable to those reported by Nimsky in his group including 35 cerebral glioma patients in whom the intra-operative tractography technique employed was similar to that of our patients. In this study, the onset of a new, post-procedural, neurological symptomatology was recorded in one patient only (2.9% of cases).
Despite its intrinsic limitations and the problems mentioned earlier, the DTI technique combined with 3D tractography for the evaluation of cerebral white-matter tracts proved to be helpful to our patients for pre-operative planning. According to the results of our study, in 21% of cases the technique modified the corticotomy surgical approach, and in 64% of patients the awareness of myelinated fibre positions both before and during the procedure provided indications on the resection margins, thus permitting an overall procedural “modification” in 82% of cases. Because of the small number of patients and the unavailability of a control group, we cannot state that tractography improves the clinical outcome of a patient undergoing a neurosurgical procedure. However, we believe that the high incidence of patients in whom the surgical intervention was modified (in terms of the corticotomy approach and, above all, in the definition of the resection margins) together with the low rate of procedure-related complications indicates that further investigation is warranted.
Conclusions
MR-tractography provides the neurosurgeon with a new anatomical view that can change the surgical resection planning of a cerebral mass. Despite the high incidence of cases in which the pathological features may determine specific modifications preventing the possibility of reconstructing white-matter tracts, in our patients this technique modified the surgical approach to corticotomy (21%), permitted the definition of the resection margins (64%) and resulted in an overall modification of the procedure in 82% of cases. According to our study, the improvement in the pre-existing symptomatology (80% of cases) and the absence of new post-procedure complaints (with the exception of one patient only) suggest the potential of this technique.
References
Yu CS, Li KC, Xuan Y, Ji XM, Qin W (2005) Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Rad 56:197–204
Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL (2004) Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy and tumor imaging patterns. AJNR Am J Neuroradiol 25:356–369
Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B (2004) Diffusion tensor eigenvector directional colour imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging 20:555–562
Sunaert S (2006) Presurgical planning for tumor resectioning. J Magn Reson Imaging 23:887–905
Nimsky C, Ganslandt O, Merhof D, Sorensen AG, Fahlbusch R (2006) Intraoperative visualization of pyramidal tract by diffusion-tensor imaging-based fiber tracking. Neuroimage 30:1219–1229
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fiber tract navigation. Neurosurgery 58:292–304
Stadlbauer A, Nimsky C, Buslei R, Salomonowitz E, Hammen T, Buchfelder M, Moser E, Ernst-Stecken A, Ganslandt O (2007) Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage 34:949–956
Nimsky C, Ganslandt O, Von Keller B, Romstöck J, Fahlbusch R (2004) Intraoperative high-field strength MR imaging: implementation and experience in 200 patients. Radiology 233:67–78
Hori M, Ishigame K, Shiraga N, Kumagai H, Aoki S, Araki T (2008) Mean diffusivity, fractional anisotropy maps, and three-dimensional white matter tractography by diffusion tensor imaging. Comparison between single-shot fast spin-echo and single-shot echo-planar sequences at 1.5 Tesla. Eur Radiol 18:830–834
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Mori S, Van Zjil PC (2002) Fiber tracking: principles and strategies - a technical review. NMR Biomed 15:468–480
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M, Mori S (2001) Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 14:723–735
Bello L, Gambini A, Castellano A, Carrabba G, Acerbi F, Fava E, Giussani C, Cadioli M, Blasi V, Casarotti A, Papagno C, Gupta AK, Gaini S, Scotti G, Falini A (2008) Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage 39:369–378
Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC (2002) Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 47:215–223
Catani M, Jones DK, Fytche DH (2004) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Laundre BJ, Jellison BJ, Badie B, Alexander AL, Field AS (2005) Diffusion tensor imaging of corticospinal tract before and after mass resection as correlated with clinical motor findings: preliminary data. AJNR Am J Neuroradiol 26:791–796
Kober H, Nimsky C, Möller M, Hastreiter P, Fahlbusch R, Ganslandt O (2001) Correlation of sensorimotor activation with functional magnetic resonance imaging and magneto-encephalography in presurgical functional imaging: a spatial analysis. Neuroimage 14:1214–1228
Nimsky C, Ganslandt O, Kober H, Moller M, Ulmer S, Tomandl B, Fahlbusch R (1999) Integration of functional magnetic resonance imaging supported by magneto-encephalography in functional neuronavigation. Neurosurgery 44:1249–1256
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2005) Intraoperative diffusion tensor MR imaging: shifting of white matter tracts during neurosurgical procedures - initial experience. Radiology 234:218–225
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2004) Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 56:130–137
Kinoshita M, Yamada K, Hashimoto N, Kato A, Izumoto S, Baba T, Maruno M, Nishimura T, Yoshimine T (2005) Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage 25:424–429
Kamada K, Todo T, Masutani Y, Aoki S, Ino K, Takano T, Kirino T, Kawahara N, Morita A (2005) Combined use of tractography-integrated functional neuronavigation and direct fiber stimulation. J Neurosurg 102:664–672
Gambini A, Bello L, Falini A (2006) Corrispondenza tra Fiber Tracking e siti di neurostimolazione sottocorticale intraoperatoria dei circuiti motori e del linguaggio in pazienti affetti da tumori cerebrali. Neuroradiol J 19(Suppl. 1):70–71
Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS (2004) Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg 100:369–375
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romano, A., D’Andrea, G., Minniti, G. et al. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur Radiol 19, 2798–2808 (2009). https://doi.org/10.1007/s00330-009-1483-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-009-1483-6