Abstract
The purpose was to compare the findings of multi-detector computed tomography (MDCT) in prosthetic valve disorders using the operative findings as a gold standard. In a 3-year period, we prospectively enrolled 25 patients with 31 prosthetic heart valves. MDCT and transthoracic echocardiography (TTE) were done to evaluate pannus formation, prosthetic valve dysfunction, suture loosening (paravalvular leak) and pseudoaneurysm formation. Patients indicated for surgery received an operation within 1 week. The MDCT findings were compared with the operative findings. One patient with a Björk-Shiley valve could not be evaluated by MDCT due to a severe beam-hardening artifact; thus, the exclusion rate for MDCT was 3.2% (1/31). Prosthetic valve disorders were suspected in 12 patients by either MDCT or TTE. Six patients received an operation that included three redo aortic valve replacements, two redo mitral replacements and one Amplatzer ductal occluder occlusion of a mitral paravalvular leak. The concordance of MDCT for diagnosing and localizing prosthetic valve disorders and the surgical findings was 100%. Except for images impaired by severe beam-hardening artifacts, MDCT provides excellent delineation of prosthetic valve disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mechanical prosthetic heart valve replacement is an important surgical treatment for severe valvular heart disease [1, 2]. Because the underlying disease in the patient group is usually severe and mechanical failure of the prosthetic valve might occur, the prognosis of patients after prosthetic valve replacement is still not good. The 10-year survival rates are 65% for aortic valve replacement and 55% for mitral and combined valve replacement [1, 2]. Thus, regular evaluation of signs and symptoms and arranging accurate examinations to check prosthetic valve function are very important for improving the prognosis [3]. With good compliance to anti-coagulant therapy, thrombosis is less likely to occur, but prosthetic valves might still have mechanical failure problems, such as pannus formation, prosthetic valve dysfunction, suture loosening (paravalvular leak) [3] and pseudoaneurysm formation [4]. Transthoracic or transesophageal echocardiography is usually limited by severe acoustic shadowing caused by the prosthetic valve itself, resulting in an incomplete evaluation [5, 6]. Furthermore, the detection rate of pannus formation is so poor that a preoperative diagnosis is almost impossible [7]. Cine fluoroscopy is only useful in evaluating valve leaflet dynamics and is not useful in detecting suture loosening or pannus formation. In clinical practice, if echocardiography or cine fluoroscopy cannot provide a definite diagnosis or exclude a patient with suspected prosthetic valve disorders, then surgical exploration with redo valve replacement is the only solution [7].
In recent years, multi-detector-row CT (MDCT) has been used to provide more precise diagnostic information in several clinical situations such as neonatal coronary anomalies [8], neonatal congenital heart disease [9, 10] and metallic prosthetic devices [11]. In prosthetic heart valve evaluation, several case reports and case series have used MDCT with encouraging results [12–17]. These reports suggest that MDCT could be a good noninvasive imaging modality for prosthetic heart valve evaluation. However, there has been no prospective evaluation of the correctness or limitations of MDCT in various prosthetic valve disorders, including pannus formation, prosthetic valve dysfunction, suture loosening (paravalvular leak) [3] and pseudoaneurysm formation [4]. The goal of our study was to prospectively evaluate MDCT in diagnosing valve disorders, using operative findings as the gold standard.
Materials and methods
Patient enrollment
From March 2005 to March 2008, we prospectively enrolled all patients that accepted prosthetic valve replacement and had suspected mechanical prosthetic valve disorders (Fig. 1). The patients were recruited consecutively from a 1,500-bed tertiary hospital with a specialized cardiovascular institute. The presenting symptoms included dyspnea, abdominal distension, leg edema, back pain, chest tightness, chronic cough and easy fatigue. Prosthetic valve disorders included pannus formation, prosthetic valve dysfunction, suture loosening (paravalvular leak) [3] and pseudoaneurysm formation [4].
Exclusion criteria were a history of asthma, severe chronic obstructive pulmonary disease, total heart block, atrial flutter or fibrillation with a heart rate higher than 100 bpm after oral propranolol 40 mg, inability to perform a breath-hold for more than 15 s, pregnancy, allergy to contrast material, and renal insufficiency (creatinine level >1.4 mg/dl). The institutional review board approved the study, and all patients gave informed consent.
Demographic data collection
The age, sex, previous indication and date of prosthetic valve replacement, the valves replaced and the current chief complaint were all recorded.
Transthoracic echocardiography
After enrollment, transthoracic echocardiography (TTE) and MDCT were performed within 1 week. TTE was performed on either one of two specialized echocardiography machines (Sonos 5500 and iE 33; Philips Medical Systems, Best, The Netherlands) by one of three qualified technologists with more then 10 years of experience and was supervised by two cardiologists with 10 years of experience. The prosthetic valve motion, presence of stenotic jet flow and regurgitation, transvalvular mean pressure gradient (calculated by a simplified Bernoulli equation), effective prosthetic valve area (calculated by continuity equation), presence of paravalvular leak and pseudoaneurysm were carefully evaluated. The general diagnostic criterion for prosthetic valve dysfunction is a mean pressure gradient ≥30 mmHg or effective orifice area ≤1.0 cm2 [5]. A more detailed diagnostic criterion for each prosthetic valve was also used for confirmation [5]. The diagnosis of paravalvular leak was established if the flow jet signal was identified outside of the suture ring.
MDCT of prosthetic heart valves
All patients with an initial heart rate more than 60 bpm were given a 10-40 mg dose of oral propranolol (Cardilol; Veteran’s Pharmaceutical Factory, Taoyuan, Taiwan, Republic of China) 1 h before MDCT by an experienced cardiac radiologist. Cardiac CT studies were performed by using a 40-detector-row CT system (Brilliance 40; Philips, Best, The Netherlands). The parameters were a tube voltage of 120 kV, an effective tube current of 400-950 mA per section according to the patient’s body weight [10], a pitch of 0.2 and a rotation time of 0.42 s. In addition, electrocardiographic gating was employed from the carina to the lower border of the heart in a craniocaudal direction. The online electrocardiography-based dose modulation (DoseRight Cardiac, Philips) was not applied, because of potential impairment of systolic phase images, which are crucial for evaluation of prosthetic valve motion. The CT protocol was the same as that used for the ‘ischemic heart routine’ [18].
A 20-gauge intravenous catheter was placed in the right antecubital vein, and 100 ml of iohexol (Omnipaque 350; Amersham, Cork, Ireland) was injected at a flow rate determined by the ‘contrast-covering time’ concept [11, 19]. This was followed by a 30-ml saline bolus given at the same flow rate. The ‘contrast-covering time’ concept has proven to be useful in evaluating intracardiac prosthetic devices and structures in patients of different body weights [8–11, 19]. To synchronize imaging with the injection of contrast agent, we used a bolus-tracking technique with a threshold of 150 HU and a region of interest placed in the ascending aorta. Two senior CT technologists with more than 10 years of experience with CT performed all the CT studies. Images were reconstructed from 0% to 90% of the RR interval with 10% intervals.
The initial heart rate, heart rate after propranolol, heart rate during the scan, scan time and dose-length product were recorded. A factor of 0.014 was used to convert the dose-length product data into the effective dose [23].
For evaluation of the MDCT examinations, a cardiac radiologist with 4 years of experience with intracardiac prosthetic device evaluation [11] used a dedicated MDCT workstation (Extended Brilliance Workspace; Philips, Best, The Netherlands) to interpret the CT data. The ten-phase images were loaded into the cine viewer software (Cardiac viewer; Extended Brilliance Workspace, Philips, Best, The Netherlands) to evaluate the prosthetic valve motion in various planes with a focus on leaflet motion and residual opening angle between leaflets. Special attention was also paid to the relationship between the suture ring and the surrounding valve annulus for detecting suture loosening, pannus and pseudoaneurysm formation.
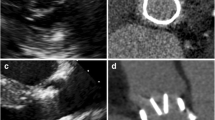
Pannus formation was defined according to previous case reports [13, 16, 17] and our clinical experience as a small low-density region located at the inflow side of the prosthetic valve suture ring. Care was taken to exclude the beam-hardening artifact, which was defined as a black shining line projecting outwards from the dense portion of the device (Fig. 2).
Björk-Shiley valve that could not be evaluated by MDCT. A 69-year-old female patient with a Björk-Shiley tilting disk aortic valve that could not be evaluated by MDCT due to severe beam-hardening artifact. a Three-chamber view of MDCT during systolic phase shows the prosthetic valve (arrow) in the aortic position, but with severe beam-hardening artifact (arrowheads). The beam-hardening artifact impaired visualization of the valve structure, making it impossible to diagnose the prosthetic valve disorder. Because TTE showed a transvalvular mean pressure gradient of 55.0 mmHg and an effective aortic valve area of 1.0 cm2, prosthetic valve dysfunction was diagnosed by TTE. Severe pannus formation with prosthetic valve dysfunction was found during redo valve replacement surgery. b Cross-sectional MDCT image of the Björk-Shiley tilting disk valve in vitro using 80 kV and 800 mAs/slice. Severe blooming artifact of the metallic prosthesis (arrow) is noted with a severe adjacent beam-hardening artifact (arrowhead). The window width and level are set at 850/150 in b, c and d for comparison. c Cross-sectional MDCT image of the Björk-Shiley tilting disk valve in vitro using 120 kV and 800 mAs/slice. Compared with b, the blooming and beam-hardening artifacts are better controlled, but are still severe. The detailed prosthesis structure still could not be evaluated. d Cross-sectional MDCT image of the Björk-Shiley tilting disk valve in vitro using 140 kV and 800 mAs/slice. With the 140-kV technique, the blooming (arrow) and beam-hardening artifacts (arrowhead) are similar to those obtained with the 120 kV technique in c. No more diagnostic information could be obtained using the high-radiation 140-kV technique. (Ao: aorta, LA: left atrium, LV: left ventricle)
Prosthetic valve dysfunction was defined according to previous literature on cine fluoroscopy and MDCT [6, 13, 16, 17]. For a bileaflet valve, the residual angle must be less than 20 degrees [17]. For a tilting disk valve, the normal opening angle is different with different vendors, and the diagnostic criterion is based on the information provided by the vendors [20]. If the residual angle was larger than 20 degrees in bileaflet valves or the defined normal angle of tilting disk valves, prosthetic valve dysfunction was diagnosed.
Paravalvular leak was evaluated by using the most quiescent systolic and diastolic phases of each valve. If the suture ring was not directly attached to the surrounding tissue, and contrast material filled in the spaces between, paravalvular leak was diagnosed.
Pseudoaneurysm was defined as a cavity that was not lined by the native cardiovascular endothelium surrounding the implanted prosthetic heart valve with an entry site caused by tearing from the suture on the native heart tissue.
The location of the pannus formation and prosthetic valve loosening were also recorded. Because the operative finding was the gold standard for our study, we use a localizing system based on the surgical view (Fig. 3) The involved zones of pannus formation and suture loosening were recorded for comparison with operative findings.
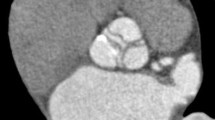
Illustration of the localization system. Localization of pannus and suture loosening in prosthetic aortic, mitral and tricuspid valves. a MDCT image of prosthetic aortic valve viewed from ascending aorta (case 3). After identifying the right coronary artery orifice (arrowhead), left main coronary artery orifice (arrow) and the mid-point of non-coronary cuspid of aortic valve, we divide the prosthetic aortic valve into three zones as shown in the figure (dashed line). Starting from the right coronary artery orifice (arrowhead), zones 1, 2 and 3 are defined in a clockwise rotation. b MDCT image of prosthetic mitral valve viewed from left atrium (case 3). After identifying the mid-point of aortic valve (arrow) at the 12 o’clock position, the prosthetic mitral valve was virtually divided into four zones as shown in the figure (dashed line). Zones 1, 2, 3 and 4 are indicated in a clockwise rotation. c MDCT image of prosthetic tricuspid valve viewed from right atrium (case 7). After identifying the coronary sinus orifice (CS) at the 6 o’clock position, the prosthetic tricuspid valve was virtually divided into four zones (dashed line). Starting from the coronary sinus orifice (CS), zones 3, 4, 1 and 2 are defined in a clockwise rotation. (CS: coronary sinus, RA: right atrium)
Operation
After completing the image evaluation, if there were positive findings on either MDCT or TTE, the patient was enrolled into the ‘positive group.’ The findings were discussed together with the cardiac radiologist and cardiovascular surgeons to clarify the indication for surgery. Then, the condition was explained to the patient. If the patient was willing to receive an operation, it was arranged within 1 week, and the patient was assigned to the ‘operative group.’ During the operation, we also recorded the presence and the locations of prosthetic valve disorders and compared them with MDCT and TTE findings.
The surgical definitions of prosthetic valve disorders were defined as the following. Pannus formation was defined as the focal soft tissue growth over the inflow portion of the suture ring. Prosthetic valve dysfunction was defined as the occurrence of resistance before the leaflets fully opened when the leaflets were probed. When the heart was opened during initial inspection, if the suction tip could easily probe into the defect between the suture ring and the valve annulus, the diagnosis of suture loosening was made. For pseudoaneurysm, the intra-operative diagnosis was made with clear identification of the entry point and successful probing of the pseudoaneurysm cavity.
For patients with suture loosening that decided to have a minimally invasive procedure, a team with endovascular device delivery experience [21] performed the Amplatzer ductal occluder for prosthetic valve suture loosening defect occlusion [22]. During the procedure, loosening and the location were confirmed by both transesophageal echocardiography and left ventriculography. Then, the presence of the defect was further confirmed by guidewire passage and occluder implantation.
Statistics
Paired t-test was used to compare the initial heart rate and heart rate during the exam (after oral propranolol). P < 0.05 was considered to indicate a statistically significant difference. The exclusion rate of MDCT prosthetic valve evaluation was defined as the valves that could not be evaluated divided by the total number of valves examined. After excluding the cases that could not be evaluated, the diagnosis correctness and localization correctness were determined. The diagnostic correctness was defined as the number of cases with correct diagnosis on MDCT divided by the number of cases in the operative group. The localization correctness was defined as the number of zones with correct interpretation divided by the total number of zones evaluated.
Results
Patient demographics
Initially, 29 patients were recruited, but 2 of them had surgery after echocardiography, and 2 required an emergent operation for the diagnosis of infective endocarditis with suture loosening. Therefore, a total of 25 patients (14 male) and 31 valves were enrolled in the study (Table 1). The effective dose of the CT examination was 15.8 ± 3.1 mSv, which is similar to previous cardiac MDCT studies [18].
Valve distribution
A total of 31 valves were examined: 13 of them were mitral, 16 aortic and 2 tricuspid. The valves were manufactured by various vendors: 13 were On-X bi-leaflet valves (On-X, Medical Carbon Research Institute, Austin, TX), 8 were CarboMedics bi-leaflet valves (CarboMedics, Inc., Austin, TX), 8 were St. Jude Medical bi-leaflet valves (St. Jude Medical valve, St. Jude Medical Inc, St. Paul, MN), 1 was a Medtronic Hall tilting disk valve (Medtronic Hall valve, Medtronic, Inc., Minneapolis, MN), and 1 was a Björk-Shiley tilting disk valve (Björk-Shiley spherical disk valve, Shiley Inc., Irvine, CA). Only the Björk-Shiley tilting disk valve could not be evaluated by MDCT due to severe beam-hardening artifact (Fig. 2). Echocardiography was positive in 6 patients, and MDCT was positive in 12. The positive group contained 12 patients.
We searched our storehouse and found an unused Björk-Shiley valve manufactured 22 years ago (July 1987). An in vitro MDCT exmaination was done with the Björk-Shiley valve embedded in a bottle of water. Even with the highest tube voltage of 140 kV in our current clinical CT system, the valve could still not be evaluated due to severe beam-hardening artifact (Fig. 2).
Only one of the 31 valves could not be evaluated, resulting in an exclusion rate of 3.2%.
Operations and comparisons
Based on a combined discussion with the cardiac radiologist and surgeons in charge, all 12 patients in the positive group were indicated for surgery. Six patients were indicated for surgery due to suture loosening, two for pannus with prosthetic valve dysfunction, two for pseudoaneurysm surrounding the suture ring, one for prosthetic valve dysfunction without pannus formation, and one for pannus formation, but no prosthetic valve dysfunction. Five patients received open heart surgery within 1 week, including three aortic (Figs. 4 and 5) and two mitral redo replacements. One patient decided to undergo a minimally invasive operation with an Amplatzer ductal occluder for prosthetic mitral valve loosening (Fig. 6) [22]. A total of six patients were included in the ‘operative group’ for further analysis (Table 2). One patient received open heart mitral valve replacement 2 months later, because the time interval between MDCT and surgery was too long; thus, this patient was excluded from further analysis.
Suture loosening. A 59-year-old male post-aortic valve (On-X) replacement with extensive suture loosening (case 4). a Three-chamber view of TTE. The bi-leaflet (arrowheads) prosthetic aortic valve is clearly identified. However, the severe acoustic shadow (arrow) produced by the prosthesis itself impaired the evaluation of the valve. The TTE was interpreted as normal! The motion cine is provided as animation 4a. The extensive suture loosening was missed by TTE. b MDCT image of the prosthetic aortic valve viewed from the ascending aorta clearly shows suture loosening. Only four stitches with pledgets are still intact (arrowheads); otherwise, the sutures are all loosened. The extensive suture loosening involves all three zones. c Three-chamber view of MDCT clearly shows separation of the suture ring (white arrowhead) and aortic valve annulus (black arrowhead). During systole, paravalvular leak is identified (dashed arrow). Compared with the TTE image in a, the loosening is in the region of severe acoustic shadowing, which explains the missed diagnosis by TTE. The motion cine is provided as animation 4c. d The intra-operative photograph. After exposing the ascending aortic graft (arrow) of the previous Bentall operation, the suction tip (arrowhead) can easily probe the loosening region. Suture loosening and its involved zones are confirmed. e The hand-drawn surgical finding. After the operation, the surgeon recorded the surgical findings with a hand-drawn picture on the operation notes. Not only the involved zones are totally compatible, but also the remaining intact four stitches (arrowheads) are exactly the same as the MDCT findings shown in b. (Ao: aorta, L: left coronary cuspid, LA: left atrium, LV: left ventricle, N: non-coronary cuspid, R: right coronary cuspid)
Pannus formation. A 41-year-old female post-prosthetic aortic valve (CarboMedics) replacement with pannus formation and prosthetic valve dysfunction (case 1). a MDCT image of the left ventricular outflow tract. The pannus (arrow) is identified as a small black area just below the suture ring extending into the housing. The pannus is formed extensively and involves all three zones. The complete serial sections are shown in animation 5a. All pannuses identified are indicated by red arrows. b MDCT image of left ventricular outflow tract during systole is used for measuring the residual angle between the leaflets (arrowheads). The residual angle is 66 degrees, which indicates prosthetic valve dysfunction. c The intra-operative photograph. The extensive pannus formation (white arrowhead) is also identified with only a small region spared (black arrowhead), which is compatible with the MDCT findings. (Ao: aorta, LV: left ventricle)
Suture loosening occluded by Amplatzer ductal occluder. A 16-year-old female post-prosthetic mitral valve (On-X) replacement with focal suture loosening (case 6) occluded by the Amplatzer ductal occluder. a Four-chamber view of TTE demonstrating paravalvular leak (arrow) during systole at the superior portion of the prosthetic valve, indicating suture loosening. Also notice the severe tricuspid regurgitation (arrowhead). b Two-chamber view of MDCT demonstrates suture loosening by identifying the separation of mitral annulus (white arrowhead) and suture ring (black arrowhead). c Virtual endoscopy of MDCT viewed from the left ventricle. The suture loosening is identified (arrow). The view is different from the surgical approach from the left atrium. With the location definition in our study, the suture loosening is defined as zone 4. d Right anterior oblique view of catheter angiography during ductal occluder deployment. After confirming the suture-loosening location (arrow) by transesophageal echocardiography and left ventriculography, the Amplatzer ductal occluder was successfully placed in the superior aspect of the prosthetic mitral valve (arrowhead). e Virtual endoscopy of MDCT viewed from the left ventricle after the procedure. The Amplatzer ductal occluder (arrow) is successfully occluding the suture loosening of the prosthetic mitral valve (arrowhead). (APM: anterior papillary muscle, LA: left atrium, LAA: left atrial appendage, LV: left ventricle, LVOT: left ventricular outflow tract, PPM: posterior papillary muscle, RA: right atrium, RV: right ventricle)
MDCT correctly diagnosed all the valve disorders in the six patients in the operative group; thus, the diagnostic correctness was 100%. In addition, a total of 24 zones were evaluated, and MDCT correctly identified the presence or absence of disorders in all 24 zones; thus, the localization correctness was also 100%.
In the six patients in the operative group, TEE could not identify severe aortic valve loosening in one patient; thus, the diagnostic correctness of TEE using the operative findings as a gold standard was only 83.3%. However, MDCT clearly and correctly identified the loosening (Fig. 4).
Although one patient with a Björk-Shiley valve could not be evaluated by MDCT, TTE correctly identified the prosthetic valve dysfunction in this patient by showing an effective orifice area of 1.0 cm2 and a transvalvular pressure gradient of 55 mmHg. Pannus formation and prosthetic valve dysfunction were also noted during redo valve replacement in this patient.
Discussion
Our study is the first to prospectively confirm the diagnostic correctness of MDCT for prosthetic valve disorders in real clinical conditions. Except for some outdated Björk-Shiley tilting valve disks, MDCT can evaluate all prosthetic valve disorders clearly, including pannus formation, prosthetic valve dysfunction, suture loosening and even pseudoaneurysm formation. MDCT resulted in a correctness of 100% for diagnosing the disease as well as localizing the disorder.
Our study vs. existing literature
Until now, there have been only some scattered case reports or case series in the literature on the use of MDCT to diagnose prosthetic valve dysfunction [12–17]. Although these studies reported encouraging images and diagnostic results, they may have been confounded by a severe selection bias due to the inherent limitation of the article format. Our study was based on a tertiary referral medical center with consecutive and prospective enrollments, which reflect real-world conditions. We confirmed the early encouraging results of previous case reports and also provided data on more valve disorders and imaged more different types of mechanical valves. In addition, we provided information about the limitations of MDCT.
Teshima et al. studied the usefulness of MDCT for detecting pannus formation, but only two patients were surgically confirmed [17]. In that study, the only valve disorder was pannus formation, and both patients had a St. Jude Medical bileaflet valve. Compared with Teshima’s study, we evaluated more valve disorders, had more surgically confirmed cases and included multiple brands of prosthetic heart valves. Therefore, our results are more applicable to routine clinical practice.
The Björk-Shiley valve
For the Björk-Shiley spherical valve that could not be evaluated by MDCT, the severe beam-hardening artifact persisted even at the highest tube voltage in the clinical CT system [24]. This means that even if the routine protocol was shifted to scanning with 140 kV, the diagnostic correctness and exclusion rate would probably have been the same. Thus, using the same protocol as for CT coronary angiography, 120 kV should be sufficient for prosthetic heart valves.
The other prosthetic valves included the On-X, St. Jude Medical, CarboMedics bileaflet valves and Medtronic Hall tilting disk valve and could all be evaluated by MDCT in our study.
Implications for clinical practice
It is possible that MDCT could be a major imaging modality in prosthetic heart valve disorders. In our study, a patient with Björk-Shiley valve was excluded due to severe beam-hardening artifact. Except in this one case, all patients with prosthetic valve disorders were successfully and accurately diagnosed by MDCT with operative confirmation. This means that when MDCT can see the prosthetic valve structure clearly, the evaluation should be accurate. Thus, in clinical practice, when TTE and TEE cannot evaluate the prosthetic valve confidently because of the inherent limitation of ultrasound, such as poor acoustic window, metallic shadowing or limited penetration, MDCT could be used as a reliable investigation for further assessment.
Limitations
There are several limitations of our study. First, it was only a single center and single country study with limited types of prosthetic valves (only five types). Some countries are still using Björk-Shiley valves extensively, which would limit the use of our study for that type of valve. Furthermore, there are still many different kinds of prosthetic valves that were not encountered in our study, even though our study did provide clinically useful information for those countries using On-X, St. Jude Medical, Carbemedics bileaflet or even Medtronic Hall tilting disk valves. Based on our protocol and interpretation methods, other investigators might also use MDCT to scan patients with different kinds of prosthetic valves, which might further extend our knowledge in this field. Secondly, we did not perform transesophageal echocardiography for prosthetic valve evaluation. The reason is that transesophageal echocardiography is considered an invasive study that causes considerable discomfort if not performed under anesthesia. Also, there are already many studies of prosthetic valves using transesophageal echocardiography in the literature [5]. Thus, we decided to use only TTE and MDCT in our study. Thirdly, there are only six patients with surgical correlation in our study. However, this is already the largest series in the literature and is the only prospective correlation study up to the time of writing this manuscript.
Conclusions
In conclusion, except for the images impaired by severe beam-hardening artifacts, MDCT provides excellent concordance with surgical findings in prosthetic valve disorders. Our study confirmed the clinical usefulness of MDCT for prosthetic valve disorders.
References
Lindblom D, Lindblom U, Qvist J, Lundström H (1990) Long-term relative survival rates after heart valve replacement. J Am Coll Cardiol 15:566–573
Groves P (2001) Surgery of valve disease: late results and late complications. Heart 86:715–721
Seiler C (2004) Management and follow up of prosthetic heart valves. Heart 90:818–824
Gupta R, Jammula P, Huang MH, Atar S, Ahmad M (2006) An unusual complication after aortic valve replacement. J Clin Ultrasound 34:361–364
Aslam AK, Aslam AF, Vasavada BC, Khan IA (2007) Prosthetic heart valves: types and echocardiographic evaluation. Int J Cardiol 122:99–110
Muratori M, Montorsi P, Teruzzi G, Celeste F, Doria E, Alamanni F, Pepi M (2006) Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol 97:94–100
Sakamoto Y, Hashimoto K, Okuyama H, Ishii S, Shingo T, Kagawa H (2006) Prevalence of pannus formation after aortic valve replacement: clinical aspects and surgical management. J Artif Organs 9:199–202
Tsai IC, Lee T, Chen MC, Fu YC, Jan SL, Wang CC, Chang Y (2007) Visualization of neonatal coronary arteries on multidetector row CT: ECG-gated versus non-ECG-gated technique. Pediatr Radiol 37:818–825
Lee T, Tsai IC, Fu YC, Jan SL, Wang CC, Chang Y, Chen MC (2006) Using multidetector-row CT in neonates with complex congenital heart disease to replace diagnostic cardiac catheterization for anatomical investigation: initial experiences in technical and clinical feasibility. Pediatr Radiol 36:1273–1282
Tsai IC, Chen MC, Jan SL, Wang CC, Fu YC, Lin PC, Lee T (2008) Neonatal cardiac multidetector row CT: why and how we do it. Pediatr Radiol 38:438–451
Lee T, Tsai IC, Fu YC, Jan SL, Wang CC, Chang Y, Chen MC (2007) MDCT evaluation after closure of atrial septal defect with an Amplatzer septal occluder. AJR Am J Roentgenol 188:W431–W439
Leborgne L, Renard C, Tribouilloy C (2006) Usefulness of ECG-gated multi-detector computed tomography for the diagnosis of mechanical prosthetic valve dysfunction. Eur Heart J 27:2537
Teshima H, Aoyagi S, Hayashida N, Shojima T, Takagi K, Arinaga K, Yoshikawa K (2005) Dysfunction of an ATS valve in the aortic position: the first reported case caused by pannus formation. J Artif Organs 8:270–273
Faletra FF, Alain M, Moccetti T (2007) Blockage of bileaflet mitral valve prosthesis imaged by computed tomography virtual endoscopy. Heart 93:324
Numata S, Okada H, Kitahara H, Kawazoe K (2007) Four-dimensional evaluation of implanted mechanical valve with 64-row multi-detector computed tomography. Eur J Cardiothorac Surg 31:934
Teshima H, Hayashida N, Enomoto N, Aoyagi S, Okuda K, Uchida M (2003) Detection of pannus by multidetector-row computed tomography. Ann Thorac Surg 75:1631–1633
Teshima H, Hayashida N, Fukunaga S, Tayama E, Kawara T, Aoyagi S, Uchida M (2004) Usefulness of a multidetector-row computed tomography scanner for detecting pannus formation. Ann Thorac Surg 77:523–526
Tsai IC, Lee T, Lee WL, Tsao CR, Tsai WL, Chen MC, Ting CT (2007) Use of 40-detector row computed tomography before catheter coronary angiography to select early conservative versus early invasive treatment for patients with low-risk acute coronary syndrome. J Comput Assist Tomogr 31:258–264
Tsai IC, Lee T, Chen MC, Tsai WL, Lin PC, Liao WC (2007) Homogeneous enhancement in pediatric thoracic CT aortography using a novel and reproducible method: contrast-covering time. AJR Am J Roentgenol 188:1131–1137
Cedars-Sinai Medical Center Cardiology staff (2008) Cedars-Sinai Medical Center Prosthetic Heart Valve Information. Available at: http://www.csmc.edu/pdf/Heart_Valves.pdf Accessed on March 23, 2008
Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE, Balzer D, Cao QL, Hijazi ZM (2006) Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol 47:319–325
Shapira Y, Hirsch R, Kornowski R, Hasdai D, Assali A, Vaturi M, Sievert H, Hein R, Battler A, Sagie A (2007) Percutaneous closure of perivalvular leaks with Amplatzer occluders: feasibility, safety, and short term results. J Heart Valve Dis 16:305–313
McCollough C, Cody D, Edyvean S, et al for the Diagnostic Imaging Council CT Committee (2008) AAPM report no. 96 The Measurement, Reporting, and Management of Radiation Dose in CT. American Association of Physicists in Medicine. Jan 2008
Brendzel AM, Rambod E, Jorgensen SM, Reyes DA, Chmelik MS, Ritman EL (2002) Three-dimensional imaging of fractures in outlet struts of Björk-Shiley convexo-concave heart valves by microcomputed tomography in vitro. J Heart Valve Dis 11:114–120
Acknowledgements
This research was supported in part by Taichung Veterans General Hospital under grants TCVGH-975506C and TCVGH-975504A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Correctness of multi-detector-row computed tomography for diagnosing mechanical prosthetic heart valve disorders using operative findings as the gold standard
Electronic supplementary material
Below is the link to the electronic supplementary material.
Animation 4a
Three-chamber view of TTE showing normal prosthetic bileaflet valve motion. See Fig. 4a for annotation. Compared with animation 4c, the loosening regions of the prosthetic aortic valve were all blocked by the acoustic shadowing caused by the prosthesis itself (MPG 355 kb)
Three-chamber view of MDCT clearly demonstrating the loosening region near the fibrous continuity between the mitral and aortic valves. During systole, the valve is displaced superiorly, exposing the loosening defect. See Fig. 4c for annotation (MPG 522 kb)
Animation 5a
Serial MDCT images of the left ventricular outflow tract. The pannus (red arrows) is identified as a small black area just below the suture ring extending into the housing. The pannus is formed extensively, involving all three zones (MPG 1762 kb)
Rights and permissions
About this article
Cite this article
Tsai, IC., Lin, YK., Chang, Y. et al. Correctness of multi-detector-row computed tomography for diagnosing mechanical prosthetic heart valve disorders using operative findings as a gold standard. Eur Radiol 19, 857–867 (2009). https://doi.org/10.1007/s00330-008-1232-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1232-2