Abstract
The aim of this study was to evaluate the value of comprehensive renal ultrasound (US), i.e., combining greyscale US and amplitude-coded color Doppler sonography (aCDS), for assessment of urinary tract infection (UTI) in infants and children, compared to (1) 99mTc DMSA scintigraphy and (2) final diagnosis. Two hundred eighty-seven children with UTI underwent renal comprehensive US and DMSA scintigraphy. The results were compared with regard to their reliability to diagnose renal involvement, using (1) DMSA scintigraphy and (2) final diagnosis as the gold standard. Sixty-seven children clinically had renal involvement. Sensitivity increased from 84.1% using only aCDS to 92.1% for the combined US approach, using DMSA scintigraphy as the reference standard. When correlated with the final diagnosis, sensitivity for DMSA scintigraphy was 92.5%; sensitivity for comprehensive US was 94.0%. Our data demonstrate an increasing sensitivity using the combination of renal greyscale US supplemented by aCDS for differentiation of upper from lower UTI. Sensitivity for DMSA and comprehensive US was similar for both methods compared to the final diagnosis. Comprehensive US should gain a more important role in the imaging algorithm of children with acute UTI, thereby reducing the radiation burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is a common disease in childhood and may cause diagnostic problems in routine pediatric practice [1]. Especially in neonates and infants, there are no specific clinical signs or reliable symptoms; even laboratory findings may be confusing. In older children upper UTI usually occurs with fever, whereas in younger patients the differentiation of lower from upper UTI can be difficult just based on clinical and laboratory findings. However, early detection of renal involvement is essential for intensity and duration of treatment, as acute pyelonephritis (aPN) may result in irreversible renal damage. APN can induce renal scars as a complication; the importance of aPN-associated risks is under debate, but long-term complications such as hypertension and chronic renal failure have to be considered [2]. Therefore, an early, reliable, and accurate as well as easily accessible imaging method for aPN assessment may be valuable [3].
Renal technetium-99m dimercaptosuccinic acid (99mTc DMSA) scintigraphy has been shown to be highly sensitive and specific for the diagnosis of acute inflammatory renal changes [4–7]; currently DMSA scintigraphy is considered the gold standard (i.e., the reference investigation) for the diagnosis of aPN and renal scarring [8–10]. Contrast-enhanced spiral CT has been reported to be an efficient diagnostic tool, but is reluctantly used in children due to its high radiation burden [11, 12]. Both DMSA and particularly CT studies have the disadvantage of ionizing radiation exposure and intravenously injected agents. Additionally, they are relatively expensive, and DMSA is not available at night hours and during the weekend in some areas. MRI is also highly effective, but the routine use is hindered by the high costs, its restricted availability, and the need for sedation in small children and infants [13].
Ultrasound (US) is widely available, inexpensive, and in general the first imaging study performed in children with suspected UTI, though availability of reliable high-quality US is also restricted. However, greyscale US is reported to be poor in detection of renal involvement [1]. More than a decade ago amplitude-coded color Doppler sonography (aCDS), also called “power Doppler,” was introduced. It has a higher sensitivity to low-flow velocities even at high insolation angles (provided sufficient flow volume, as encountered in the peripheral renal parenchyma) [14]. Also, aCDS has successfully been used for assessment of particularly focal or segmental renal perfusion alteration [15]. Most pyelonephritic lesions have an ischemic component or exhibit associated perfusion disturbances, with a reportedly higher sensitivity of aCDS for the detection of (focal) aPN [16, 17]. However, all these studies focus exclusively on the use of aCDS, not including additional information retrieved from greyscale US that may also be beneficial for the diagnosing upper UTI.
The aim of this retrospective analysis was to evaluate if combining renal greyscale US and aCDS findings may improve US potential for depiction of upper UTI in infants and children compared to (1) DMSA scintigraphy and (2) final diagnosis.
Materials and methods
The picture archiving and communication system of our hospital was used to retrospectively identify pediatric patients with acute UTI who underwent comprehensive US (i.e., including aCDS) and DMSA for the assessment of renal involvement. Patients with congenital abnormalities, hydronephrosis, recurrent UTI, known vesico-ureteral reflux (VUR), or scars were excluded.
Tc99m DMSA scintigraphy was performed using a standard protocol according to the guidelines on renal cortical scintigraphy in children with UTI of the European Association of Nuclear Medicine [18]. Two to three hours after intravenous injection of 80 μCi/kg (2,92 MBq/kg), planar anterior, posterior, and right as well as left oblique images of the kidneys were obtained using an ELSCINT Helix dual-head camera (GE Medical Systems, Milwaukee, WI); since 2002 some patients were examined using a E.CAM dual-head device (Siemens Medical Solutions, Erlangen, Germany). Images were obtained for 300,000 to 500,000 counts on a 256 × 256 matrix. The consensus criteria of the International Radionuclides in Nephrourology Group were used for interpretation of DMSA results [18]. The scintigraphic diagnosis of an “upper UTI” was defined by totally or partially reversible lesions on DMSA; if the first DMSA examination was abnormal, the examination was repeated after 6 and 12 months. A specialist in nuclear medicine independently read the images. No SPECT studies were performed for radiation protection issues.
Comprehensive US was performed using an Acuson Sequoia 512 Ultrasound device (Acuson/Siemens, Mountain View, CA) with various curved array and/or linear multi-frequency transducers (1–14.0 MHz). Initially, a thorough greyscale US study was performed. This always started with the sufficiently filled urinary bladder after physiological hydration of the patient, included a view of the posterior urethra, and continued with the kidneys [19]. Subsequently, aCDS was applied manipulating of color gain until noise become apparent. Frequency, gate, filter, scale, and persistence were optimized to visualization of the peripheral intrarenal vasculature, keeping the focus zone in the lower third of the color box. Both axial and longitudinal scans were obtained to provide a vascular map of the kidneys. In each patient both kidneys-when present-were assessed using the healthy kidney for comparison; in patients with bilateral disease, the spleen was used for intraindividual comparison. In all kidneys the largest amount of depictable vasculature throughout the entire kidney was assessed in either prone or supine position. Criteria for aPN diagnosis on greyscale US included changes such as mild dilatation with thickened pelvic wall and increased echogenicity of the renal sinus as well as nephromegaly (Fig. 1a, b), and triangular hyper-echogenicities or round hypo-echoic areas (Fig. 2a, b) [20]. On aCDS, the presence of any zone of decreased or absent flow in the parenchyma (compared with other parts of the same kidney at the same depth) was considered indicative for aPN, provided it could be demonstrated in two planes (Fig. 3). No patient was sedated. Special manoeuvres such as breath-holds were performed in older patients capable of cooperation. All US examinations were performed either by pediatric radiologists or specially trained pediatric surgeons. In case of repetitive US studies, the examination closest to the DMSA scan was used for analysis. Repeated studies as well as duplex Doppler velocity measurements and inter-/intra-observer variability have not been evaluated.
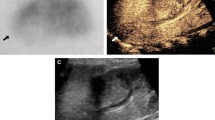
Greyscale US in acute febrile urinary tract infection with renal involvement. Transverse (a) and longitudinal (b) greyscale US view demonstrates the thickened urothelium (+ +) with increased echogenic sinus in an enlarged kidney with reduced cortico-medullary differentiation and slight lax dilatation of pelvocaliceal system with some sludge
Amplitude CDS in UTI. (a) Transverse aCDS view of a kidney in acute UTI depicts some decent peripheral focal perfusion defects. (b) Longitudinal view of the lower pole: aCDS demonstrates a polar perfusion defect in the same patient as Fig. 2., consistent with acute pyelonephritis (+ +)
Original readers of US did not know the result of any other imaging modalities, as US was generally the first examination performed. For image review, the US readers were blinded to all other imaging results and used the captured and stored US images available on PACS. DMSA examinations were read based on anatomical information obtained from US, as demanded by the guidelines.
Clinical presentation data with non-specific symptoms (such as fever; lethargy, irritability, malaise, vomiting, poor feeding, abdominal pain) and more specific symptoms (such as frequency, dysuria, loin tenderness, dysfunctional voiding, changes to continence, hematuria, and offensive or cloudy urine), as well as laboratory findings (such as blood count, C-reactive protein, urinalysis, and urine culture) were available. This data as well as the patient treatment, response to treatment, imaging findings (including US, acute DMSA, and late DMSA follow-up), and the discharge diagnosis were used in synopsis for establishing a “final diagnosis.” This final diagnosis was then implemented into further statistical workup.
Further imaging was performed depending on the diagnosis, the initial findings, and the clinical course. The standard imaging algorithm included voiding cystourethrography, contrast-enhanced CT, and/or MRI [21]; these results were not included or analyzed in this study.
Sensitivity, specificity, and predictive values were calculated using the MS Office 2003 Excel 11.0 statistical package (Microsoft, Redmond, WA).
Results
The database search identified 287 children (all demographic data are shown in Table 1). Two hundred nineteen children showed no sign of upper UTI, neither clinically nor on imaging (US and DMSA). The average time interval between US and DMSA was 2.9 ± 4.75 days.
In 68 patients either US and/or DMSA displayed abnormal findings; 67 of them had also clinical signs of renal involvement, were treated accordingly, and were attributed the final diagnosis of upper UTI.
In a first step we analyzed the abnormal imaging findings using DMSA scintigraphy as the reference standard. DMSA matched greyscale US findings in 54 children. In 53 patients aCDS showed irregular vascularity (Fig. 4), and in the 58 of them the combined results of both US modalities showed abnormal findings (Table 2). Sensitivity increased from 84.1% for using only aCDS, and 85.7% for greyscale US, respectively, to 92.1% in the combined approach (applying renal greyscale US supplemented by aCDS) for the number of patients, but also increased for the number of individual renal units (Table 3). Specificity was 97.8% and 98.8%, respectively, with a positive predictive value of 88% and 92%.
Acute febrile urinary tract infection with acute pyelonephritis. Defect in the upper pole of the right kidney on DMSA scintigraphy (a) and corresponding perfusion defect on aCDS (b) Note that the time interval between the examinations was 2 days, which may explain the relative different in lesion size
A detailed analysis of these data showed five patients where only the DMSA showed renal involvement. From these five patients with six affected renal units who had a positive DMSA and a negative US, four kidneys (66.6%) were on the left and two (33.3%) on the right side. This analysis also showed five children where only the combined US (i.e., including aCDS) was abnormal, demonstrating signs that may be attributed to upper UTI. When analyzing these last five patients with negative DMSA and abnormal US findings, all of them also had clinical signs of renal involvement, had been treated and followed accordingly, and have been attributed the clinical discharge diagnosis “upper UTI.”
In a second step, we also analyzed the imaging data (DMSA scintigraphy and US) with respect to the final diagnosis of the patients. Greyscale US alone matched the final diagnosis in 57 patients, aCDS in 58 patients, and the combined approach in 63 patients. DMSA scintigraphy matched the final diagnosis in 62 patients. Using the “final diagnosis” as the reference (as established by clinical, laboratory, and imaging findings), sensitivity increased from 85% using only greyscale US and 86% using only aCDS information, respectively, to 94% for the combined US approach, with a specificity for comprehensive US of 100%. Sensitivity for DMSA scintigraphy compared to final diagnosis was 92.5% and specificity 99.5% (Table 4).
Discussion
UTI is the second most common bacterial infection in children and occurs in as many as 5% of girls and approximately 0.5% of boys, with a maximum prevalence in male infants and school age girls [22]. VUR and renal scarring are major concerns, especially during the first years of life [23]. Differentiation of upper from lower UTI in childhood based on clinical and laboratory findings may be difficult, especially for infants in whom physical findings are often ambiguous. Commonly used laboratory markers, such as leukocyte count, C-reactive protein, or urinalysis, can not reliably differentiate upper from lower UTI. An easily accessible and accurate diagnostic tool for reliable diagnosis of renal involvement would be helpful for selecting those patients with UTI who require intensified treatment, more intensive imaging workup, and long-term follow-up [21]. A precise differentiation of aPN and cystitis is helpful to avoid overtreatment and might help to decrease the prevalence of renal scarring and its long-term complications [24].
Multiple experimental and clinical studies compared the ability of different imaging modalities to diagnose upper UTI in the pediatric population [1, 2, 4, 15, 16, 25]. Renal involvement and scarring has a multifactorial pathophysiology. One common factor that may be responsible for DMSA, CT, MRI, and aCDS findings appears to be the focal perfusion alteration resulting from intense vasoconstriction of peripheral arterioles and consequently reduced blood flow. Additionally, the (focal) decrease in renal perfusion is aggravated by edema from inflammatory response of the kidney to bacterial invasion, which may result in vascular compression [26, 27].
The use of DMSA for the detection of renal involvement in acute UTI was first described in 1972 [28]. Majd et al. compared DMSA findings with histopathology in a pig model and found a sensitivity of 92.1% and specificity of 93.8% [4]. Since then, this technique has been considered the gold standard for the diagnosis of upper UTI, with relatively good availability in many centers at reasonable cost. However, restrictions to scintigraphy, especially in children, are the use of ionizing radiation, the need of intravenous injection, and the restricted anatomical resolution (anatomic-topographic information is usually provided by US). DMSA cannot reliably exclude upper UTI [29] and may have limitations in small kidneys in very young children [30]. This may explain the findings in five of our patients with renal lesions revealed by comprehensive US, but normal DMSA findings. One possible explanation for this observation is the young age of this patient subpopulation (mean age 2.74 ± 4.55; median 0.78). Especially in small kidneys, the relative DMSA uptake is decreased, and it therefore may sometimes be hard to distinguish a normal kidney even in the absence of focal abnormalities on DMSA scintigraphy [29]. Beside the restrictions of DMSA in small kidneys, this phenomenon may also be due to the better US conditions in these very young patients (e.g., higher resolution transducers applicable, less disturbing fat and gas, etc.).
Multi-detector CT (MDCT) and MRI have also been shown to be sensitive methods for the depiction of upper UTI in experimental and in clinical studies [4, 21, 22, 31, 32]. Their sensitivity and specificity were reported to be 86.8% and 87.5% for CT and 89.5% and 87.5% for MRI, compared to histopathology [4]. However, both of these methods have several disadvantages that hinder routine clinical use. The greatest disadvantage of MDCT is the high radiation burden (the absorbed radiation dose at CT is significantly higher than of DMSA), particularly in pediatric imaging. MRI, enhanced by modern approaches such as BOLD or diffusion imaging, holds great potential, allowing both anatomic and functional assessment, and can realistically be accomplished in a time frame suitable for a clinical imaging examination [33]. But access to this modality is rather restricted, particularly for pediatric radiology in many places, it is relatively expensive, and it may require sedation in infants and young children. Thus-although in the future MRI may play an increasing role-MRI cannot be promoted presently as a general approach for routine imaging in UTI.
Ultrasound is non-invasive, feasible at bedside, does not deliver ionizing radiation, and is relatively inexpensive. New greyscale US methods, such as high-resolution US and harmonic imaging, have enhanced US potential. US is the established imaging tool for assessment of urinary tract anatomy and usually the first imaging modality performed in children with (suspected) UTI. Various signs are known that may indicate upper UTI; using modern techniques and new ‘extended criteria’ for US interpretation, our data suggest that greyscale US has become better than reported in the past. US remains operator dependent, also potentially influencing our results, as pediatric radiologists as well as pediatric surgeons with different levels of experience performed and read the US examinations. Furthermore, availability of high-quality pediatric US is restricted in some areas. And US depends on the availability or use of various and potentially different scanning techniques (prone and/or supine position, color and/or power Doppler, sedation, high-frequency US, or harmonic imaging).
Conventional color Doppler sonography is based on the mean frequency shift. ACDS sums up the venous and arterial Doppler activities, but this does not affect its accuracy in showing flow and perfusion. In the last decade some encouraging reports have been published regarding the use of aCDS in renal disease in children and infants [2, 34–38]. ACDS reportedly has a significant advantage in identifying hypovascular areas in aPN due to vascular compression by inflammatory edema. ACDS with its ability to demonstrate perfusional disturbances can be applied easily at the same investigation improving US diagnostic potential in children with UTI. Restrictions of aCDS are caused by the lack of a reference or a general baseline standard for gain setting; also quantification is impossible at present.
When analyzing our false-negative US results, two-thirds affected the left kidney; this may be attributed to an often inferior visualization by the absence of the hepatic acoustic window and interference from intestinal gas on the left side when using the flank approach.
When analyzing our five patients with false-positive US reports, the most important factor was false positive aCDS. This may be explained either by flash artifacts or by the partial venous obstruction caused by edema in the early phase of aPN that leads to increased volume and sluggish blood flow in the involved kidney, with still perfused and functioning parenchyma [4], as aCDS cannot differentiate venous from arterial flow, and low flow statuses may be difficult to properly assess in uncooperative toddlers with a high respiratory rate.
Five patients with signs of renal involvement on comprehensive US, but normal DMSA, who presented clinically as having upper UTI, had matching laboratory findings; they were treated accordingly and were discharged with the clinical diagnosis “upper UTI.” Because of this observation and in the absence of a histopathological reference standard, we evaluated-as a second step of our study–the sensitivity and specificity of both imaging techniques (US and DMSA) with regard to the final diagnosis as the reference standard. This calculation showed similar results for both modalities, with slightly better sensitivity and specificity for comprehensive US than for DMSA scintigraphy.
Our results show that the combination of both US modes (i.e., aCDS and greyscale US) increases US sensitivity and accuracy (Table 2). We observed in our study population that, using comprehensive US, a similar sensitivity and specificity can be achieved as offered by other imaging modalities, particularly if correlated with clinical data and final diagnosis. Based on our results and in the light of the aspects discussed above, we propose that US should gain a more important role in the imaging algorithm in children with acute UTI, not only to get some first anatomic information, but also to depict signs of renal involvement. Future technical US developments and the introduction of echo-enhancing materials into clinical use enabling dynamic perfusion studies will probably further expand the role of US in this condition. We acknowledge that-in a child with known normal urinary tract anatomy and a clinically evident diagnosis-early imaging may be unnecessary. But initial US is helpful in all other cases for demonstration of urinary tract anatomy, detection of renal involvement, or early recognition of a complicated course such as abscess formation or pyonephrosis [39]. Children with equivocal or unreliable US examinations (e.g., due to complicated conditions or insufficient cooperation) or mismatch between clinical, laboratory, and US results will need additional imaging by DMSA or-particularly in patients with suspected complications–MDCT/MR.
In conclusion our data demonstrate an increased US sensitivity when using information from both greyscale US and aCDS for differentiation of upper from lower UTI in infants and children. When comparing DMSA scintigraphy and the combined US method to the final diagnosis, we found similar results for comprehensive US and for DMSA. This has been integrated in the recently proposed imaging algorithm for pediatric UTI of the European Society of Urogenital Radiology (ESUR) that also has been adopted by the European Society for Pediatric Radiology (ESPR) [40]. Thus, the combination of the two US modalities may obviate scintigraphy in the majority of patients, especially when accounting for future US development in terms of imaging technique and equipment as well as the potential use of new US contrast agents. DMSA as well as CT and MR will still play a role in evaluating children with inconclusive, discordant, or unreliable US results, as well as in patients with complications and for differential diagnoses (e.g., infected and/or complicated cyst, xanthogranulomatous, pyelonephritis or renal tuberculosis). In summary, we postulate that comprehensive US should gain a more important role in the imaging algorithm of infants and children with acute UTI, helping to reduce cost and radiation burden in the pediatric population.
References
Hoberman A, Charron M, Hickey RW et al (2003) Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 348:195–202
Halevy R, Smolkin V, Bykov S et al (2004) Power Doppler ultrasonography in the diagnosis of acute childhood pyelonephritis. Pediatr Nephrol 19:987–991
Jacobson SH, Eklof O, Lins LE, Wikstad I, Winberg J (1992) Long-term prognosis of post-infectious renal scarring in relation to radiological findings in childhood–a 27-year follow-up. Pediatr Nephrol 6:19–24
Majd M, Nussbaum Blask AR, Markle BM et al (2001) Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology 218:101–108
Majd M, Rushton HG (1992) Renal cortical scintigraphy in the diagnosis of acute pyelonephritis. Semin Nucl Med 22:98–111
Majd M, Rushton HG, Chandra R et al (1996) Technetium-99m-DMSA renal cortical scintigraphy to detect experimental acute pyelonephritis in piglets: comparison of planar (pinhole) and SPECT imaging. J Nucl Med 37:1731–1734
Verboven M, Ingels M, Delree M, Piepsz A (1990) 99mTc-DMSA scintigraphy in acute urinary tract infection in children. Pediatr Radiol 20:540–542
Moorthy I, Wheat D, Gordon I (2004) Ultrasonography in the evaluation of renal scarring using DMSA scan as the gold standard. Pediatr Nephrol 19:153–156
Rushton HG, Majd M (1992) Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies. J Urol 148:1726–1732
Rushton HG, Majd M, Jantausch B, Wiedermann BL, Belman AB (1992) Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with 99mtechnetium-dimercaptosuccinic acid scintigraphy. J Urol 147:1327–1332
Sattari A, Kampouridis S, Damry N et al (2000) CT and 99mTc-DMSA scintigraphy in adult acute pyelonephritis: a comparative study. J Comput Assist Tomogr 24:600–604
Talner LB, Davidson AJ, Lebowitz RL, Dalla Palma L, Goldman SM (1994) Acute pyelonephritis: can we agree on terminology. Radiology 192:297–305
Papanicolaou N, Pfister RC (1996) Acute renal infections. Radiol Clin North Am 34:965–995
Weskott HP (1997) Amplitude Doppler US: slow blood flow detection tested with a flow phantom. Radiology 202:125–130
Bude RO, Rubin JM, Adler RS (1994) Power versus conventional color Doppler sonography: comparison in the depiction of normal intrarenal vasculature. Radiology 192:777–780
Dacher JN, Pfister C, Monroc M, Eurin D, LeDosseur P (1996) Power Doppler sonographic pattern of acute pyelonephritis in children: comparison with CT. AJR Am J Roentgenol 166:1451–1455
Sakarya ME, Arslan H, Erkoc R, Bozkurt M, Atilla MK (1998) The role of power Doppler ultrasonography in the diagnosis of acute pyelonephritis. Br J Urol 81:360–363
Piepsz A, Blaufox MD, Gordon I et al (1999) Consensus on renal cortical scintigraphy in children with urinary tract infection. Scientific Committee of Radionuclides in Nephrourology. Semin Nucl Med 29:160–174
Riccabona M (2006) Imaging of the neonatal genito-urinary tract. Eur J Radiol 60:187–198
Dacher JN, Avni F, Francois A et al (1999) Renal sinus hyperechogenicity in acute pyelonephritis: description and pathological correlation. Pediatr Radiol 29:179–182
Riccabona M, Fotter R (2005) Modern imaging technology for childhood urinary tract infection. Radiologe 45:1078–1084
Dacher JN, Hitzel A, Avni FE, Vera P (2005) Imaging strategies in pediatric urinary tract infection. Eur Radiol 15:1283–1288
Vachvanichsanong P (2007) Urinary tract infection: one lingering effect of childhood kidney diseases–review of the literature. J Nephrol. 20:21–28
Glauser MP, Lyons JM, Braude AI (1978) Prevention of chronic experimental pyelonephritis by suppression of acute suppuration. J Clin Invest 61:403–407
Stogianni A, Nikolopoulos P, Oikonomou I et al (2007) Childhood acute pyelonephritis: comparison of power Doppler sonography and Tc-DMSA scintigraphy. Pediatr Radiol 37:685–690
Nosher JL, Tamminen JL, Amorosa JK, Kallich M (1988) Acute focal bacterial nephritis. Am J Kidney Dis 11:36–42
Roberts JA (1991) Etiology and pathophysiology of pyelonephritis. Am J Kidney Dis 17:1–9
Davies ER, Roberts M, Roylance J, Penry JB, Stadden G (1972) The renal scintigram in pyelonephritis. Clin Radiol 23:370–376
Hitzel A, Liard A, Vera P et al (2002) Color and power Doppler sonography versus DMSA scintigraphy in acute pyelonephritis and in prediction of renal scarring. J Nucl Med 43:27–32
Estorch M, Torres G, Camacho V, Tembl A, Prat L, Mena E, Flotats A, Carrió I (2004) Individual renal function based on 99mTc dimercaptosuccinic acid uptake corrected for renal size. Nucl Med Commun 25:167–170
Lonergan GJ, Pennington DJ, Morrison JC et al (1998) Childhood pyelonephritis: comparison of gadolinium-enhanced MR imaging and renal cortical scintigraphy for diagnosis. Radiology 207:377–384
Kavanagh EC, Ryan S, Awan A, McCourbrey S, O’Connor R, Donoghue V (2005) Can MRI replace DMSA in the detection of renal parenchymal defects in children with urinary tract infections. Pediatr Radiol 35:275–281
Riccabona M (2007) (Paediatric) magnetic resonance urography: just fancy images or a new important diagnostic tool. Curr Opin Urol 17:48–55
Riccabona M (2006) Modern pediatric ultrasound: potential applications and clinical significance. A review. Clin Imaging 30:77–86
Riccabona M, Ring E, Schwinger W, Aigner R (2001) Amplitude coded-colour Doppler sonography in paediatric renal disease. Eur Radiol 11:861–866
Yucel C, Ozdemir H, Akpek S et al (2001) Renal infarct: contrast-enhanced power Doppler sonographic findings. J Clin Ultrasound 29:237–242
Riccabona M, Preidler K, Szolar D et al (1997) Evaluation of renal vascularization using amplitude-coded Doppler ultrasound. Ultraschall Med 18:244–248
Winters WD (1996) Power Doppler sonographic evaluation of acute pyelonephritis in children. J Ultrasound Med 15:91–96
Dacher JN, Hitzel A, Avni FE, Vera P (2005) Imaging strategies in pediatric urinary tract infection. Eur Radiol 15:1283–1288
Riccabona M, Avni FE, Blickman JG, Dacher JN, Darge K, Lobo ML, Willi U, (= ESUR Paediatric guideline subcommittee and ESPR paediatric uroradiology work group) (2008) Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup-session on urinary tract infection, foetal hydronephrosis, urinary tract ultrasound and voiding cysto-urethrography. ESPR-Meeting, Barcelona/Spain, June 2007. Pediatr Radiol 38:138–145
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brader, P., Riccabona, M., Schwarz, T. et al. Value of comprehensive renal ultrasound in children with acute urinary tract infection for assessment of renal involvement: comparison with DMSA scintigraphy and final diagnosis. Eur Radiol 18, 2981–2989 (2008). https://doi.org/10.1007/s00330-008-1081-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1081-z