Abstract
This article is focused on the controversial topic of imaging strategies in pediatric urinary tract infection. A review of the recent literature illustrates the complementary roles of ultrasound, diagnostic radiology and nuclear medicine. The authors stress the key role of ultrasound which has recently been debated. The commonly associated vesicoureteric reflux has to be classified as congenital or secondary due to voiding dysfunction. A series of frequently asked questions are addressed in a second section. The proposed answers are not the product of a consensus but should rather be considered as proposals to enrich the ongoing debate concerning the evaluation of urinary tract infection in children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary tract infection (UTI) is a common bacterial disease in children, observed at least once in 5% of girls and 0.5% of boys. Maximum prevalence is observed in male neonates and school age girls [1–3]. Diagnostic imaging plays a quadruple role in this situation: (1) pre- and postnatal ultrasound can identify fetuses and infants with a malformation of the urinary tract. Prevention of UTI can be started in those infants immediately after birth. However, the major preventive role of imaging is not within the scope of this review; (2) different techniques have been shown useful to diagnose acute pyelonephritis (APN) as well as its early complications (pyohydronephrosis, renal or para renal abscess); (3) nuclear medicine studies are considered the reference tool for the diagnosis and follow-up of late complications, e.g. scarring, which is thought to favor arterial hypertension, eclampsia, and renal insufficiency; (4) lastly, imaging plays a key role in the follow-up of children who had a previous UTI in order to detect underlying disease [vesicoureteric reflux (VUR), voiding dysfunction, obstruction].
Imaging modalities which have been described in the context of pediatric UTI are: ultrasound (US) (including grey scale, color and power Doppler), 99mTc-dimercaptosuccinic acid (DMSA) scintigraphy scan and reflux studies [including voiding cystourethrography (VCUG), voiding urosonography, direct and indirect isotopic cystography]. Intravenous urography (IVU) was previously considered a reference examination of the urinary tract, whereas enhanced CT and MRI have been proposed by some authors.
The aims of this article were (1) to describe the advantages and limitations of those different modalities and (2) to suggest diagnostic strategies in common clinical situations. The latter are not the product of a consensus. In fact, they should be considered as proposals to enrich the ongoing debate concerning this common disease. The ALARA principle, cost effectiveness and availability of equipment were taken into consideration.
Imaging modalities
Ultrasound
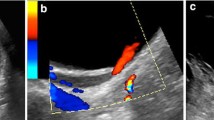
US scanning is absolutely non-invasive, feasible at bedside, does not deliver ionizing radiation, and remains relatively inexpensive. However, in a recent study, the role of US in the evaluation of UTI in children was critically evaluated [4]. Hoberman et al. [4] reported a 12% rate of sonographic abnormalities in a population of children with UTI. Moreover, these authors stated that prenatal US could detect most children with urinary tract malformation. They concluded that in a child referred for UTI, renal US would not be relevant if his/her prenatal US examinations were normal. This approach can be criticized for several reasons. First of all, prenatal US was shown to be unable to detect all children with congenital vesicoureteric reflux, the most frequent malformation associated with UTI [5–7]. Secondly, hydronephrosis as a consequence of uretero–pelvic junction obstruction can be diagnosed at any age of life in patients who had had normal prenatal sonograms, and can become complicated with severe infection. Thirdly, communication between pre- and postnatal medical teams can fail for several different reasons [8, 9]. In addition, during the acute phase of infection, US was shown to be effective in the diagnosis of infectious emergencies such as renal abscess, pyohydronephrosis with or without kidney stone. Ultrasound (or CT) can also be employed as a treatment to guide percutaneous drainage. Lastly, in neonates, urosepsis can be life threatening and the role of ultrasound is even greater as bedside examination at the intensive care unit is possible, as well as high frequency scanning allowing excellent resolution and optimal diagnosis. The main drawback of US in the context of pediatric UTI is its reported sensibility. US sensibility varied among the different published studies regarding acute pyelonephritis [4, 10–14]. This may be explained due to different operators (expert pediatric radiologists involved in this field in some studies versus residents or sonographers in others), different techniques (use of prone and supine position scanning, of color/power Doppler, of sedation, of injection of contrast medium, of high frequency scanning) as well as variable equipment. Even under optimal technical conditions, the diagnostic accuracy of color Doppler US for APN ranged from 80 to 90% [13, 14] and remained below that of DMSA scintigraphy, enhanced CT or MRI. A normal US examination cannot definitely eliminate renal involvement in a child with clinical signs of upper UTI.
Under optimal conditions, subtle abnormalities should be investigated in the context of an APN. US signs can be divided into two categories: (i) signs of pyelitis include mild dilatation, thickened pelvic wall (non-specific), and increased echogenicity of the renal sinus [15]; (ii) signs of nephritis include nephromegaly, triangular hyper-echogenicity or a rounded hypo-echoic area. Decreased perfusion on color/power Doppler (spontaneously or after IV infusion of contrast medium) is common [10, 11]. In addition, in school age girls with UTI and voiding dysfunction, bladder US completes the flowmetry examination (or urodynamics) to search for residual urine [16].
In summary, in spite of a recent study [4], we estimate that US should remain the first line examination in children with UTI. Moreover, follow-up examinations could be conditioned by the US results, as shown in the following sections.
Reflux studies
VCUG remains a reference examination, since it permits rapid and effective detection of VUR when performed with cyclic filling [17, 18]. VCUG is the only available technique to analyze the male urethra. Furthermore, VCUG can detect problems in micturition and help diagnose bladder instability or dysfunctional voiding [16]. However, VCUG delivers radiation and requires either an indwelling catheter or a suprapubic puncture. For these reasons, VCUG should be considered on an individual basis and limited in its use and indications. Retrograde or suprapubic approaches do not differ in terms of sensitivity. Retrograde catheterization is usually considered as more invasive, but this technique is also easier to perform, and does not require preliminary bladder ultrasound.
Isotopic methods to detect VUR include direct and indirect cystography. Direct cystography is obtained by retrograde filling of the bladder. Indirect cystography is obtained via intravenous injection. Due to their poor anatomic resolution, these techniques should be reserved for the follow-up of children with VUR. The recently reported voiding urosonography [19] could compete with isotopic methods in VUR follow-up. However, it should be mentioned that the latter remains an expensive and time-consuming technique. In addition, US contrast medium is still not available (or authorized for that study) throughout Europe.
After VUR is detected, it is necessary to classify the condition as congenital or secondary [20]. Congenital reflux, a consequence of ureteral meatus malformation, mainly occurs in boys. Prenatal diagnosis is common. The grade of that frequently bilateral reflux is usually high at the time of diagnosis and tends to decrease with growth. The rate of children who can be managed conservatively is inversely proportional to the grade of VUR at the age of diagnosis. After 3 years of age, congenital reflux in a previously healthy child becomes a questionable diagnosis.
Secondary reflux is a frequent consequence of bladder instability and dysfunctional voiding. Neurogenic bladder should be considered and eliminated. VUR is usually the consequence of anatomical distortion of the ureterovesical junction due to continued high bladder pressure [21]. Secondary reflux mainly occurs in preschool and school age girls. Grade is usually low and resolution occurs frequently with the treatment of bladder sphincter disorder. Surgery is certainly not the first line treatment in these children, but it still may be required in some children [22].
DMSA scintigraphy
Currently, DMSA scintigraphy is considered the reference examination in the diagnosis of post-infectious scarring [23]. Intravenous urography in this instance is no longer required. Several authors have suggested that DMSA could also be useful in the diagnosis of APN. The renal uptake of DMSA is conditioned by intra-renal blood flow and proximal tubular cell membrane transport function. In depth description of the technique has been provided by international experts [23–25]. Images are performed 1.5–6 h following tracer injection. The quantification of relative function (left to right DMSA uptake ratio) can be performed on the posterior view, but is preferably calculated by geometric means using both anterior and posterior views [26]. The absolute uptake of DMSA is generally not used, as its reproducibility has not been proven. In normal kidneys, the cortical uptake of DMSA is homogeneous and renal contours are smooth. In APN, defects in the renal outline of varied degrees are observed. Defects are not associated with any loss of volume. DMSA needs to be performed within a brief delay after the occurrence of clinical signs in the context of APN. Conversely, in cortical scarring, the defect is associated with a focal loss of volume (cortical thinning, flattening of the renal contour, or wedge-shaped defect) [23–25, 27, 28]. For detection and follow-up of scarring, the accepted recommendation is to perform DMSA scintigraphy at least 6 months after the last episode of APN. DMSA scintigraphy is limited in diagnosing post-infectious scar which cannot be differentiated from a pre-existing developmental one [29]. Those “scar-like” foci of dysplasia have been described in some neonates without any history of previous infection. Such developmental lesions can be severe and associated with poor renal growth. Vis-à-vis post-infectious scarring, we previously demonstrated a 100% negative predictive value for DMSA performed during the acute phase of infection [14]. In fact, none of the children with a normal DMSA performed during APN was shown to have scarring 6 months later (follow-up DMSA). Recently, we showed that the automatic calculation of an index measured on acute DMSA scintigraphy, could help to detect infected kidneys at risk of subsequent scarring [30]. In children with acute pyelonephritis, we performed a mapping of DMSA studies based on the activity value of kidney pixels. Isocounts were automatically calculated for each infected kidney: n% isocount was the region of interest containing all the pixels with a value ≥n% of the maximal intensity value pixel. The Cn% ratio was the n% isocount density divided by the 20% isocount density. The C70% was shown to be the best index for prediction of scarring. A cut-off value of 0.45 was able to predict scarring with a sensitivity of 0.83, a specificity of 0.78, a positive predictive value of 0.85, and a negative predictive value of 0.77.
MRI and CT
Enhanced CT scanner [10] and MRI [31] were shown to be effective means to diagnose APN and scarring. Lonergan et al. [31] reported in 1998 that gadolinium enhanced inversion recovery sequences enabled detection of more pyelonephritic lesions that did renal cortical scintigraphy, and had superior interobserver agreement. Up to now, for reasons of limited availability, MRI has not found the place that it deserves. Due to the radiation dose, enhanced CT could be reserved for patients with severe or complicated disease.
Diagnostic strategies
There are many different subsets of children with UTI and all do not require the same imaging management. Factors to be considered whether or not to perform imaging are: fetal US, age, gender, previous medical history, physical signs, voiding dysfunction, renal function, and course of the disease under treatment.
Some basic rules deserve to be underlined. (1) Neonates and infants with fever and a positive urinary culture should be evaluated during the acute infection stage to rule out the possibility of severe disease (abscess, pyohydronephrosis or APN). The high rate of complications and the well-known risks of urosepsis in that age range justify imaging. US is particularly effective in these thin patients. (2) The high prevalence of congenital VUR in children under 3 years of age with an initial proven APN justifies carrying out a VCUG. Congenital reflux predominates in male infants, and in cases of positive family history. (3) US scanning could be sufficient in older children or adolescents with typical uncomplicated acute pyelonephritis. Other imaging modalities (DMSA, CT) should be reserved for children with doubtful diagnosis, an uncommon course of treatment, or infected with rare bacteria. (4) At least, if one considers that UTI could present a risk of late complication (e.g. hypertension, eclampsia, renal failure), a delayed DMSA scan should be performed in children with a previous medical history of proven acute pyelonephritis.
In order to illustrate our argument, we have chosen to address ten frequently asked questions regarding this topic.
Should we perform DMSA in children with clinically suspected upper UTI and normal sonography?
A clear-cut diagnosis of the presence or absence of renal involvement has to be performed. Maximal effort should be made to establish the diagnosis of APN based on US. In cases of positive US, DMSA would be less useful. However, it was shown that a normal US examination could not completely eliminate APN. Therefore, DMSA (or MR) could be advocated during the acute stage of upper UTI in a child with equivocal diagnosis and normal US.
Should we perform VCUG in children with clinical criteria of UTI and normal acute stage examinations?
The association of US and DMSA can definitely eliminate renal infectious involvement. As the negative predictive value of acute stage DMSA was shown to be excellent, VCUG could no longer be performed in this context (even in a child under 3 years of age). The exception would be a case of recurrent UTI.
How long after APN should VCUG be performed in children under 3 years of age?
VCUG can be performed as soon as the follow-up urinary culture has become negative. There was no demonstrated advantage of a delayed VCUG.
Is VCUG relevant in cases of significantly dilated urinary tract on ultrasound?
Obviously, question 2 excludes children with a dilated urinary tract malformation due either to high grade VUR or obstruction. US and VCUG remain the first line examinations in children with a dilated urinary tract.
Should we perform VCUG in all children with positive US or DMSA scan?
We do not believe so. VCUG could be considered based on age and gender. In a child under 3 years of age with an initial episode of febrile UTI, we would recommend VCUG rather than any other reflux test to detect congenital reflux. Conversely, in the particular case of preschool or school age girls with APN, it is extremely important to diagnose voiding dysfunction first (oriented interview, flowmetry) and to detect scarring by DMSA [16, 32, 33]. The frequently associated reflux is the consequence of a long-standing high-pressure bladder. In most cases, VCUG which is not well tolerated in this subset of children, is of limited interest. The challenge in those children with voiding dysfunction is to preserve the kidneys (preventing recurrent infections) and the bladder sphincter function (e.g. education, diet, biofeedback physiotherapy). We should spare those children as well as possible procedures requiring an indwelling catheter. Hence VCUG should be discussed on an individual basis in girls with voiding dysfunction.
Should we perform VCUG in adolescent girls with an initial APN?
The clinical diagnosis of APN is usually straightforward in this age range. VCUG is not routinely performed in adults, as congenital reflux is rare and functional bladder disorders have spontaneously resolved. From a pathophysiological perspective, nothing differentiates a 15-year-old adolescent girl from a young woman. Hence, we postulate that imaging strategy should be the same. US is useful to eliminate dilation of the urinary tract. When US is normal, VCUG should not be performed, except in cases of recurrent infections.
When should enhanced CT be performed?
Radiation dose and injection of a potentially nephrotoxic agent do not justify routine CT in children with APN. CT should be used for children with unusual course of disease on treatment or a very severe disease. CT can be employed to evaluate a child with intra- or pararenal abscess. Unenhanced CT can also be performed in children with UTI complicating kidney stone.
Which technique should be used for follow-up of children with VUR?
In this context, the high anatomic resolution of VCUG is not required. Isotopic techniques or voiding urosonography should be employed. The less invasive examination is indirect isotopic cystography, the non-radiating one is voiding urosonography.
Which technique should be used to detect scarring?
DMSA scintigraphy is the reference examination in this field. However, we think that it should be coupled with US, which can clarify some difficult situations. A common occurrence is a scarred kidney with an intra-renal Bertin’s column compensatory hypertrophy. MRI could compete with DMSA scan, since it can provide detailed anatomic depiction and functional assessment [34]. It should be stressed that the role of APN in the development of hypertension, eclampsia or chronic renal failure has not been supported by recent studies [35].
What about the usefulness of MR?
Since the study reported by Lonergan et al. [31], MR has had limited use in children with APN or in the evaluation of scarred kidneys. The explanation is perhaps due to MR limited availability and high cost contrasting with the high prevalence of the disease. In our opinion, the role of MRI would deserve reconsideration since it could probably address practically all questions.
There is no consensus in 2004 regarding the optimal strategy to evaluate and follow up pediatric patients with UTI. However, the association of US and DMSA should be able to detect all children with APN who could deserve reflux studies and/or DMSA follow-up. In addition, continuous effort has to be made to distinguish between children with different kinds of vesicoureteric reflux. It is probable that the role of congenital VUR has been over emphasized in the past, as compared with that of secondary reflux due to dysfunctional voiding. In fact, the management is different in those two categories of children. Lastly, MR should certainly play a more important role in a close future, and could probably compete with DMSA.
References
Jodal U (1994) Urinary tract infections (UTI): significance, pathogenesis, clinical features and diagnosis. In: Postelthwaite RJ (ed) Clinical pediatric nephrology, 2nd edn. Butterworth-Heineman, Oxford, pp 151–159
Jodal U, Lindberg U (1999) Guidelines for management of children with UTI and VUR. Recommendations from a Swedish state of the art conference. Acta Paediatr 431:87–89
Hellerstein S (1995) Urinary tract infection: old and new concepts. Pediatr Clin N Am 42:1433–1457
Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER (2003) Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 348:195–202
Moorthy I, Joshi N, Cook JV, Warren M (2003) Antenatal hydronephrosis: negative predictive value of normal postnatal ultrasound: a 5-year study. Clin Radiol 58:964–970
Phan V, Traubici J, Hershenfield B, Stephens D, Rosenblum ND, Geary DF (2003) Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol 18:1224–1228
Anderson NG, Wright S, Abbott GD, Wells JE, Mogridge N (2003) Fetal renal pelvic dilatation: poor predictor of familial vesico–ureteral reflux. Pediatr Nephrol 18:902–905
Nishisaki A (2003) Imaging studies after a first febrile urinary tract infection in young children (comment). N Engl J Med 348:1812
Dacher JN, Mandell J, Lebowitz RL (1992) Urinary tract infections in infants in spite of prenatal diagnosis of hydronephrosis. Pediatr Radiol 22:401–405
Dacher JN, Pfister C, Monroc M, Eurin D, Le Dosseur P (1996) Power Doppler sonographic pattern of acute pyelonephritis in children. Am J Roentgenol 166:1451–1455
Riccabona M, Fotter R (2004) Urinary tract infection in infants and children: an update with special regard to the changing role of reflux. Eur Radiol 14:L78–L88
Alon US, Ganapathy S (1999) Should renal ultrasonography be done routinely in children with urinary tract infection? Clin Pediatr 38:21–25
Morin D, Veyrac C, Kotzki PO et al. (1999) Comparison of ultrasound and DMSA scintigraphy changes in acute pyelonephritis. Pediatr Nephrol 13:219–222
Hitzel A, Liard A, Vera P, Manrique A, Menard JF, Dacher JN (2002) Color and power Doppler sonography versus DMSA scintigraphy in acute pyelonephritis and in prediction of renal scarring. J Nucl Med 43:27–32
Dacher JN, Avni EF, François A et al. (1999) Renal sinus hyperechogenicity in acute pyelonephritis: description and pathological correlation. Pediatr Radiol 29:179–182
Dacher JN, Savoye-Collet C (2004) Urinary tract infection and functional bladder sphincter disorders in children. Eur Radiol 14:L101–L106
Paltiel HJ, Rupich RC, Kiruluta HG et al. (1992) Enhanced detection of vesico–ureteral reflux in infants and children with use of cyclic voiding cystourethrography. Radiology 184:753–755
Franchi-Abella S, Waguet J, Aboun M, Sariego F, Pariente D (2000) Cyclic filling cystourethrography in the study of febrile urinary tract infection in children. J Radiol 81:1615–1618
Darge K (2002) Diagnosis of vesicoureteric reflux with ultrasonography. Pediatr Nephrol 17:52–60
Allen TD (1992) Commentary: voiding dysfunction and reflux. J Urol 148:1706–1707
Fotter R (2001) Functional disorders of the urinary tract. In: Fotter R (ed) Pediatric uroradiology. Springer, Berlin, Heidelberg, New York, pp 185–200
Badachi Y, Pietrera P, Liard A, Pfister C, Dacher JN (2002) Vesicoureteric reflux and dysfunctional voiding in children. J Radiol 83:1823–1827
Goldraich NP, Goldraich IH (1995) Update on DMSA renal scanning in children with urinary tract infection. Pediatr Nephrol 9:221–226
Piepsz A, Colarinha P, Gordon I et al. (2001) Paediatric Committee of the European Association of Nuclear Medicine. Guidelines for 99 mTc-DMSA scintigraphy in children. Eur J Nucl Med 28(3):37–41
Piepsz A, Blaufox MD, Gordon I et al. (1999) Consensus on renal cortical scintigraphy in children with urinary tract infection. Scientific Committee of Radionuclides in nephrourology. Semin Nucl Med 29:160–174
Fleming JS, Cosgriff PS, Houston AS, Jarritt PH, Skrypniuk JV, Whalley DR (1998) UK audit of relative renal function measurement using DMSA scintigraphy. Nucl Med Commun 19:989–997
Majd M, Rushton HG (1992) Renal cortical scintigraphy in the diagnosis of acute pyelonephritis. Semin Nucl Med 22:98–111
Patel K, Charron M, Hoberman A, Brown ML, Rogers KD (1993) Intra- and interobserver variability in interpretation of DMSA scans using a set of standardized criteria. Pediatr Radiol 23:506–509
Ditchfield MR, Grimwood K, Cook DJ et al. (2004) Persistent renal cortical scintigram defects in children 2 years after urinary tract infection. Pediatr Radiol 34:465–471
Hitzel A, Liard A, Dacher JN et al. (2004) Quantitative analysis of 99 m Tc-DMSA during acute pyelonephritis for prediction of long term renal scarring. J Nucl Med 45:285–289
Lonergan GJ, Pennington DJ, Morrison JC et al. (1998) Childhood pyelonephritis: comparison of gadolinium enhanced MR imaging and renal cortical scintigraphy for diagnosis. Radiology 207:377–384
Naseer SR, Steinhardt GF (1997) New renal scars in children with urinary tract infections, vesico–ureteral reflux and voiding dysfunction: a prospective evaluation. J Urol 158:566–568
Pfister C, Dacher JN, Gaucher S, Liard-Zmuda A, Grise P, Mitrofanoff P (1999) The usefulness of minimal urodynamic evaluation and pelvic floor biofeedback in children with chronic voiding dysfunction. Br J Urol 84:1054–1057
Rohrschneider WK, Haufe S, Clorius JH, Tröger J (2003) MR to assess renal function in children. Eur Radiol 13:1033–1045
Wennerstrom M, Hansson S, Hedner T, Himmelmann A, Jodal U (2000) Ambulatory blood pressure 16–26 years after the first urinary tract infection. J Hypertens 18:485–491
Acknowledgements
The authors thank Richard Medeiros, Rouen University Hospital Medical Editor, for his advice in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dacher, JN., Hitzel, A., Avni, F.E. et al. Imaging strategies in pediatric urinary tract infection. Eur Radiol 15, 1283–1288 (2005). https://doi.org/10.1007/s00330-005-2702-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-005-2702-4