Abstract
Percutaneous thermal ablation is increasingly applied in the therapy of renal tumors. Various techniques are available, allowing a safe and accurate therapy of renal tumors either using hyperthermia such as radiofrequency ablation (RFA), laser-induced thermotherapy (LITT) and microwave ablation (MW) or by hypothermia (cryoablation). As thermal ablation is a minimally invasive and nephron-sparing procedure, it is ideally suited for patients with a single kidney, multiple tumors or contraindications for resective surgery. Although cryotherapy is the most extensively studied technique, RFA has become the most accepted thermal ablation technique over the last years. Modern RFA probes allow ablation volumes between 2 and 5 cm in diameter. A major advantage of RFA is the ability to avoid tract bleeding and tumor seeding by coagulating the puncture channel during RF probe withdrawal. The increasing number of clinical reports on RFA of the kidney show the promising potential of renal RFA for minimally invasive tumor treatment. Due to its technical benefits, RFA seems to be advantageous when compared to cryoablation or laser ablation. However, there are no long-term follow-up or comparative data proving an equal effectiveness to surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During 2002, the American Cancer Society reported 31,800 new cases and 11,500 deaths from renal cancer [1]. While historically renal cell carcinoma (RCC) was detected by flank pain and hematuria, the development of imaging techniques such as ultrasound (US) and computed tomography (CT) have led to an increased detection rate of small renal tumors [2]. Further advances in cross-sectional imaging with the introduction of multislice spiral CT (MSCT) and high-field magnetic resonance imaging (MRI) in clinical routine and the more widespread availability of cross-sectional imaging have resulted in an even earlier tumor detection. About two-thirds of all RCCs are now detected incidentally [3]. However, the differentiation of small renal tumors remains difficult [4]. The natural history of renal tumors is variable, but histological tumor type and tumor stage are important prognostic factors, with a survival advantage attributed to smaller tumors [5, 6]. Moreover, tumors less than 4 cm in diameter rarely metastasize.
The traditional treatment of RCC was open radical nephrectomy [7]. More frequent detection of small tumors pushed the development of less invasive techniques such as nephron-sparing partial nephrectomy via an open or laparoscopic approach. These techniques were successfully introduced in clinical routine with results equal to radical nephrectomy [8]. The 10-year tumor-free survival rates were greater than 85% following nephron-sparing surgery [9, 10]. Initially limited to patients with bilateral disease or solitary kidney, nephron-sparing surgery is now being applied to patients with healthy contralateral kidney also [11]. These convincing results pushed the development of new therapeutic concepts and the introduction of even less invasive, energy-based treatment options. Several of these thermal ablation techniques are now subject to clinical investigation.
Thermal ablation techniques
For patients with contraindications or those who refuse open surgery, thermal ablation techniques are a promising option. Like nephron sparing surgery, energy-based non-surgical ablation techniques are suited for patients with solitary kidney or compromised renal function. If these techniques prove as effective as nephron-sparing surgery, they can be applied to patients with small renal masses and normal contralateral kidney also. There is a variety of thermal ablation techniques available that can be applied under analgo-sedation or general anesthesia. All of these techniques are energy-based either by hyperthermia—radiofrequency ablation (RFA), laser-induced thermotherapy (LITT) and microwave ablation (MW)—or by hypothermia (cryoablation). In all techniques, hyperthermal or hypothermal effects on tumor tissue result in coagulation necrosis, which scars over time. In order to completely destroy the tumor, thermal effects must exceed the tumor margin into healthy renal parenchyma. Adversely, thermal damaging of the calices and renal pelvis must be avoided in any case. Therefore, centrally located tumors are a contraindication for thermal tumor ablation. Unlike open or laparoscopic surgery, thermal therapy allows no direct visual control of the critical structures during the intervention. Consequently, an optimal monitoring system (US, CT or MRI) is mandatory.
Radiofrequency ablation (RFA)
Over the last years, RFA has become the most commonly used thermal ablation technique. It is mainly applied for therapy of hepatic malignancies. Among other tumor locations, renal RFA has become increasingly accepted over the last few years. First applied in 1997 [12], renal RFA is now the most commonly applied percutaneous ablation technique in renal malignancies.
In RFA high-frequency electrical current (375–480 kHz) is used, which is applied through needle electrodes. It causes molecular friction with heat production that leads to a coagulation necrosis with surrounding inflammatory changes. Over time, renal RF lesions show progressive fibrosis with variable reabsorption [13]. The effect of the applied RF energy decreases with increasing distance from the active tip of the probe. As induction of necrosis with RFA requires a temperature >60°C, highly vascular tissue such as the renal parenchyma may limit the size of the necrosis because of a heat sink. In combination with different probe designs, this effect has to be considered when planning the ablation procedure. Various probe designs are available, including differently shaped needles or expandable probes [14]. Some probes are internally cooled to reduce desiccation and charring around the active tip of the probe; others directly infuse saline into the tissue parenchyma to allow a better electrical conduction and thereby larger necrosis volumes. Contrary to cryotherapy, results of animal experiments indicate that temporary occlusion of the renal artery allows for the modulation of the size of the necrosis [15, 16]. Tumor embolization with coils or particles prior to RFA uses the same effect. The size and configuration of the ablated tissue mainly depends on the tissue impedance, ablation time, amount of delivered energy and surface area of the electrodes. Mostly, the effect of the ablation is controlled by monitoring of the temperature or the impedance at the tip of the electrode. To avoid damage to neighboring organs such as the colon, pararenal injection of air, CO2, water or saline might be useful [17].
Among all hyperthermal ablation procedures, RFA became the most accepted, presumably because of its superior relation between probe diameter and size of ablated tissue. Modern RF probes allow for ablation volumes between 2 and 5 cm in diameter using a probe shaft diameter of 7F or less. When compared to other thermal ablation techniques, the ability to avoid tract bleeding and tumor seeding by coagulating the puncture channel during RF probe withdrawal is an important advantage of RFA. Consequently, the risk of bleeding complications is low. Opposite to cryotherapy, however, the heat extension during ablation cannot be monitored directly and therefore requires a thorough planning of the procedure. While placement of the probes is possible under US, CT and MR guidance, the formation of micro-bubbles limits the use of US during and immediately following the intervention [18]. As coagulated and necrotic tissue is not perfused, contrast administration before probe retraction helps to estimate the ablation result during CT-guided or MR-guided RFA. In one study comparison between MR imaging and pathologic examination allowed for differentiation between treated and untreated renal parenchyma within a range of 2 mm [19].
Several approaches have been reported to apply RFA to the kidney. These include open surgical exposure, laparoscopic exposure and an entirely percutaneous approach. Animal experiments showed no difference in the results using different approaches towards the kidney [20].
The increasing number of clinical reports on renal RFA outline the promising potential of renal RFA for minimally invasive tumor treatment. The first case of percutaneous RFA of an exophytic RCC prior to radical open nephrectomy was reported by Zlotta et al. in 1997 [12]. In 1999, McGovern et al. reported on the first case of RFA as sole treatment for renal tumor with 3 months of follow-up [21].
The largest series with 34 patients with 42 RCC undergoing 54 percutaneous RFA treatments was reported by Gervais et al. in 2003 [22]. The tumor size ranged from 1.1 to 8.9 cm with a mean diameter of 3.2 cm. With a mean follow-up period of 13.2 months, this study proved RFA to be effective. Most important, this study demonstrated the influence of tumor location on the ablation results. Therefore, exophytic tumors, even if they are bigger than 3 cm in diameter, can be treated effectively by use of RFA, while parenchymal and central tumors recur more frequently if the diameter exceeds 3 cm. Thus, devascularizaion of tumors exceeding a diameter of 3 cm by embolization seems to be advantageous, as less energy is required and the remaining renal parenchyma will be protected [23, 24]. Furthermore, animal experiments indicate an influence of tumor location on the complication rate, with central tumors prone to major complications, including renal artery injury [25]. Table 1 summarizes the preliminary results and clinical reports on renal RFA.
Complications are rare and limited to hematoma, ureteral obstruction and fistulas, which can be treated conservatively. Gervais et al. reported complications in 4 out of 54 visits, including bladder outlet obstruction because of hematuria in prostatic hyperplasia (n=1), ureteral obstruction for several reasons that required ureteral stent placement (n=2) and one case of perirenal hematoma. In another study, a single 5-mm cutaneous metastasis along the puncture tract was reported, which was resected without any complications [26].
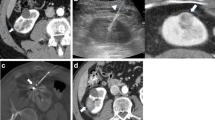
In two studies, concern was raised regarding the efficiency of renal RFA. In both studies, RFA was performed prior to nephrectomy. On histology, Michaelis et al. found viable tumor in all specimens of all 13 tumors included in this study [27]. Rendon et al. found viable residual tumor in four of five tumors undergoing partial or radical nephrectomy immediately after RFA and in three of five tumors undergoing nephrectomy 1 week after RFA [28]. These observations have to be taken seriously, although other studies, including the authors’ own experience, do not support these findings. In contrast, Jacomides et al. performed tumor resection secondary to RFA in 5 of 17 laparoscopically treated RCC and did not find residual tumor on histology [29]. Thus, concern has to be raised regarding the ablation protocols used by Rendon and Michaelis, as this has the most severe impact on the efficiency of the procedure. From our experience, it is crucial to chose an ablation protocol and RF probe suited to create a necrosis large enough to keep a sufficient safety margin (Fig. 1).
a RF ablation in a 71-year-old female patient with renal cell carcinoma. Multiplanar reformat (MPR) from a pre-interventional MSCT depicts a 4.1-cm tumor at the lower pole of the left kidney. b Angiography shows the hypervascular tumor at the lower pole of the left kidney. c The day before RFA embolization with coils and microspheres was performed, and the feeding vessels were occluded. d The RF probe was placed percutaneously under CT guidance via a dorsal approach. e One day after RFA, MPR from a contrast-enhanced MSCT shows a hypodense tumor without contrast enhancement, corresponding to a complete tumor necrosis. A sufficient safety margin without contrast enhancement around the tumor is visible
After the intervention, it is important to follow a stringent imaging surveillance with contrast-enhanced MSCT or MRI to detect local or systemic recurrence, as this can be treated successfully by repeated percutaneous RFA.
Alternative thermal ablation techniques
Cryotherapy is the most extensively studied technique for thermal ablation of renal tumors. Initially used to treat hepatic or prostate tumors, it took until 1995 to be described for percutaneous ablation of renal tissue [30]. The mechanism of tissue destruction is complex and contains immediate and delayed effects [31, 32]. Using either liquid argon or liquid nitrogen, core temperatures of −187 or −195°C are achieved at the tip of the cryoprobe. As the ice ball volume depends on the diameter of the cryoprobe, tumors exceeding 2 cm in size require large (>3 mm) or multiple cryoprobes with an increased risk for bleeding with enlarged diameter and number of the probes. As the cold does not coagulate blood vessels, hemostyptic techniques have to be applied after cryoablation. Thus, percutaneous cryotherapy is limited to tumors less than 2 cm in diameter. Larger lesions require a surgical approach, with the majority of all renal cryoablations being performed via a laparoscopic or an open surgical approach. In detail, different clinical studies on renal cryotherapy are summarized in Table 2.
Using different approaches towards renal cryotherapy, several complications have been reported. Most important are bleeding complications, either as renal laceration [33]—requiring suturing—or small perinephric hemorrhage [34]. Laceration of the liver has also been described [35]. To avoid injury of adjacent organs, protection from the cryoprobe is needed. Directly related to cryotherapy pancreatic injury [36], ureteropelvic junction stricture [37] and complete bowel obstruction [38] have been described. From animal experiments, urinary extravasation was reported. However, until now no urinary leak was seen in patient series, even in case of involvement of the collecting system in the ice ball [33].
LITT has also been shown to be a possible treatment of RCC. However, for lesions exceeding 2 cm in diameter, a cooling catheter is necessary, which increases the diameter of the device up to 9F. On the other hand, LITT is principally MR-compatible and allows MR-thermometry during ablation. Therefore, this technique is well suited for MR-guided interventions. However, the technical effort of LITT is higher than that of RFA and cryotherapy, and thus, there is only a small number of clinical reports, which are limited to patients with unresectable tumors [39, 40].
Microwave ablation is characterized by rapid induction of small necrosis volumes and has been used intra-operatively exclusively. Although technically very interesting, there are no MW probes commercially available that are suited for percutaneous induction of thermal lesions greater than 2 cm in diameter. The main advantage of this technique is its ability to avoid bleeding from the renal parenchymal incision. Nevertheless, there is only limited patient data available from this treatment option [41, 42].
In animal studies, high intensity focused ultrasound has been applied also to create renal coagulation necrosis [43, 44]. Only recently has preliminary experience in a clinical setting been reported [45, 46]. However, this technique is still highly experimental, with several problems concerning visualization of the target lesion as well as control of the lesion size, and until now high intensity focused ultrasound lacks sufficient clinical experience.
Summary
In summary, percutaneous thermal tumor ablation is a rapidly increasing technique, allowing a safe and accurate therapy of renal tumors. As it is a minimally invasive and nephron-sparing procedure, it is ideally suited for patients with a single kidney, multiple tumors (Hippel-Lindau’s disease) or contraindications for radical surgery. Due to its technical benefits, RFA seems to be advantageous compared to cryoablation or laser ablation. However, it lacks long-term follow-up data or comparative studies proving an equal effectiveness of RFA when compared to surgery.
References
Jemal A, Thomas A, Murray T, Thun M (2002) Cancer statistics. CA Cancer J Clin 52:23–27
Pantuck AJ, Zisman A, Belldegrun AS (2001) The changing natural history of renal cell carcinoma. J Urol 166:297–301
Homma Y, Kawabe K, Kitamura K et al (1995) Increased incidental detection and reduced mortality in renal cancer—recent retrospective analysis at eight institutions. Int J Urol 2:77–80
Zagoria RJ, Dyer RB (1998) The small renal mass: detection, characterization, and management. Abdom Imaging 23:256–265
Guinan PD, Vogelzang NJ, Fremgen AM et al (1995) For the members of the cancer incidence and end results committee. Renal cell carcinoma: tumor size, stage and survival. J Urol 153:901–903
Thrasher JB, Paulson DF (1993) Prognostic factors in renal cancer. Urol Clin North Am 20:247–262
Robson CJ, Churchill BM, Anderson W (1969) The results of radical nephrectomy for renal cell carcinoma. J Urol 101:297–301
Uzzo RG, Novick AC (2001) Nephron sparing surgery for renal tumors: indications techniques and outcomes. J Urol 166:6–18
Fergany AF, Hafez KS, Novick AC (2000) Long-term results of nephron-sparing surgery for localized renal cell carcinoma: 10-year follow-up. J Urol 163:442–445
Herr HW (1999) Partial nephrectomy for unilateral renal cell carcinoma and a normal contralateral kidney: 10-year follow-up. J Urol 161:33–35
Moll V, Becht E, Ziegler M (1993) Kidney preserving surgery in renal cell tumors: indications, techniques and results in 152 patients. J Urol 150:319–323
Zlotta AR, Wildschutz T, Wood BJ et al (1997) Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol 11:251–258
Hsu THS, Fidler ME, Gill IS (2000) Radiofrequency ablation of the kidney: acute and chronic histology in porcine model. Urology 56:872–875
Pereira PL, Trübenbach J, Schmidt D (2003) Radiofrequency ablation: basic principles, techniques and challenges (German). Fortschr Röntgenstr 175:20–27
Aschoff AJ, Sulman A, Martinez M et al (2001) Perfusion-modulated MR imaging-guided radiofrequency ablation of the kidney in a porcine model. Am J Roentgenol 177:151–158
Corwin TS, Lindberg G, Traxer O et al (2001) Laparoscopic radiofrequency thermal ablation of renal tissue with and without hilar occlusion. J Urol 166:281–284
Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML (2003) Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. Am J Roentgenol 181:1315–1317
Rendon RA, Gertner MR, Sherar MD et al (2001) Development of a radiofrequency based thermal therapy technique in an in vivo porcine model for the treatment of small renal masses. J Urol 166:292–298
Merkle EM, Shonk JR, Duerk JL et al (1999) MR-guided RF thermal ablation of the kidney in a porcine model. Am J Roentgenol 173:645–651
Crowley JD, Shelton J, Iverson AJ et al (2001) Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology 57:976–980
McGovern FJ, Wood BJ, Goldberg SN et al (1999) Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol 161:599–600
Gervais DA, McGovern FJ, Arellano RS, McDougal SW, Mueller PR (2003) Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology 226:417–424
Hall WH, McGahan JP, Link DP, deVere White RW (2000) Combined embolization and percutaneous radiofrequency ablation of a solid renal tumor. Am J Roentgenol 174:1592–1594
Tacke J, Mahnken A, Bucker A, Rohde D, Gunther RW (2001) Nephron-sparing percutaneous ablation of a 5 cm renal cell carcinoma by superselective embolization and percutaneous RF-ablation. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb Verfahr 173:980–983
Lee JM, Kim SW, Chung GH, Lee SY, Han YM, Kim CS (2003) Open radio-frequency thermal ablation of renal VX2 tumors in a rabbit model using a cooled-tip electrode: feasibility, safety, and effectiveness. Eur Radiol 13:1324–1332
Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ (2003) Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. Am J Roentgenol 180:1503–1508
Michaelis MJ, Silverman M, Libertino JA (2001) Absence of total tumor necrosis in radiofrequency ablated renal tumors (abstract). J Urol 165:21
Rendon RA, Kachura JR, Sweet JM et al (2002) The uncertainity of radiofrequency treatment of renal cell carcinoma: findings at immediate and delayed nephrectomy. J Urol 167:1587–1592
Jacomides L, Ogan K, Watumull L, Cadeddu JA (2003) Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol 169:49–53
Uchida M, Imaide Y, Sugimoto K et al (1995) Percutaneous cryotherapy for renal tumors. Br J Urol 75:132–136
Hoffmann NE, Bischof JC (2002) The cryobiology of crysurgical injury. Urology 60 [2 Suppl 1]:40–49
Stephenson RA, King DK, Rohr LR (1996) Renal cryoablation in a canine model. Urology 47:772–776
Rukstalis DB, Khorshandi M, Garcia FU et al (2001) Clinical experience with open renal cryoablation. Urology 57:34–39
Shingelton WB, Sewell PE Jr (2001) Percutaneous renal tumor cryoablation with magnetic resonance imaging guidance. J Urol 165:773–776
Gill IS, Novick AC, Meraney AM et al (2000) Laparoscopic renal cryoablation in 32 patients. Urology 56:748–753
Lee DI, McGinnis DE, Feld R, Strup SE (2003) Retroperitoneal laparoscopic cryoablation of small renal tumors: intermediate results. Urology 61:83–88
Campbell SC, Krishnamurti V, Chow G et al (1998) Renal cryosurgery: evaluation of treatment parameters. Urology 52:29–33
Desai MM, Gill IS (2002) Current status of cryoablation and radiofrequency ablation in the management of renal tumors. Curr Opin Urol 12:387–393
Dick EA, Joarder R, De Jode MG, Wragg P, Vale JA, Gedroyc WM (2002) Magnetic resonance imaging-guided laser thermal ablation of renal tumours. BJU Int 90:814–822
de Jode MG, Vale JA, Gedroyc WM (1999) MR-guided laser thermoablation of inoperable renal tumors in an open-configuration interventional MR scanner: preliminary clinical experience in three cases. J Magn Reson Imaging 10:545–549
Hirao Y, Fujimoto K, Yoshii M et al (2002) Non-ischemic nephron-sparing surgery for small renal cell carcinoma: complete tumor enucleation using a microwave tissue coagulator. Jpn J Clin Oncol 32:95–102
Murota T, Kawakita M, Oguchi N et al (2002) Retroperitoneoscopic partial nephrectomy using microwave coagulation for small renal tumors. Eur Urol 540–545
Paterson RF, Barret E, Siqueira TM Jr et al (2003) Laparoscopic partial kidney ablation with high intensity focused ultrasound. J Urol 169:347–351
Chapelon JY, Margonari J, Theillere Y et al (1992) Effects of high-energy focused ultrasound on kidney tissue in the rat and the dog. Eur Urol 22:147–152
Wu F, Wang ZB, Chen WZ, Bai J, Zhu H, Qiao TY (2003) Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J Urol 170:2237–2240
Köhrmann KU, Michel MS, Gaa J et al (2002) High intensity focused ultrasound as noninvasive therapy for multilocular renal cell carcinomas: a case study and review of literature. J Urol 167:2397–2403
Walther MM, Shawker TH, Libutti SK et al (2000) A phase 2 study of radiofrequency interstitial tissue ablation of localized renal tumors. J Urol 163:1424–1427
Ogan K, Jacomides L, Dolmatch BL et al (2002) Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology 60:954–958
Matlaga BR, Zagoria RJ, Woodruff RD, Torti FM, Hall MC (2002) Phase II trial of radio frequency ablation of renal cancer: evaluation of the kill zone. J Urol 168:2401–2405
Pavlovich CP, Walther MM, Choyke PL et al (2002) Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol 167:10–15
de Baere T, Kuoc V, Snmayra T et al (2002) Radiofrequency ablation of renal cell carcinoma: preliminary clinical experience. Urology 167:1961–1964
Roy-Choudhury SH, Cast JE, Lee-Elliott CE, Breen DJ (2002) Percutaneous radiofrequency (RFA) ablation of small renal cell carcinoma (RCC)—medium term outcome (abstract). Radiology 225:S387
Su LM, Jarrett TW, Chan DY, Kavoussi LR, Solomon SB (2003) Percutaneous computed tomography-guided radiofrequency ablation of renal masses in high surgical risk patients: preliminary results. Urology 61[Suppl 4A]:26–33
Farrell MA, Charboneau WJ, DiMarco DS et al (2003) Imaging-guided radiofrequency ablation of solid renal tumors. Am J Roentgenol 180:1509–1513
Delworth MG, Pisters LL, Fornage BD, von Eschenbach AC (1996) Cryotherapy for renal cell carcinoma and angiomyolipoma. J Urol 155:252–254
Bishoff JT, Chen RB, Lee BR et al (1999) Laparoscopic renal cryoablation: acute and long-term clinical, radiographic, and pathologic effects in an animal model and application in a clinical trial. J Endourol 13:233–239
Harada J, Dohi M, Mogami T et al (2001) Initial experience of percutaneous renal cryosurgery under the guidance of a horizontal open MRI system. Radiat Med 19:291–296
Korshandi M, Foy RC, Chong W, Hoenig D, Cohen JK, Rukstalis DB (2002) Preliminary experience with cryoablation of renal lesions smaller than 4 centimeters. J Am Osteopath Assoc 102:277–281
Lowery PS, Nakada SY (2003) Renal cryotherapy: 2003 clinical status. Curr Opin Urol 13:193–197
Nadler RB, Kim SC, Rubenstein JN, Yap RL, Campbell SC, User HM (2003) Laparascopic renal cryosurgery: the Northwestern experience. J Urol 170:1121–1125
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahnken, A.H., Günther, R.W. & Tacke, J. Radiofrequency ablation of renal tumors. Eur Radiol 14, 1449–1455 (2004). https://doi.org/10.1007/s00330-004-2360-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2360-y