Abstract
The glacier retreat in the Antarctic Peninsula is opening new ice-free areas and providing an excellent opportunity to study successional processes. Antarctic terrestrial ecosystems have the particular characteristic of being dominated almost exclusively by lichens and mosses. The aim of the present study was to analyze the diversity, cover and composition of a lichen community on a deglaciated gradient on Potter Peninsula, King George Island (maritime Antarctica), and to investigate how microsite variables influence these patterns. Total lichen cover, species richness, and the frequency and cover of lichens species were measured in five 50 × 50 cm grids in 24 sites covering the whole Peninsula from the coast to the glacier front. Microsite conditions were also registered: slope, aspect, and proportion of different substrates (rocks, soil or bryophytes). We recorded a highly diverse and complex lichen community arranged in three assemblages of species. The lichen communities showed clear variations along the studied gradient, related to the distance to the glacier, the slope, the type of substrate, and the interaction between them. We consider that the patterns of these Antarctic lichen communities are dynamic and very heterogeneous, since they depend on macroclimatic variables but there is also a strong influence of microsite factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are several reports on the retreat of glaciers in maritime Antarctica, i.e. the Antarctic Peninsula and adjacent archipielago (King and Harangozo 1998; Cook and Vaughan 2010; Rückamp et al. 2011; Turner et al. 2013), in spite of recent evidence that suggest a temperature decline since 1998 (Turner et al. 2016). In particular, Rückamp et al. (2011) recorded the retreat of the Fourcade Glacier in Potter Cove and Potter Peninsula since 1956, near the Carlini (ex. Jubany) Argentinean base, detecting the reduction of the ice mass.
Antarctic terrestrial ecosystems have the the particular characteristic of being dominated almost exclusively by lichens and mosses. Many studies have focused on the lichen diversity of the Antarctic continent, especially on maritime Antarctica where the milder climate conditions harbor a higher number of species (Inoue 1995; Seppelt 1995; Øvstedal and Smith 2001; Smykla et al. 2007; Johansson and Thor 2008; Favero-Longo et al. 2011; Rai et al. 2011). In particular, King George Island (South Shetland Archipelago) has been intensively studied (Redón 1985; Olech 2004; Piñeiro et al. 2012; Spielmann and Pereira 2012). However, relatively few studies have focused on the ecology of lichen communities and how they have changed after the retreat of glaciers (Sancho and Valladares 1993; Smith 1995; Valladares and Sancho 1995; Poelking et al. 2015; Sancho et al. 2017).
Bacteria, algae and lichens initiate the succession of terrestrial communities beyond the glacier boundaries with a subsequent progressive increase in species diversity and colony size (Longton 1988; Fernández-Martínez et al. 2017). The types of vegetation that colonize the ice-free areas depend on the substrata and other factors. Antarctic vegetation distribution is primarily determined by environmental factors such as temperature, moisture availability, snow melt, and micro-topography (Robinson et al. 2003; Schroeter et al. 2017). Lichen communities, for example, tend to be particularly rich on north-facing rock sites, where the temperature is consistently warmer (Kappen 1985).
In spite of the many studies on the lichen flora, the patterns and processes of lichen succession after a rapid retreat of glaciers are still far from being understood in the highly diverse lichen communities from maritime Antarctica.
The aim of the present study is to analyze the variation of the diversity, cover and composition of a lichen community within a deglaciation gradient, and to investigate how microsite variables influence these patterns.
Materials and methods
Study site
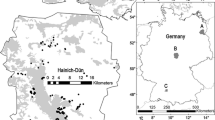
This study was performed in the Potter Peninsula, located between Potter Cove and Stranger Point in the southwest of King George Island (Isla 25 de Mayo), in the South Shetland Islands, north of the Antarctic Peninsula. Average annual air temperature is − 2.8 °C, with the summer temperature ranging from − 1.3 to 2.7 °C and in winter from − 15.5 to − 1.0 °C (Ferron et al. 2004). The geology of the Potter Peninsula belongs to the Warszawa tectonic block, which is dominated by a volcanic rock sequence, mainly basalts and basaltic andesites, formed between 50.6 and 49.1 Ma (Kraus and del Valle 2008). The peninsula has been shaped by glacial action, with moraines forming with typical rock outcrops, and different levels of terraces (Birkenmajer 1998; Kraus and del Valle 2008). The highest point in the area is Three brothers Hill (196 m. a.s.l.). For a more detailed description, see Birkenmajer (1998). The soils of the Potter Peninsula are poorly developed, typical for a periglacial environment, with coarse sand and gravel, a sandy texture, and ornithogenic soils in marine beaches; permafrost was found at about 90–100 cm depth (Poelking et al. 2015). Although snow usually melts completely during summer, it is possible to find some isolated permanent snow banks and ice patches throughout this area (Vinocur and Maidana 2010). The Peninsula is characterized by strong and humid westerly and easterly winds.
Sampling
Systematic sampling was conducted using a 2-km2 grid with 25 sampling stations located 500 m from each other. The grid was designed to cover the largest possible area free of ice of the Potter Peninsula (Fig. 1). Each sampling station was marked with GPS for future reference and monitoring. At each sampling station, five 50 × 50 cm quadrats were randomly located within a 25-m-diameter circle. Each quadrat was divided into 25 10 × 10 cm squares, and the frequency and cover of lichen species in each were measured. The frequency is the number of 10 × cm squares occupied by each lichen species. The percentage of each type of substrate—rocks, soil or bryophytes—was also measured. The frequency and cover of vascular plants and bryophytes (as a group) were measured and incorporated into the multivariate matrix. When the sampling station was inaccessible (i.e. aquatic habitats, permanent snow banks), the station was relocated to the closest place outside the constraint.
In addition, we registered environmental variables for each sample point: altitude, slope angle, aspect, distance to glacier front, distance to coast (both measured on a map) and the presence of bird nests. The distances were calculated with the latest satellite images available (Google Earth January 2014). Twenty-four sampling points were measured. Point number 25 was inaccessible.
Identification of species
The species were identified following standardized methods in lichenology (Nash et al. 2002). In general, we analyzed morphological, anatomical, reproductive and chemical characteristics following routine techniques, including macroscopic and microscopic observations of sections of the thallus and the identification of secondary metabolites by thin layer chromatography (Orange et al. 2001). A specimen of each identified species was deposited in the BCRU and CORD Herbaria. The identification of a few crustose specimens was impossible at the genus or species level due to the lack of sexual structures. These specimens were processed with artificial names. The nomenclature mainly follows Øvstedal and Smith (2001).
Data analysis
Sample points were ordinated using nonmetric multidimensional scaling (NMS; McCune and Grace 2002) separately for frequency and cover. In order to filter noise that could obscure the underlying structure of the data and to reduce the stochastic effects of rare species, we excluded lichen species present in five or fewer sample points (10% of frequency; McCune and Grace 2002). Pearson’s correlation coefficients were calculated to compare environmental variables and multivariate axes.
The NMS analysis was run with 500 iterations per run and 999 runs in total, using 0.005 as the stability criterion and 20 iterations to evaluate the stability using the relative Sørensen index of dissimilarity.
Total lichen cover and species richness were modeled by fitting generalized linear models (Guisan et al. 2002). The significance of each predictor was estimated by means of deviance tests. Predictors were excluded from the model when the level of significance was higher than 0.05. Previously, all residuals were tested for normality and homoscedasticity. Model selection was conducted using nonlinear fittings in R project software v.2.6.2 and INFOSTAT (Di Rienzo et al. 2014).
We also performed all the same analyses for lichen diversity measured as the Shannon diversity index, but we always obtained the same pattern of results as with species richness, with which it is highly correlated (Pearson rank correlation; 0.89, P < 0.001).
Results
A total of 65 lichen species were identified from 24 measured sampling stations (Table 1). Four stations did not contain lichen species or any other type of vegetation. A total of 36 species were present in less than 10% of sample points and were therefore excluded from the multivariate analysis (Table 1).
The diversity and coverage of lichens increased with the distance from the glacier front and from the coast (Fig. 2). However, habitat features were also found to be important explanatory variables. According to the fitted generalized model, the main variables explaining the species richness are the glacier and coast distances, their interaction, the altitude, the slope and the % of rock cover (Table 2). The fitted generalized model for total coverage of lichens showed that the explanatory variables are the glacier distance, the altitude, the percentage of bryophyte cover and the interaction between the last two variables (Table 2).
The NMS analysis for the frequency and coverage of lichen species (Fig. 3) showed that the sample points near the coast are grouped together, and that the vascular plants are grouped together and related to the points near the coast. Also, the species Thelenella mawsonii (C.W. Dodge) H. Mayrhofer & P.M. McCarthy, Carbonea vorticosa (Flörke) Hertel, Lecanora polytropa (Ehrh.) Rabenh., Huea diphyella (Nyl.) C.W. Dodge and Caloplaca schofieldii C.W. Dodge are related to the sample points closer to the front of the glacier.
Non–metrical multidimensional scaling plots of frequency (a) and cover of species (b) versus sampling points. Arrows indicate correlations of principal axes with environmental variables. Aust Austrolecia sp., B isa Buellia isabellina, C sch Caloplaca schofieldii, C sub C. sublobulata, C vor Carbonea vorticosa, Colo Colobanthus quitensis, Desch Deschampsia antarctica, Hima Himatormia lugubris, H cer Huea cerussata, H dyp Huea diphyella, L gri Lecanora aff. griseosorediata, L poly Lecanora polytropa, L spA Lecanora sp. A, Lept Leptogium puberulum, Massa Massalongia carnosa, Ochro Ochrolechia frigida, Pert c Pertusaria corallophora, Pert e Pertusaria erubescens, Plac an Placopsis antarctica, Plac co Placopsis contortuplicata, Ps bu Psoroma buchananii, Ps hi Psoroma hypnorum, Rh ge Rhizocarpon geminatum, Rh geo Rhizocarpon geographicum, Rh obs Rhizocarpon obscuratum, Rh sp Rhizocarpon sp., Rh sup Rhizocarpon superficiale, Thell Thelenella aff. mawsonii, U ant Usnea antarctica, U aur Usnea aurantiaco-atra, Altitud altitude, Cov. Mus coverage of bryophytes, Cov. Soil coverage of soil, Coast distance from the coast, Glacier distance from the glacier
The richest communities are those far from the glacier and the coast. Some points are closer to the coast but in elevated terrains. In these communities, the type of substrate conditioned the presence of the species. The NMS coverage analysis (Fig. 3b) showed a better separation of the points and species depending on the type of substrate. Points with higher coverage of mosses were associated with Himatormia lugubris (Hue) I.M. Lamb, Austrolecia sp., Psoroma hypnorum (Vahl) Gray and Caloplaca sublobulata (Nyl.) Zahlbr. On the other hand, stations with rocks or soil as the main substrate are separated according to the distance from the glacier front. The genera and species of these groups closer to the glacier are Leptogium puberulum Hue, Rhizocarpon obscuratum (Ach.) A. Massal., Placopsis antarctica D.J. Galloway, R.I.L. Sm. & Quilhot and Usnea antarctica Du Rietz. Finally, Massalongia carnosa (Dicks.) Körb., Usnea aurantiaco-atra (Jacq.) Bory, Psoroma buchananii (C. Knight) Nyl., Ochrolechia frigida (Sw.) Lynge, Pertusaria sp., etc. belong to the group of species related to sample points more distant from the glacier and with the highest richness and coverage of lichens.
Discussion
The present investigation reports on the structure of a lichen community in maritime Antarctica in a deglaciation scenario. The results showed a highly diverse and complex lichen community. Similar ecological studies have reported fewer numbers of species, 18–40 compared to the 65 species found in the present study (Valladares and Sancho 1995; Kim et al. 2006, 2007; Piñeiro et al. 2012). However, the total lichen species cited for King George Island exceeds 300 taxa (Olech 2004) and there are still new records being found (Passo et al. 2015; De la Rosa et al. 2016). In fact, it has been reported that the terrestrial biota of Antarctica is poorly described in detail (Convey 2010).
Very few species and low lichen coverage were recorded at those sampling points closest to the front of the glacier. It can be assumed that this is a clear sign that the retreat of the glacier on the Potter Peninsula is very recent (Lagger et al. 2017), considering the rapid lichen response reported for similar places in maritime Antarctica (Smith 1995; Sancho et al. 2017). This is in agreement with the observations of the ice masses on King George Island (Rückamp et al. 2011).
At sites near the coast, we found low coverage and species numbers. However, our sampling area did not include those rich lichen communities near penguin rookeries and bird colonies with a high nutrient input, which have a typical and different species assemblage (Smykla et al. 2007). Thus, the community near the coast is very poor in lichen species. Sea tides and unstable substrata are probably the cause of this pattern. On the other hand, our results from the multivariate analysis show that these points near the coast are related to the frequency and cover of vascular plants. Vera (2011) argued that at the coast the temperature is higher and that this provides better conditions for the expansion of vascular plants.
According to the linear models, the environmental variables that best explain the species richness and total cover of lichens are the distance to the glacier and the altitude. When considering the species richness alone, the distance to the coast explains not only this but also the interaction between both distances considered, the slope and the coverage of rocks. These results show that not only are very few species found near the coast or near the glacier front but also the combination of both showed the lowest number of species (e.g., sites 4, 5 and 10 in Fig. 1). At these sites, located at the inner side of Potter Cove and near the glacier front, the richness is very low while the cover is almost imperceptible. In the same way, places with a steep slope without rocks are almost devoid of lichens. The stability of the substrate in such conditions may be playing a key role in lichen colonization (Matthews and Vater 2015). On the other hand, the total lichen cover is explained by the interaction between the coverage of bryophytes and altitude: the higher the site and bryophyte cover, the higher the lichen diversity.
Scarce ice-free areas in Antarctic territory have a diverse lichen biota, usually not reflected in large-scale ecological works (Poelking et al. 2015). Classical works on Antarctic vegetation are based on physiognomic approaches that show different community assemblages only considering dominant species (Longton 1988; Piñero et al. 2012). This is usually due to mainly two factors: first, the considerable necessary taxonomic effort for the identification of species, mainly crustose lichens, and second, the need to cover microhabitat scale conditions such as aspect or slope (Colesie et al. 2014; Laguna-Defior et al. 2016). In our work, the species assemblages showed a greater variability than previous studies. Our results showed that richness and coverage of lichens depends on many driving variables that work at different scales (Alfredsen and Hoiland 2001; Casanovas et al. 2013). On the one hand, the distance to the glacier front, the distance to the coast and the altitude, and on the other hand, the type of substrate and the slope. The type of substrate generates microclimatic modifications that facilitate the establishment of other plant species (Groeneveld et al. 2007; Casanova-Katny and Cavieres 2012). Laguna-Defior et al. (2016) found higher cover of some species of macrolichens (such as H. lugubris) at higher sites together with an increase of the humidity.
We considered that the succession dynamic of this community responds more to a turnover of species (Garibotti et al. 2011) rather than a nested pattern (Nascimbene et al. 2017), probably due to the microsite variables acting as ecological filters (Keddy 1992). Accordingly, from the multivariate analysis, we can recognize at least three species assemblages, with only a few species present in the whole gradient. The first one is near the front of the glacier, with a low coverage and pioneer species. The next assemblage, observed at medium distances from the glacier front, has a low cover but higher number of species than the first assemblage. Species with cyanobacteria, such as the main photobiont or in cephalodia, such as L. puberulum and Placopsis spp., are common in this assemblage, which may be playing an important role in developing the community through the input of nitrogen (Sancho et al. 2011; Raggio et al. 2012). However, sites where these species were found had moderate slopes. In contrast, sites with a similar distance to the glacier, but with steeper slopes and a low proportion of rocks, which were very unstable and had a smaller number of species. It is clear that the slope and the type of substrate are also very important (Favero-Longo et al. 2011). The third assemblage is a more developed community, far from the front of the glacier (or at higher altitudes) and with a composition depending on the type of substrate. We speculate that in this last assemblage the competition could be another filter for species (Trenbirth and Matthews 2010), although we do not have enough data to be sure about this factor. Finally, near the coast and far from the glacier, without bird enrichment, the lichen species are almost absent with the conspicuous presence of the two vascular plants.
We consider that the patterns in these Antarctic communities are dynamic and very heterogeneous, since they depend on macroclimatic variables but there is also a strong influence of microsite factors. It is essential to perform further evaluations of the responses to these factors in studies that assess the impact of climate change in Antarctic terrestrial communities.
References
Alfredsen G, Hoilan K (2001) Succession of terrestrial macrofungi along a deglaciation gradient at Glacier Bllisen, South Norway. Nordic J Bot 21:19–37
Birkenmajer K (1998) Geology of volcanic rocks (Upper Cretaceous-Lower Tertiary) at Potter Peninsula, King George Island (South Shetland Islands, West Antarctica). Bull Pol Acad Earth Sci 46:147–155
Casanova-Katny MA, Cavieres LA (2012) Antarctic moss carpets facilitate growth of Deschampsia antarctica but not its survival. Polar Biol 35:1869–1878. https://doi.org/10.1007/s00300-012-1229-9
Casanovas P, Lynch HJ, Fagan WF (2013) Multi-scale patterns of moss and lichen richness on the Antarctic Peninsula. Ecography 36:209–219
Colesie C, Green TGA, TürkI R, Hogg D, Sancho LG, Büdel B (2014) Terrestrial biodiversity along the Ross Sea coastline, Antarctica: lack of a latitudinal gradient and potential limits of bioclimatic modeling. Polar Biol 37:1197–1208
Convey P (2010) Terrestrial biodiversity in Antarctica: recent advances and future challenges. Polar Sci 4:135–147
Cook AJ, Vaughan DG (2010) Overview of areal changes of the ice shelves on the Antarctic Peninsula over the past 50 years. Cryosphere 4:77–98
De la Rosa IN, Passo A, Rodríguez JM, Chiapella JO, Messuti MI (2016) A new species and new records of Lecanora (Lecanoraceae, lichenized Ascomycota) with usnic acid from the Antarctic region. Phytotaxa 261:185–193
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2014) InfoStat versión 2014. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. http://www.infostat.com.ar
Favero-Longo SE, Cannone N, Roger Worland M, Convey P, Piervittori R, Guglielmin M (2011) Changes in lichen diversity and community structure with fur seal population increase on Signy Island, South Orkney Islands. Antarct Sci 23:65–77
Fernández-Martínez MA, Pérez-Ortega S, Pointing SB, Green TGA, Pintado A, Rozzi R, Sancho LG, de Los Ríos A (2017) Microbial succession dynamics along glacier forefield chronosequences in Tierra del Fuego (Chile). Polar Biol 40:1939–1957. https://doi.org/10.1007/s00300-017-2110-7
Ferron FA, Simões JC, Aquino FE, Setzer AW (2004) Air temperature time series for King George Island, Antarctica. Pesqui Antart Bras 4:155–169
Garibotti I, Pissolito C, Villalba R (2011) Vegetation development on deglaciated rock outcrops from Glaciar Frías, Argentina. Arct Antarc Alp Res 43:35–45
Groeneveld EVG, Masse A, Rochefort L (2007) Polytrichum strictum as a nurse-plant in Peatland restoration. Rest Ecol 15:709–719
Guisan A, Edwards TC TC Jr, Hastie T (2002) Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 57:89–100
Inoue M (1995) The lichen flora and habitats of the Syowa region, continental Antarctica. Lichenologist 27:451–462
Johansson P, Thor G (2008) Lichen species density and abundance over ten years in permanent plots in inland Dronning Maud Land, Antarctica. Antarct Sci 20:115–121
Kappen L (1985) Vegetation and ecology of ice-free areas of northern Victoria Land, Antarctica. 2. Ecological conditions in typical microhabitats of lichens at Birthday Ridge. Polar Biol 4:227–236
Keddy PA (1992) Assembly and response rules: two goals forpredictive community ecology. J Veg Sci 3:157–164
Kim JH, Ahn Y, Hong SG, Andreev M, Lim K, Oh MJ, Koh YJ, Hur J (2006) Lichen flora around the Korean Antarctic Scientific Station, King George Island, Antarctic. J Microbiol 44:480–491
Kim JH, Ahn Y, Lee KS, Chung H, Choi H (2007) Vegetation of Barton Peninsula in the neighbourhood of King Sejong Station (King George Island, maritime Antarctic). Polar Biol 30:903–916
King JC, Harangozo SA (1998) Climate change in the western Antarctic Peninsula since 1945: observations and possible causes. Ann Glaciol 27:571–575
Kraus S, del Valle R (2008) Geological map of Potter Peninsula (King George Island, South Shetland Islands, Antarctic Peninsula). Instituto Antártico Chileno, Punta Arenas, Chile and Instituto Antártico Argentino, Buenos Aires. https://doi.org/10.1594/PANGAEA.667386
Lagger C, Nime M, Torre L, Servetto N, Tatián M, Sahade R (2017) Climate change, glacier retreat and a new ice-free island offer new insights on Antarctic benthic responses. Ecography 40:001–012. https://doi.org/10.1111/ecog.03018
Laguna-Defior C, Pintado A, Green TGA, Blanquer JM, Sancho LG (2016) Distributional and ecophysiological study on the Antarctic lichens species pair Usnea antarctica/Usnea aurantiaco-atra. Polar Biol 39:1183–1195. https://doi.org/10.1007/s00300-015-1832-7
Longton RE (1988) The Biology of Polar Bryophytes and Lichens. Cambridge University Press, Cambridge
Matthews JA, Vater AE (2015) Pioneer zone geo-ecological change: observations from a chronosequence on the Storbreen glacier foreland, Jotunheimen, southern Norway. CATENA 135:219–230
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Nascimbene J, Mayrhofer H, Dainese M, Bilovitz PO (2017) Assembly patterns of soil-dwelling lichens after glacier retreat in the European Alps. J Biogeog 44:1393–1404. https://doi.org/10.1111/jbi.12970
Nash TH III, Ryan BD, Gries C, Bungartz F (2002) Lichen Flora of the Greater Sonoran Desert Region. Lichens Unlimited, Arizona State University, Tempe, p 532
Olech M (2004) Lichens of King George Island, Antarctica. The Institute of Botany, Jagiellonian University, Krakow
Orange A, James PW, White FJ (2001) Microchemical methods for the identification of Lichens. British Lichen Society, London
Øvstedal DO, Smith RIL (2001) Lichens of Antarctica and South Georgia. Cambridge University Press, Cambridge
Passo A, Rodriguez JM, Chiapella J (2015) New records of Antarctic lichens. New Zeal J Bot 53:216–223. https://doi.org/10.1080/0028825X.2015.1057185
Piñeiro V, Eguren G, Pereira I, Zaldúa N (2012) Líquenes del entorno de la base científica Antártica Artigas, Bahía Collins, Isla Rey Jorge, Antártida: Estudio preliminar. Polibotanica 33:105–116
Poelking EL, Schaefer CER, Fernandes Filho EI, de Andrade AM, Spielmann AA (2015) Soil landform plant-community relationships of a periglacial landscape on Potter Peninsula, maritime Antarctica. Solid Earth 6:583–594
Raggio J, Green TGA, Crittenden PD, Pintado A, Vivas M, Pérez-Ortega S, De los Ríos A, Sancho LG (2012) Comparative ecophysiology of three Placopsis species, pioneer lichens in recently exposed Chilean glacial forelands. Symbiosis 56:55–66
Rai H, Khare R, Nayaka S, Upreti DK, Gupta RK (2011) Lichen synusiae in East Antarctica (Schirmacher Oasis and Larsemann Hills): substratum and morphological preferences. Czech Polar Rep 1:65–77
Redón J (1985) Líquenes Antárticos. INACH (Instituto Antártico Chileno), Santiago de Chile
Robinson SA, Wasley J, Alyson KT (2003) Living on the edge—plants and global change in continental and maritime Antarctica. Glob Change Biol 9:1681–1717. https://doi.org/10.1046/j.1529-8817.2003.00693.x
Rückamp M, Braun M, Suckro S, Blindow N (2011) Observed glacial changes on the King George Island ice cap, Antarctica in the last decade. Glob Planet Change 79:99–109
Sancho LG, Valladares F (1993) Lichen colonization of recent moraines on Livingston Island (South Shetland I., Antarctica). Polar Biol 13:227–233
Sancho LG, Palacios D, Green TGA, Vivas M, Pintado A (2011) Extreme high lichen growth rates detected in recently deglaciated areas in Tierra del Fuego. Polar Biol 34:813–822
Sancho LG, Pintado A, Navarro F, Ramos M, De Pablo MA, Blanquer JM, Raggio J, Valladares F, Green TGA (2017) Recent warming and cooling in the Antarctic Peninsula region has rapid and large effects on lichen vegetation. Sci Rep 7:5689. https://doi.org/10.1038/s41598-017-05989-4
Schroeter B, Green TGA, Pintado A, Türk R, Sancho LG (2017) Summer activity patterns for mosses and lichens in Maritime Antarctica. Antarct Sci 29:517–530. https://doi.org/10.1017/S095410201700027X
Seppelt RD (1995) Phytogeography of continental Antarctic lichens. Lichenologist 27:417–431
Smith RIL (1995) Colonization by lichens and the development of lichen-dominated communities in the maritime Antarctic. Lichenologist 27:473–483
Smykla J, Wołek J, Barcikowski A (2007) Zonation of vegetation related to penguin rookeries on King George Island, Maritime Antarctic. Arct Antarc Alp Res 39:143–151
Spielmann AA, Pereira AB (2012) Lichens on the maritime Antarctica. Glalia 4:1–28
Trenbirth HE, Matthews JA (2010) Lichen growth rates on glacier forelands in southern Norway: preliminary results from a 25-year monitoring programme. Geogr Ann 92:19–39
Turner J, Barrand NE, Bracegirdle TJ, Convey P, Hodgson DA, Jarvis M, Jenkins A, Marshall GJ, Meredith MP, Roscoe H, Shanklin J (2013) Antarctic climate change and the environment: an update. Polar Rec 50:237–259
Turner J et al (2016) Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535:411–415
Valladares F, Sancho LG (1995) Lichen colonization and recolonization of two recently deglaciated zones in the maritime Antarctic. Lichenologist 27:485–493
Vera ML (2011) Colonization and demographic structure of Deschampsia antarctica and Colobanthus quitensis along an altitudinal gradient on Livingston Island, South Shetland Islands, Antarctica. Polar Res 30:7146
Vinocur A, Maidana NI (2010) Spatial and temporal variations in moss-inhabiting summer diatom communities from Potter Peninsula (King George Island, Antarctica). Polar Biol 33:443–455. https://doi.org/10.1007/s00300-009-0719-x
Acknowledgements
We thank the staff of the Carlini (ex. Jubany) station, the Dirección Nacional del Antártico (DNA) and the Instituto Antártico Argentino (IAA) for providing logistics support during the the fieldwork. The authors would like to thank three anonymous reviewers and the the editor for their contributions to improving the manuscript. The authors are supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina. This work was funded by Project PICTO 2010–0095 (ANPCyT-DNA) to the third author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodriguez, J.M., Passo, A. & Chiapella, J.O. Lichen species assemblage gradient in South Shetlands Islands, Antarctica: relationship to deglaciation and microsite conditions. Polar Biol 41, 2523–2531 (2018). https://doi.org/10.1007/s00300-018-2388-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2388-0