Abstract
Cape Hallett (72°19′S; 170°13′E) lies at the northern end of the western coastline of the Ross Sea region, and, to date, there appears to be no full description of its terrestrial flora despite its probable importance in understanding links between biodiversity and latitude. Here we present information about lichens and mosses from published papers, herbarium collections and personal surveys for Cape Hallett and seven nearby sites. A total of 59 lichen and 11 moss species are reported for these eight sites. Cape Hallett is one of the richest sites for terrestrial biodiversity in the Ross Sea region with about 46 lichen species and nine species of bryophytes. Lichens have their greatest diversity on the upper scree and summit area (30 species, 330 m), the least within the large penguin colony at sea level (one species). The station at Cape Hallett was established in 1957, and some of the earliest ecological and ecophysiological studies in Antarctica were carried out there. Historical comparisons are possible and have revealed considerable changes in vegetation in the lower flush area, a high level of frost heave disturbance, new lichen growth rate estimates for northern Victoria Land and extreme stability of the snow banks on the scree slopes. Cape Hallett represents a very important site for studies on links between terrestrial flora and the environment as well as on possible effects of climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in the distribution of terrestrial vegetation in Antarctica continues to grow because of the potential use of biodiversity as an indicator or predictor of the effects of climate change. Although terrestrial biodiversity is confined to ice-free areas that actually make up only 0.34 % of Antarctica (Peat et al. 2007), the particular value of Antarctica is its relatively pristine nature with almost no anthropogenic influences on the present distribution of terrestrial vegetation. There is also the relative simplicity of the vegetation itself, being composed of bryophytes, lichens, microscopic algae, cyanobacteria and fungi, with the exception of the presence of two vascular plants in the Antarctic Peninsula region (Longton 1988). Major research projects over the past decades have focussed on biodiversity distribution and its controls. These include the main biological programme in SCAR (Scientific Committee on Antarctic Research), Evolution and Biodiversity in the Antarctic (EBA http://www.eba.aq), and the New Zealand Latitudinal Gradient Project (LGP http://www.lgp.aq) set up specifically to enhance our understanding of the distribution and diversity of both marine and terrestrial biota, particularly in the Ross Sea region, as well as factors that control present-day distributions (Howard-Williams et al. 2006, 2010).
The Ross Sea region is an interesting area to investigate links between terrestrial biodiversity and latitude. Its western coastline spans around 15° latitude, from Cape Adare in the north (71°10′S; 164°30′E) to La Gorce Mountains (86°45′S; 146°00′W) to the south. The Transantarctic Mountains run along the whole coast line and not only provide the largest area of ice-free landscape in continental Antarctica, around 21,000 km2, but also include the Dry Valleys, at 77°S–78°S, with an area of 4800 km2, representing the largest contiguous ice-free area in continental Antarctica.
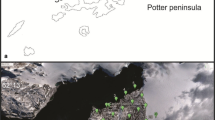
Cape Hallett is a particularly interesting site because of its long history of scientific research. Hallett Station (72°19′S; 170°13′E) was established on Seabee Hook in 1957 as a co-operative base by New Zealand and the USA for the International Geophysical Year 1957–1958 (Fig. 1).
Maps showing the location of Cape Hallett and other sites: A representation of the area surrounding Cape Hallett. The inset shows the location of this area on the Antarctic continent, B is a more detailed map of Seabee Hook with the start of the scree slope to the right. The Rudolph vegetation plot was established in 1962 (Rudolph 1963) and resurveyed in 2004 (Brabyn et al. 2006)
Although research undertaken at this station was primarily concerned with atmospheric physics and geology, the presence of the rich cryptogamic flora meant that biological studies became more prominent in the 1960s, especially after 1964 when summer-only operations started. Many of the studies were both novel and prophetic. The first CO2 exchange measurements made in the field in Antarctica were carried out in 1966 (Gannutz 1968, 1971; see Lange 1972) on lichens growing at the Hallett scree slope (see Fig. 2). This research formed part of a project directed by V Ahmadjian and led by TP Gannutz and S Frishman. OL Lange acted as consultant (Ahmadjian et al. 1967; Ahmadjian 1970). This work was extended, under controlled laboratory conditions with samples of Umbilicaria decussata (see Table 1 for lichen and bryophyte authorities) and Physcia caesia (named “Parmelia coreyi”) that ED Rudolph had collected at Hallett (Lange 1965; Lange and Kappen 1972). Positive net photosynthesis was found at temperatures well below 0, −12.5 °C for Buellia frigida, −16.5 °C for Rhizoplaca melanophthalma (“Lecanora melanophthalma”) and Xanthomendoza borealis (“Xanthoria mawsonii”), and −18.5 °C for Usnea sphacelata (“Neuropogon acromelanus”). Optimal net photosynthesis of R. melanophthalma was attained at around 0 °C. The Hallett lichens showed an extremely high resistance to cold temperatures. Dry thalli of all species tested survived cooling to −196 °C, whilst thalli of Rusavskia elegans (“Xanthoria elegans”) and R. melanophthalma even survived −196 °C when hydrated (Kappen and Lange 1970, 1972; Lange and Kappen 1972). In an early demonstration of a now well-known effect, it was also shown that, undercontrolled conditions, dry Antarctic lichens are able to reactivate their photosynthetic metabolism through water vapour uptake alone (Gannutz 1968). Comparative studies showed that the temperature dependence of gross photosynthesis of Ramalina maciformis (Negev Desert) and Xanthomendoza borealis (Hallett) (as Xanthoria mawsonii) was almost identical (Lange 1969). Habitat conditions were suggested to differ little when the lichens were active—a proposal that has since been confirmed by long-term monitoring studies (Schroeter et al. 2010). Innovative studies on isolated photobionts and mycobionts of Antarctic lichens were also made on samples from Hallett (Ahmadjian 1958; Schofield and Ahmadjian 1973).
Over the past two decades there has been a major improvement in our knowledge of the distribution of terrestrial vegetation in the Ross Sea region. A major study by the Italian Antarctic Research Programme has reported 57 species of lichens from central Victoria Land, extending from Stefania Cirque (72°28′S; 169°42′E) to Tripp Island (76.67°S), covering 41 localities centred around the Italian Mario Zuccelli Base at Terra Nova Bay (74°50′S; 164°30′E) (Castello and Nimis 1995b; Castello 2003). Other studies include Seppelt et al. (1995) for the Kar Plateau (76°56′S; 162°20′E), Seppelt et al. (2010) for Botany Bay, Granite Harbour (77°00′S; 162°35′E), Green et al. (2011b) for Mt. Kyffin (83°44′S; 171°46′E), Perez-Ortega et al. (2012) for the McMurdo Dry Valleys. There have also been some floristic analyses of lichen distribution: Castello and Nimis (1995b) for the Terra Nova Bay region and Cannone and Seppelt (2008) for central and northern Victoria Land. The distribution of mosses in the Ross Sea region has recently been analysed by Cannone et al. (2013) using collections from 63 sites along the Ross Sea coast and including the same 41 localities used above for the lichen studies. One overall result, for both lichens and mosses, is that within the Ross Sea region there seems to be no apparent relationship between biodiversity (number of species) and latitude. Similar totals for lichen species, around 35–40 species, are reported for Terra Nova Bay 74°S), Botany Bay (77°S) and Mt. Kyffin (84°S) (Peat et al. 2007; Green et al. 2011b). Green et al. (2011a) proposed that the lack of an obvious distributional pattern within the Ross Sea is a consequence of the vegetation being confined to suitable microhabitats, the location of which is more dependent on local landscape than on macroclimate. Cannone et al. (2013) support this conclusion for bryophyte distribution. The next site to the north which has been comprehensively surveyed is at Birthday Ridge (70°48′S; 170°00′E) where, despite it being an open granite ridge, 42 species of lichens and five bryophytes were recorded by Kappen (1985a, b). This site is not included in the analysis in this paper as it is about 250 km distant and outside the Ross Sea region.
The occurrence and arrival of species within the Ross Sea is controversial at present with several suggestions available which are not all exclusive. Fraser et al. (2014) suggest that the present biodiversity is linked to the presence of geothermal areas offering refugia through past more extensive glaciation periods. In contrast, Pisa et al. (2014) suggest that several colonisation events have occurred for the cosmopolitan moss Bryum argenteum, and Hills et al. (2010) suggest Pleistocene survival for the same species. Green et al. (2011a) also suggest relict occurrence of several species in the Beardmore Glacier area, 83°S. Knowledge of the terrestrial biodiversity at Cape Hallett is extremely relevant to this discussion.
The first general surveys of the flora of Cape Hallett and vicinity were also made during the International Geophysical Year and in subsequent early years of occupancy of the base (Pryor 1962; Rudolph 1963, 1966a, b; Murray 1963). However, once Hallett Station was closed (1967) vegetation surveys effectively stopped. The surveys which form part of the current work commenced in the 1998/1999 austral summer and were extended in 2004 and 2005 as part of the LGP. During our studies we were able to relocate ED Rudolph’s original vegetation study plot, established and first mapped in 1962, and this has provided a unique opportunity to assess vegetation change over a 42-year time frame (Rudolph 1963; Brabyn et al. 2006). This site now has special protection under the management plan for Antarctic Specially Protected Area 106 (ASPA 106) as it is the oldest monitored site in continental Antarctica. The ASPA also contains photographic sites established by ED Rudolph in 1962, and this has provided the longest period for lichenometric growth measurements in continental Antarctica (0.07 mm radial per year for B. frigida, Brabyn et al. 2005). Another example of the use of historical data has been the estimation of historical ozone levels from the flavonoid content of moss samples collected at various times at Cape Hallett (Ryan et al. 2009).
Despite the long history of research and its important location at the northern end of the Ross Sea coastal latitudinal gradient, there appears to be no detailed published list of lichens or mosses for Cape Hallett. The major study by Castello (2003) does not include Cape Hallett as a site and visits by other groups appear to have been brief with no detailed collecting. Smykla et al. (2011) list only four species of lichens, and Cannone and Seppelt (2008) only three species. The most complete biodiversity list appears to be an informal one of 18 lichen species and four moss species in the ASPA 106 management plan.
Peat et al. (2007) make the point that some parts of the Ross Sea are probably still undercollected. Surprisingly, this includes Cape Hallett for which no list of the flora or vegetation description has yet been published although an approximate total number of species has recently been published (Colesie et al. 2014). In the present publication we look at the diversity of lichens and bryophytes at Cape Hallett (72°19′S; 170°16′E) and nearby sites that, at around 72°S, are close to the northern limit of the Ross Sea. We have generated lists of lichens and mosses for Cape Hallett and seven other collected sites nearby. The lists are constructed from personal collecting by the authors in 1966, 1999, 2003, and 2004, from published collections in herbaria, from published collections by scientists at the time Hallett Station was active, and from published papers. Our results show Cape Hallett to be one of the richer vegetation sites along the Ross Sea coast.
Cape Hallett—Site description
Geomorphology
Cape Hallett (72°19′S; 170°16′E) (Fig. 1) discovered by James Clark Ross in 1841 and named after Thomas R. Hallett, purser on one of the expedition ships, Erebus, is located at the southern end of Mowbray Bay, northern Victoria Land, in the western Ross Sea region, and is the northern tip of the 32-km-long, 8-km-wide, north–south-trending Cape Hallett Peninsula. It was formed from a basalt shield volcano, Mt. Geoffrey Markham (72°23′S; 170°07′E) (Harrington et al. 1967) dated at 5.49 ± 0.12 and 6.6 ± 0.4 Ma (McIntosh and Kyle 1990). Hallett Peninsula extends south to Cape Wheatstone (72°37′S; 170°13′E) and connects with the mainland by a narrow ridge between Tucker Glacier and Edisto Inlet, the low point of the ridge being the broad Football Saddle (72°31′S; 169°46′E), which reaches 700 m altitude.
Cape Hallett can be divided into four zones based on landscape (Fig. 2). The first is Seabee Hook (name derived from American abbreviation “CB”, for “Construction Battalion”), a low (<5 m asl), recurved spit 130–575 m wide, composed of coarse volcanic debris, with a total area of about 41.1 ha and extending about 1200 m west from the high ridge forming Cape Hallett. The soils of Seabee Hook have been described by Hofstee et al. (2006). Seabee Hook is almost completely occupied by an Adélie penguin (Pygoscelis adeliae Hombron and Jacquinot 1841) colony (see below). The second is a relatively flat area with some hummocks, many of which are abandoned penguin nesting sites, composed of scree fans of basaltic gravel and sandy deposits on the north-east side of Willett Cove, a small bay enclosed by Seabee Hook. This area has several melt streams crossing it in summer and can be very wet with areas covered by surface water. The third is the scree slope which is steep at around 30°, composed of basaltic rocks and gravel with a north-west aspect that has a steady rise to about 330 m. The fourth area is the summit immediately above the scree slope. This is a more or less level area sloping gently southwards towards the snow-covered slopes leading to the summit of the peninsula, covered with snow for much of the year, except for a period in the later part of the summer season.
Adélie penguin colony
Seabee Hook (Fig. 3) supports a large Adélie penguin colony which, when the area was first occupied for Hallett Station, numbered some 62,900 pairs (Reid 1964). The colony was subjected to substantial impacts from the establishment and operation of Hallett Station (Fig. 3) and declined to a low of 37,000 pairs in 1968. Closure of the station and its final removal in 2006 has led to a recovery of colony numbers close to the original colony size. Breeding pairs were estimated at 39,000 in a 1998/1999 breeding season census by K. Barton and around 64,041 breeding pairs in a major survey in 2009 (ASPA 106 Management plan). The penguin colony extends to scree fans on the north-east side of Willett Cove (Gordon 2003).
A Seabee Hook in 2003; the photograph was taken in late January looking to the northeast from the scree slopes. The lighter colour is guano and delineates the area occupied by the penguin colony. The white arrow points to the remains of a large diesel storage tank (since completely removed). The black arrow points to the LGP camp (2004) on the southern shore of Willett Cove. B Hallett Station in 1966 when it was in operation, the geomagnetic dome is on the left and buildings are red. C A general view (looking approximately west) of the station area in December 1966 showing the close proximity of the station and penguin colony. (Color figure online)
The penguin colony is a major source of ammonia, and this enhanced nitrogen input results in dominance of the vegetation by nitrophilic lichen species occurring nearby. Theobald et al. (2013) have estimated ammonia production at 1.1 g NH3 per breeding pair per day, 1.9 % of the estimated nitrogen excretion of the penguins. This estimate is lower than in warmer coastal areas of Antarctica and is most likely due to lower temperatures at Cape Hallett. Atmospheric ammonia was measured as 1 μg m−3 at 1 km distance from the penguin colony. Because of the predominant wind flow the plume of NH3 forms in a north-east direction and is mainly over the sea (Crittenden et al. 2015). Although the actual NH3 levels were not measured, two lichen species, U. decussata and U. sphacelata, showed elevated phosphomonoesterase activity on the ridge at the top of the scree, indicating that NH3 levels were certainly elevated over the regional background (Site 13, in Crittenden et al. 2015).
There are also relatively large numbers of south polar skuas (Catharacta maccormicki Saunders 1893) that nest around, and even within, the penguin colonies.
Climate
Climate data for Cape Hallett are available on the LGP website (www.lgp.aq) and includes 7-year data (1957–1964) from the operating period of Hallett Station and more recent records (2003–present) from LGP and LTER (McMurdo Long-Term Ecological Research) monitoring stations. All records show a similar pattern (Fig. 4). Maximum air temperatures are above 0 °C almost every day from mid-December to the end of January (Hofstee et al. 2006). Extreme maximum temperatures can reach around 3.8 °C in January, and mean maximum temperatures are 0.9 °C in these months. No month has a mean temperature above freezing point; the warmest month is January with −1.35 °C. Mean monthly minimum temperatures are around −26 °C in July/August with an absolute minimum temperature recorded of −40.6 °C (August 1958). Snowfall totals about 1755 mm (actual snow depth) and falls on 160 days each year (averages for the years 1957 to 1963—Guthridge 1983). Most snow falls in late summer (February–April) and least in the early summer (September–December). The total snowfall is about 4–5 times more than at Granite Harbour (77°S) with around 300–400 mm per year (personal observations) and probably about 20 times more than in the Dry Valleys. This compares to a difference of 4.8 °C in mean annual temperatures, −15.3 and −19.1 °C, Cape Hallett and Wright Valley, respectively.
Upper panel Mean monthly temperatures for Cape Hallett, upper line maxima, middle line means, lower line minima. Data from LGP website (www.lpg.aq). Lower panel, monthly snowfall, solid line, number of days each month with snowfall (left hand axis); dashed line, total snowfall for month as actual snow depth in mm, right-hand axis, data are from Guthridge (1983)
The north-west, relatively protected aspect of the locality means that soil temperatures are warm and on Seabee Hook at 32 cm depth are above freezing in January. The depth to ice cement is greater at Cape Hallett (~80 cm) than at any other location in the McMurdo Sound region where it is normally no more than 60 cm deep (Campbell et al. 1997; Hofstee et al. 2006). Surface water is present for most of December and January.
Nearby sites (see Fig. 1)
Information was also gathered from personal and other collections from seven other sites that lie to the west of Cape Hallett but still within the Edisto Inlet or are directly adjacent with identical rock surfaces.
Cape Christie (72°18′S; 170°01′E): a cape situated 8 km WNW of Cape Hallett and directly across the Edisto Inlet. The underlying rocks are medisedimentary greywacke of the Robertson Group in contrast to the McMurdo volcanics at all other sites except Luther Peak.
Salmon Cliff (72°22′S; 170°06′E): the second prominent, but of small area, rock cliff within the Edisto Inlet of Seabee Hook on the W side of Hallett Peninsula.
Redcastle Ridge (72°18′S; 170°01′E): a castle-like ridge of red and black volcanic rocks between Arneb Glacier and the terminal face of Edisto Glacier at the head of Edisto Inlet.
Luther Peak (approx. 72°21′S; 169°52′E): the slopes in front of the peak running down to Edisto Inlet. Rock type is metasedimentary greywacke of the Robertson Bay Group.
Football Saddle (72°31′S; 169°46′E) is a broad pass at 700 m (2300 ft), 2 nautical miles (4 km) east-south-east of Football Mountain, on the ridge between Edisto Inlet and Tucker Glacier. Rock type is McMurdo volcanics, as at Cape Hallett and Crater Cirque although metamorphics are reported (Hertel 2007).
Crater Cirque (72°38′S; 169°22′E), named by the New Zealand Geological Survey Antarctic Expedition, 1957–1958, is a cirque on the south wall of Tucker Glacier, immediately west of its junction with Whitehall Glacier. On its floor is a lake containing various algae and cyanobacteria, and in the surrounding rock walls there are nests of Wilson’s storm petrel (Oceanites oceanicus Kuhl 1820), South polar skua and Snow petrel (Pagodroma nivea Forster 1777), as well as running streams and growths of mosses and lichens. The local rock type is McMurdo volcanics, as at Cape Hallett and Football Pass.
Cape Daniell (72°43′S; 169°55′E): Cape at the NE extremity of Daniell Peninsula which marks the S side of the entrance to Tucker Inlet, in Victoria Land. The local rock type is McMurdo volcanics.
Methodology
Species occurrence data
Lists of the lichen and moss species present at Cape Hallett and the nearby sites of Salmon Cliff, Cape Christie, Crater Cirque, Football Saddle, Cape Daniell and Luther Peak were compiled from a variety of sources (Table 1). Direct surveys were made in the seasons 1966 by OL Lange, species determined mainly by RD Seppelt, R Türk and H Hertel (Hertel 2007), and 1999, 2003, and 2004 (by RD Seppelt, C Beard, and R Türk). Samples from 1966 are archived in Herbarium Türk, Salzburg, and the others are archived in the Tasmanian Herbarium (HO). Details of about 540 samples were retrieved from the British Antarctic Survey Herbarium Database, Antarctic Plant Database (APD: http://www.antarctica.ac.uk/Resources/BSD/PlantDatabase/index.html). Individual collections made during the 1960s were obtained from online records for G Llano and TP Gannutz, and from archived information at the Ohio State University, Institute of Polar Studies, for ED Rudolph. Species were also added from the publications of Murray (1963), Castello and Nimis (1995b), Castello (2003), Cannone and Seppelt (2008), Smykla et al. (2011), Cannone et al. (2013).
Species nomenclature
Many lichens found at Cape Hallett, including many of the specimens in the BAS Herbarium, were originally named according to Dodge (1973), and some of the species were also first described by Dodge. It has, however, become clear that many of these new species are synonyms for already existing species or even of other species described by Dodge. We have attempted to correct all the original names to presently accepted names. This is difficult, however, as the review by Castello and Nimis (1995a) shows not only considerable synonymy but also missing, poor-quality, and poorly described type specimens. The first stage in checking specimen names was to apply the online correction list for the Dodge species from the Michigan State University Herbarium (http://herbarium.msu.edu/SSP/P&P/Dodge_Inovations.html). Additional and later sources were Øvstedal and Smith (2001), Castello (2003) for lichens, Ochyra et al. (2005) and Cannone et al. (2013) for mosses, Castello and Nimis (1994) for the lichen family Acarosporaceae, Seppelt et al. (1998) for Sarcogyne, Castello (2010) for Rhizoplaca, Søchting and Olech (1995), Søchting and Castello (2012), Lindblom and Søchting (2008), and Søchting and Seppelt (2003) for Caloplaca, Arup et al. (2013) for Teloschistaceae. Lists of synonyms were also available from the online key to northern Victoria Land lichens (http://dbiodbs.units.it/antartide/victoria). Names for a small number of specimens were also accepted if identifications had been reassessed recently.
There have been particular problems in the past about the use of some names, e.g. the mosses Bryum subrotundifolium A. Jaeger and B. argenteum Hedw. The former name has been extensively used for non-silvery and yellowish green specimens that are the dominant form in the Ross Sea region. Recent molecular analyses (Hills et al. 2010) and a thorough comparison by Ochyra et al. (2005) have led to the recognition that all specimens are referable to B. argenteum and they are so treated here. The common lichen Xanthoria mawsonii C.W.Dodge is also now known as Xanthomendoza borealis (C.W.Dodge) Søchting, Kärnefelt, and Kondratyuk after a reassessment by Lindblom and Søchting (2008).
Vegetation analysis
Two different approaches to describe the cryptogamic vegetation of the Hallett scree slope are reported: In 1966 the epilithic lichen community of the Hallett scree slope (see Fig. 2) was generally characterised by species occurrence in relevés distributed around the area. In the second approach the cryptogamic vegetation of the Rudolph plot was analysed by DCA-ordination.
Scree slope
Thirty phytosociological relevés were distributed over the scree slope (Fig. 5) at points selected subjectively to have a conspicuous lichen cover of at least six different species. The area of each relevé was ca. 1.5 m2 and composed of rock, gravel, and rock debris. The 21 species found and determined are sorted according to presence (in percentage) in the relevés (Table 4).
DCA-ordination of Rudolph plot
An analysis of the vegetation associations was undertaken using relevés (20 cm × 10 cm quadrats) located along the seaward long axis of the Rudolph long-term site (Brabyn et al. 2005). The location of the Rudolph plot is shown in the management plan for the Antarctic Specially Protected Area (ASPA) No. 106 (http://www.ats.aq/documents/recatt/Att443_e.pdf). The result of a topographic survey is given in Fig. 6c and shows that the majority of the plot lies on the flat area with its height increasing towards the west where the plot sits on the lower slope of the scree. Species presence was recorded, and cover estimates were obtained using point intercepts at 1-cm intervals with the frame gridded using fine fishing line and the point intercept being that point at which the lines crossed in grid corners. Field estimations of cover values maintained an accuracy of ±1 %. Within each relevé we recorded lichen substrata as saxicolous, terricolous or muscicolous and also made a visual estimate made of the water status (allocated to either wet or dry).
Results from the detrended correspondence analysis (DCA) of the Rudolph plot. Upper panel, A location of the individual relevés, species found and substratum type on the two main axes 1 and 2. Species are: cyano, cyanobacteria; brysub, Bryum argenteum; lecexp, Lecanora expectans; grealg, green algae; algmos, algae on moss; pracri, Prasiola crispa; calcit, Austroplaca darbishirei; grincr, grey indeterminate crust; xanmaw, Xanthomendoza borealis; canmur, Candelaria murrayi; and amapet, Amandinea petermannii. Middle panel B gives the vector generalised additive models (VGAM). The first axis of the DCA analysis corresponds closely to the X axis in panel (C). Lower panel C shows the topography of the Rudolph plot. Heights are in metres and coloured as black/blue/green/yellow/orange for heights of 0–2/>2–4/>4–6/>6–8/>8 m, respectively, above the lowest point of the plot (the north-east corner. (Color figure online)
A traditional vegetation table (not shown) was generated by using the generic function “vegemite” in the R library vegan (Oksanen 2004). Sites are arranged by the first axis of a detrended correspondence analysis (DCA) (Hill and Gauch 1980; Oksanen and Minchin 1997) with default specification. A DCA was calculated with the function “decorana-in library vegan”. Species are ordered relative to species scores. The table shows original abundances (frequencies). The results of the DCA-ordination were also used to estimate gradient length and to display species composition in unconstrained ordination space. Vector averages of environmental variables were fitted with the function “envfit” in “vegan”. Significance was estimated by using a permutation procedure (1000 permutations). In addition to proper eigenvalues, the function decorana also reports “decorana values” in detrended analysis. These are the values that the legacy code of decorana returns as eigenvalues.
To evaluate species optima along the first DCA-axis vector generalised additive models (VGAM) (Hastie and Tibshirani 1990; Yee and Wild 1996; Yee and Mackenzie 2002) were fitted with the implementation of the method in library VGAM. Fitted values are shown in plots for six selected species. The Gaussian family was used as the link function.
Historical change
The presence of scientists at Hallett Station in the early 1960s has meant that there are some scientific and photographic records available from that period. Brabyn et al. (2006) were able to use the vegetation surveys made by ED Rudolph in 1962 (Rudolph 1963) to show areas with vegetation change, and Brabyn et al. (2005) were able to use a photopoint established by ED Rudolph to obtain a lichen growth rate. In addition to these, we used historical photographs found in the archives of Ohio State University (taken by ED Rudolph) and by OL Lange that allow information to be gained about lichen growth, soil solifluction, and stability of snow areas.
Results
Biodiversity of lichens and mosses
Table 1 contains, with authorities, the complete list of lichens and mosses for the eight sites totalling 70 species of which 59 are lichens and 11 are mosses (Table 1). For lichens, Cape Hallett is by far the richest site with 46 species, 50 % more than found at the second richest site, Crater Cirque, 32 spp. The other sites have fewer species of lichens, 25 at Football Saddle, 22 at Luther Peak, 16 at Redcastle Ridge, 15 at Cape Christie, 11 at Cape Daniell, and only 4 at Salmon Cliffs, probably reflecting the small area of the latter site. Omitting Salmon Cliff from consideration, of the 59 lichen species, only one, Umbilicaria decussata, occurs at all seven sites, whilst nine species (15.3 %) occur at six sites (Austroplaca darbishirei, Candelariella flava, Lecanora physciella, Lecidea cancriformis, Pleopsidium chlorophanum, Rhizoplaca melanophthalma, U. decussata, Usnea sphacelata, and Rusavskia elegans, Table 2). Twenty-two lichen species (37.3 %) occur at only one site (Table 2), and of these 14 are found only at Cape Hallett and seven only at Crater Cirque.
A total of 11 species of mosses were found for the eight sites with Cape Hallett again being the richest (nine species), eight species at Crater Cirque, four at Luther Peak, and only one at Football Saddle (Tables 1, 2). Excluding again Salmon Cliff, no species occur at all seven sites, two (18.2 %) were present at six sites and three species (27.3 %) occur at only one site.
The occurrence of lichen species in the five vegetation zones at Cape Hallett is given in Table 3. Seabee Hook has only one recorded species, X. borealis. The flat area, which is very wet, has 13 species of which two, P. caesia and P. dubia, occur along the sides of ephemeral rivulets, and many of the remainder belong to known nitrophilic genera. The lower scree has 20 species, the mid-slope 12, and the upper scree slope and summit 30 species. The highest biodiversity is therefore at the top of the slope where the influence of gaseous ammonia from the penguin colony is likely to be lower although elevated above the regional background, with the lowest biodiversity directly adjacent to the penguin colony. Excluding Seabee Hook, only five lichen species (B. frigida, A. darbishirei, C. flava, P. caesia, and R. elegans) are found at all the four remaining sites (Table 3). The 12 species in the mid-slope zone include these five ubiquitous species and seven others that are otherwise only found in the upper slope and summit areas. Taking these distributions into account the four areas can be collapsed into three main lichen habitats: the flat, wet area; the lower scree slope; and the remainder of the scree and the summit. Lichens present in the upper scree and summit and not elsewhere include two Usnea species, three Umbilicaria species, P. chlorophanum, Pseudephebe minuscula, Rhizocarpon geographicum, and Rhizocarpon melanophthalma; all widespread in the Ross Sea region. It would appear that the high input of nitrogen from the penguin colony, mostly as gaseous ammonia, is sufficient to prevent the occurrence of these species at lower altitudes at Cape Hallett whilst encouraging many nitrophilous species.
Vegetation structure
Scree vegetation: the classical phytosociological study of the scree slope recorded a total of 21 lichen species with B. frigida and B. grisea being the most commonly found, 97 % and 93 % of relevés (Table 4). Lichens with yellow thalli, often linked to higher nitrogen inputs, were also common: C. flava, X. borealis, and R. elegans (93, 90, and 67 % of relevés, respectively). Lichens known to prefer more oligotrophic sites were least common, such as U. sphacelata, Acarospora gwynnii, and L. cancriformis all at 7 % (Table 4). The moss B. argenteum and the terrestrial green alga Prasiola crispa occurred in 38 and 57 % of relevés, respectively, an indication that wet conditions, even running water, can occur between the larger rocks and stones. P. crispa is also an indicator of high nitrogen input.
The DCA-ordination (Fig. 6A) and the vegetation table (not shown) show a main axis (DCA1) that approximates four standard deviations and is highly significant, P < 0.001. The axis (negative to positive) is also aligned and, in terms of vegetation, from cyanobacterial domination to lichen dominated relevés, and in moisture status from wet (water present) to dry. The axis approximates the situation of the Rudolph plot on the ground and covers the transition from a large, flat area, with water running across it, onto the scree (Fig. 6C). There is also a change from sandy to more cobbly substratum and, eventually at the higher sites, small blocks of lava.
Vector generalised additive models (VGAM) (Fig. 6B) show the smoothed response of an individual species along the first axis of the DCA which is strongly correlated with wet/dry distinction of the relevés and is aligned with the topography of the whole plot (compare Fig. 6B, C). The extracted species optima show that cyanobacteria (mainly Nostoc sp. but no full list appears to exist) are characteristic for wet sites (correlated with sand), whilst the other extreme of the axis is defined by the lichens A. darbishirei and X. borealis (Fig. 6B). B. argenteum has its optimum close to cyanobacteria, whilst the distribution of the green alga P. crispa is centred on the middle of the gradient.
The ordering of relevés in the vegetation table (not shown) is consistent with the results of the fuzzy clustering (not shown). The vegetation divides into three main groups dominated by cyanobacteria, moss, and lichens. This represents again the transition from the flat, wetter areas with cyanobacteria (coloured black and blue in Fig. 6C), through moss (coloured green) to the dry sites with lichens (coloured yellow/orange).
Changes over time
Stability
A comparison of photographs taken 36 years apart at Cape Hallett shows that the occurrence of longer-lying snow beds is almost identical over this time (Fig. 7). This has important effects on the occurrence of lichens. Snow beds are larger at the start of summer, then slowly melt back and, as they do so, reveal a ring of higher lichen cover that is normally clearly visible because of the dominant yellow lichens. When the snow beds are at their minimum in area, a pronounced snow kill area is revealed at the margin of the permanent snow cover where lichens are unable to grow.
Lichen growth
Photographs taken around 42 years apart (Fig. 8A, B, D, E) show slow but clear lichen growth at Cape Hallett. Rates of growth (all radial) for the marked lichen thalli (X. borealis) are 0.13 mm year−1 (panel A, Rudolph H42), 0.09 mm year−1 for (panel B and D, Rudolph H01 and H06). Another opportunistic example of lichen establishment and growth is shown by the numerous small thalli of X. borealis that were found in 1999 growing on a small piece of paper used by Lange in 1966 to label one of the relevés for photographing (Fig. 8F). The lichen colonisation also shows the importance of a suitable substratum, in this case paper, with probably slightly better water relations in order to allow rapid establishment.
Sets of paired photographs showing changes in lichens and their surrounding soil over around 40 years. The paired photographs A–C (1962 by Rudolph ED, 2004 by Seppelt RD) show evidence of strong cryoturbation (frost heave). Growth rates were estimated from comparisons A, B, and D, whilst E shows extensive but not quantifiable growth of Candelaria murrayi over 33 years (photographs by Lange OL and Green TGA). F Extensive lichen (Xanthomendoza borealis) growth on a piece of paper used by Lange OL to mark a relevé in 1966 (1999 by Seppelt RD). The paper is approximately 7 cm on each edge. Vertical white bars in A and B are 10 cm long, the same as the sides of the red boxes marked by Rudolph ED in 1962 (see in A, C). The actual notations used by Rudolph ED for A–D are H42, H01, H19, and H06, respectively. (Color figure online)
Frost heave (cryoturbation)
In the paired photographs those on the left were taken by ED Rudolph in 1962 and those on the right by RD Seppelt in 2004, 42 years later (Fig. 8A–C). In each case it is possible to compare the ground surface immediately adjacent to the larger rock and to see that considerable cryoturbation has taken place to the extent that there are no identical small stones in the photographs.
Discussion
Cape Hallett had an active station present from 1957 to 1967, but, despite being one of the early bases to be established in the 1958 Geophysical Year and also being close to the northern limit of the Ross Sea latitudinal gradient, no complete summary of the vegetation has been published. We report here a total of 59 lichen species for Cape Hallett and seven other sites in the near area. Cape Hallett, with around 46 lichen and nine moss species, is one of the richer sites along the Ross Sea coast. Only Terra Nova Bay (74°S), with over 40 lichen and 16 bryophyte species, and the Beardmore Glacier area (84°S), with around 42 lichen and two moss species (Green et al. 2011b), have similar vegetation richness. The two richer sites, Cape Hallett and Beardmore Glacier, occupy opposite ends of the Ross Sea coastline, the former at 72°S and the latter at 84°S, so any gradient in species richness with latitude appears unlikely confirming the suggestion of Colesie et al. (2014). The proposal that geothermal activity has preserved biodiversity in the Ross Sea is not supported by our results as the high diversity sites of Cape Hallett and Beardmore Glacier (data not used by Fraser et al. 2014) are the most distant sites from geothermal activity in the region. Existing geothermal sites also show no signs of being hotspots being colonised at most by two unusual moss species. Geothermal sites in the Ross Sea region are both at high latitude and at high altitude and thus differ from those in the Antarctic Peninsula where geothermal refugia might apply.
Cape Hallett, because of the early start of quantitative ecological research, is one of the few sites in the Ross Sea where it is possible to detect changes over a long time frame, reaching 50 years. It is one of only two sites in the Ross Sea region where lichen growth rates have been determined (Brabyn et al. 2005) and vegetation change can be clearly detected (Brabyn et al. 2006). The results presented here confirm that it is a special area for terrestrial research in the Ross Sea region.
The total numbers for lichens and bryophytes presented here have a firm basis. Of the 59 lichen species found at Cape Hallett and nearby sites the majority have been collected more than once or have been found and determined from our or other recent collections. Around 12 species are present only in the BAS Herbarium, and of these many are early collections that appear not to have been refound later. This is certainly partly due to limited collecting, both in time spent and in location studied in recent visits. An example of this is Smykla et al. (2011) who report only four lichens from Cape Hallett although one was a new record. Two species appear not to have been recently validated by other workers, Buellia pernigra and B. subtegens (Table 1), but both species have been collected at several other sites. Even allowing for these uncertainties it appears that the total of lichen species at Cape Hallett is certainly above 40. The number of bryophyte species is nine at Cape Hallett and 11 for the area, and this appears to be a verifiable number. The surprise, perhaps, is that the liverwort Cephaloziella varians (Gottsche) Steph. has so far not been reported, although it is known from much further south at Botany Bay (77°S) and Edmonson Point (74°S). Possibly this reflects the lack of a suitable habitat which, from records at Botany Bay and Edmonson Point, appears to be wet with a slightly enhanced nutrient level. The species is also very small, dark blackish purple when in full sun, and is easily confused with cyanobacterial aggregations that are common on the surface of mosses in moist habitats.
Crater Cirque, which is a small corrie facing east-south-east, is also a rich site with 30 species of lichens and eight mosses. The number of moss species certainly reflects the good water availability in the cirque, and the presence of Pohlia nutans, a common species in the northern Antarctic Peninsula but rare in continental Antarctica, is another indicator of a less extreme habitat. The species is found further south only on volcanically heated ground. Amongst the lichens, seven species are found only at Crater Cirque; one, Bryonora castanea, is known from this single occurrence in Antarctica and is otherwise circumpolar in the northern hemisphere, whilst Rinodina egentissima occurs mainly in the Antarctic Peninsula region. Several others (Acarospora flavocordia, Parvoplaca athallina, Caloplaca lewis-smithii) have been mainly collected from the Terra Nova Bay area by the same collectors as at Crater Cirque, suggesting that these species may well be undercollected.
There appear to be several possible reasons for the high terrestrial biodiversity at Cape Hallett.
First, the local landscape is particularly suitable for lichens and mosses. The scree and lower area have a north-west aspect which means not only substantial warming during the day but also shelter from most strong winds. Maximal temperatures are above 0 °C each day from mid-December through January and resulting in a deeper ice cement level than at other Ross Sea sites (Hofstee et al. 2006).
Second, the presence of a snow cap at the top of the scree from which melt water runs down through the scree provides a regular water supply, both for the scree and for wet flat area at the base. Snow patches on the scree form regularly each year (Fig. 7) and provide a regular water supply from snow melt for lichens and mosses around their edges.
Third, there is the enhanced nutrient input due to the presence of the large Adélie penguin colony on Seabee Hook. Although ammonia production, 1.1 g NH3 per breeding pair per day (Theobald et al. 2013), is less than from temperate sites, it still makes a major contribution to at least parts of this otherwise pristine area. Several nitrophilous lichen species, especially the yellow Candelaria murrayi and orange X. borealis, Austroplaca, Parvoplaca, Caloplaca, Rusavskia, and Xanthoria species, are abundant in this area and would otherwise not be present.
Fourth, there is a wide variety of habitats available at Cape Hallett that range from the very wet flat area at the base of the scree, through the well-drained scree face to the elevated level summit area. This is further influenced by probable differences in ammonia input, together with possible nitrate and phosphate transport through dust and water, which would be much higher at the base of the scree. Ten species of lichen occur only at the top of the scree, and another five species are present only in the middle or top of the scree. Several of these species, such as Umbilicaria spp., Usnea spp., Pseudephebe minuscula, Carbonea vorticosa, and Rhizocarpon geographicum, are typically associated with sites that are not nutrient enriched.
Fifth, there are species present that are at their known southern limit in Antarctica at Cape Hallett and have otherwise more northerly distributions (e.g.: Umbilicaria cristata, Lecanora flotowiana, and Buellia nelsonii). Green et al. (2011a) suggest that north of 72°S, the latitude of Cape Hallett, there will be an increase in lichen and bryophyte species due to increased precipitation and a longer physiologically active period. The presence of these species supports this assertion. The situation at Cape Hallett contrasts with that at Beardmore Glacier where one component adding to the vegetation diversity is the presence of a group of relict species (Green et al. 2011b).
Sixth, there is growing evidence that the area around Cape Hallett may have contained ice-free refugia for millions of years. The molecular studies on Collembola by both Stevens et al. (2006) and Torricelli et al. (2010) suggest long-term refugia. Genetic analysis of population samples of the collembolan Friesea grisea (Schaeffer 1891) from Cape Hallett and Crater Cirque shows that each possesses their own unique set of mitochondrial haplotypes, a result confirming the studies of Stevens et al. (2006) for other species. Torricelli et al. (2010) suggest that their results confirm the likelihood of a pre-Pleistocene origin for Antarctic springtails, which must have survived the glacial periods in multiple refugia in each of the major regions where they are currently found. Mosses are suggested to have recolonised the Ross Sea region at around 3.5 Ma (Pisa et al. 2014), and this follows the major glaciations of the Miocene and also the volcanic activity and formation of the region around Cape Hallett dated at 5.49 ± 0.12 and 6.6 ± 0.4 Ma (McIntosh and Kyle 1990).
The bryophyte flush, areas where slow moving water covers the ground and encourages substantial soil crusts of bryophyte, cyanobacterial, and algal growth, is a habitat that occurs sporadically in Victoria Land. Known sites include the Canada Glacier flush (Schwarz et al. 1992), Beaufort Island (Seppelt et al. 1999), Botany Bay (Seppelt et al. 2010). These sites differ substantially in nutrient conditions, and this has an effect on bryophyte diversity. Figure 9 shows the flush sites arranged along an axis of increasing nutrient input due to the influence of birds. The most oligotrophic site, Canada Glacier flush in the lower Taylor Valley, has two common moss species (B. argenteum and Hennediella heimii) together with B. pseudotriquetrum, which is relatively rare at the site. There is almost no influence by birds at Canada Glacier, the nearest colonies being on Ross Island some 100 km distant. At Botany Bay, where nutrients are higher due to the presence of a significant colony of breeding skuas, there are 10 bryophyte species (nine mosses and one hepatic). At Cape Hallett there is a very large colony of Adélie penguins and scattered breeding skuas. The large moss covered area at the northern end of Beaufort Island is amply supplied with melt water from snow and a backing ice cliff, and nutrients from a colony of breeding skuas and the nearby annual sea ice colony of Emperor penguins (Aptenodytes forsteri Gray 1844). At the latter two sites B. argenteum is the dominant moss with a minor occurrence of B. pseudotriquetrum at Cape Hallett, and an insignificant but expanding population of Hennediella heimii at Beaufort Island. The pattern fits the accepted ecological concepts of reduced biodiversity at both high and low nutrient levels (Herbert et al. 2004). The exact reasons for the changes in numbers are often not clear. It is noteworthy that the moss B. argenteum is common at all sites and exhibits extreme ecological versatility.
The early presence of researchers at Cape Hallett has allowed some long-term comparisons to be made. The stability of the snow patches has been mentioned above and is important for the maintenance of summer water relations. The highest density of lichens on the scree slopes is around the snow patches, with the lichens becoming hydrated and active as the melting margin of the snow patch passes over them. This phenomenon has been demonstrated for B. frigida at Botany Bay (Kappen et al. 1998). Lichens beneath the snow are not photosynthetically active because of the low light levels and also because they remain cold due to the insulating properties of the snow (Pannewitz et al. 2003). Because of this there is a lichen desert under the longer remaining snow which is only revealed in seasons of more extensive melt.
A comparison of recent photographs and historical images from 1962 (Rudolph) and 1966 (Lange) indicates radial growth rates by lichens (Fig. 8) between 0.09 and 0.13 mm year−1, in close agreement with a rate of 0.07 mm year for B. frigida (Brabyn et al. 2005). These growth rates are still about 10 % of those for crustose lichens in the climatically much milder Antarctic Peninsula and an even smaller proportion of the rates calculated for Placopsis spp. in Tierra del Fuego (Sancho et al. 2011). Sancho et al. (2007) suggested that crustose lichen growth rates, which show a 100 times difference from the Dry Valleys to the maritime Antarctic, are possibly one of the better indicators for climate change in Antarctica. Its position at 72°S makes Cape Hallett a particularly important site for lichen growth rate studies as it lies on the border between the micro- and macroenvironmental zones proposed by Green et al. (2011a). In the microenvironmental zone, which includes the entire Ross Sea region, vegetation is confined to sites which have a regular local water supply so that distribution is driven by landscape rather than macroclimatic factors. Further to the north the increase in precipitation allows extension in cover and species numbers. Additional lichen growth monitoring sites were established in 2004 at Luther Peak, away from the influence of penguins.
Frost heaving (cryoturbartion) of soils is known throughout Antarctica but is particularly important in wet sites (Cannone and Guglielmin 2010). A comparison of photographs taken by Rudolph in 1962 and Seppelt in 2004 (Fig. 8) reveals that frost heave is substantial on the wet flat area at Cape Hallett. Inspection of the photographs shows that there are no identical small stones present in the two sets of pictures, indicating that the substratum is very dynamic. The rhizoids and lower stems of B. argenteum, the only moss growing in the wet flat area, do not penetrate the substratum surface beyond a few millimetres, and this is probably one reason for its success at such high levels of surface disturbance. It also produces abundant propagules with shoot tips that readily release and are dispersed by wind or water following rewetting after the winter freeze period, allowing lower and dormant axillary buds to develop on the parent plant.
In summary, Cape Hallett is revealed as an exceptionally important research site in the Ross Sea region. For a variety of reasons it has a very rich terrestrial flora and the presence of a single but very large penguin colony provides excellent opportunities for research on nutrient inputs. The region also supports a significant and abundant invertebrate fauna, particularly collembolids, and the invertebrates have already been shown to offer important keys to the glacial history of Antarctica. Ice-free areas appear to have been present in this area through the entire glaciation of Antarctica. Cape Hallett also lies at a key latitude where the effects of any changes in climate might be expected to be easily detected and the many historical records make it a unique site for detecting climate change in continental Antarctica.
References
Ahmadjian V (1958) Antarctic lichen algae. Carol Tips 11:17–18
Ahmadjian V (1970) Adaptations of Antarctic terrestrial plants. In: Holdgate MW (ed) Antarctic ecology 2. Academic Press, London, pp 801–811
Ahmadjian V, Gannutz TP, Frishman S (1967) Photosynthesis and respiration of Antarctic lichens. Antarct J US 2:100–101
Arup U, Søchting U, Frödén P (2013) A new taxonomy of the family Teloschistaceae. Nord J Bot 31:016–083
Brabyn L, Green TGA, Beard C, Seppelt RD (2005) GIS goes nano: vegetation studies in Victoria Land, Antarctica. NZ Geogr 61:139–147
Brabyn L, Beard C, Seppelt RD, Rudolph ED, Türk R, Green TGA (2006) Quantified vegetation change over 42 years at Cape Hallett, East Antarctica. Antarct Sci 18:561–572
Campbell DI, MacCullach RJL, Campbell IB (1997) Thermal regimes of some soils in the McMurdo region, Antarctica. In: Lyons WB, Howard-Williams C, Hawes I (eds) Ecosystem processes in Antarctic ice-free landscapes. Proceedings of an international workshop on polar ecosystems, Christchurch, New Zealand, 1–4 July, 1996, A A Balkema, Rotterdam, pp 45–55
Cannone N, Guglielmin M (2010) Relationships between periglacial features and vegetation development in Victoria Land, continental Antarctica. Antarct Sci 22:703–713
Cannone N, Seppelt RD (2008) A preliminary floristic classification of southern and northern Victoria Land vegetation, continental Antarctica. Antarct Sci 20:553–562
Cannone N, Convey P, Guglielmin M (2013) Diversity trends of bryophytes in continental Antarctica. Polar Biol 36:259–271
Castello M (2003) Lichens of the Terra Nova Bay area, northern Victoria Land. Stud Geobot 22:30–59
Castello M (2010) Notes on the lichen genus Rhizoplaca from continental Antarctica and on some other species from northern Victoria Land. Lichenologist 42:429–437
Castello M, Nimis PL (1994) Critical notes on Antarctic yellow Acarosporaceae. Lichenologist 26:283–294
Castello M, Nimis PL (1995a) A critical revision of Antarctic lichens described by C.W. Dodge. Bibl Lichenol 57:71–92
Castello M, Nimis PL (1995b) The lichen vegetation of Terra Nova Bay (Victoria Land, continental Antarctica). Bibl Lichen 58:43–55
Colesie C, Green TGA, Haferkamp I, Büdel B (2014) Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. ISME J 8:2104–2115
Crittenden PD, Scrimgeour G, Minnullina G, Sutton MA, Tang YS, Theobald MR (2015) Lichen response to ammonia deposition defines the footprint of a penguin rookery. Biogeochem 122:295–311
Dodge CW (1973) Lichen flora of the Antarctic continent and adjacent islands. Phoenix Publishing, Canaan
Edwards HGM, Moody CA, Villar SEJ, Dickensheets DL, Wynn-Williams DD (2004) Antarctic analogues for Mars exploration: a Raman spectroscopic study of biogeological signatures. In: Third European workshop on exo-astrobiology, pp 33–36
Fraser CI, Terauds A, Smellie J, Convey P, Chown SL (2014) Geothermal activity helps life survive glacial cycles. PNAS 111:5634–5639
Gannutz TP (1968) Effects of environmental extremes on lichens. Bull Soc Bot Fr 115(Suppl 2):169–179
Gannutz TP (1971) Ecodynamics of lichen communities in Antarctica. In: Quam LO (ed) Research in the Antarctic. Am Assoc Adv Sci Pub, No. 93, Washington, pp 213–226
Gordon S (2003) Site description and literature review of Cape Hallett and surrounding areas. Antarctica New Zealand, Latitude Gradient Project, Internal report, pp 1–16
Green TGA, Sancho LG, Pintado A, Schroeter B (2011a) Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol 34:1643–1656
Green TGA, Sancho L, Tuerk R, Seppelt RD, Hogg ID (2011b) High diversity of lichens at 84°S suggests preglacial survival of species in the Ross Sea region, Antarctica. Polar Biol 34:1211–1220
Guthridge GG (1983) Hallett station, Antarctica, 1956–1973. Antarct J US 18:1–8
Harrington HJ, Wood BL, McKellar IC, Lensen GJ (1967) The geology of Cape Hallett—Tucker Glacier district, Antarctica NZ Geol Survey Bulletin 80
Hastie TJ, Tibshirani RJ (1990) Generalized additive models. Monographs on statistics. Chapman & Hall/CRC
Herbert DA, Rastetter EB, Gough L, Shaver GR (2004) Species diversity across nutrient gradients: an analysis of resource competition in model ecosystems. Ecosystems 7:296–310
Hertel H (2007) Notes on and records of Southern Hemisphere lecideoid lichens. Bibl Lichen 95:267–296
Hill M, Gauch HG (1980) Detrended correspondence analysis: an improved ordination technique. Vegetatio 42:47–58
Hills SFK, Stevens M, Gemmill CEC (2010) Molecular support for Pleistocene persistence of the continental Antarctic moss Bryum argenteum. Antarct Sci 22:721–726
Hofstee EH, Balks MR, Petchey F, Campbell DI (2006) Soils of Seabee Hook, Cape Hallett, northern Victoria Land, Antarctica. Antarct Sci 18:473–486
Howard-Williams C, Peterson D, Lyons WB, Cattoneo-Vietti R, Gordon S (2006) Measuring ecosystem response in a rapidly changing environment: the Latitudinal Gradient Project. Antarct Sci 18:465–471
Howard-Williams C, Hawes I, Gordon S (2010) The environmental basis of ecosystem variability in Antarctica: research in the Latitudinal Gradient Project. Antarct Sci 22:591–602
Kappen L (1985a) Vegetation and ecology of ice-free areas of northern Victoria Land, Antarctica. I. The lichen vegetation of Birthday Ridge and an inland mountain. Polar Biol 4:213–225
Kappen L (1985b) Vegetation and ecology of ice-free areas of northern Victoria Land, Antarctica. II: ecological conditions in typical microhabitats of lichens at Birthday Ridge. Polar Biol 4:227–236
Kappen L, Lange OL (1970) Kälteresistenz von Flechten aus verschiedenen Klimagebieten. Vorträge aus dem Gesamtgeb. d. Botanik (Deutsche Bot. Ges.), N.F. 4:61–65 (Cold resistance of lichens from different climatic areas)
Kappen L, Lange OL (1972) Die Kälteresistenz einiger Makrolichenen. Flora 161:1–29 (Cold resistance of some macro lichens)
Kappen L, Schroeter B, Green TGA, Seppelt RD (1998) Microclimatic conditions, meltwater moistening, and the distributional pattern of Buellia frigida on rock in a southern continental Antarctic habitat. Polar Biol 19:101–106
Lange OL (1965) Der CO2-Gaswechsel von Flechten bei tiefen Temperaturen. Planta 64:1–19 (CO 2 exchange of lichens at low temperatures)
Lange OL (1969) Die funktionellen Anpassungen der Flechten an die ökologischen Bedingungen arider Gebiete. Ber dtsch Bot Ges 82:3–22 (Functional adaptation of lichens to the ecological conditions of arid areas)
Lange OL (1972) Flechten—Pionierpflanzen in Kältewüsten. Umschau 72:650–654 (Lichens - pioneer plants in cold deserts)
Lange OL, Kappen L (1972) Photosynthesis of lichens from Antarctica. In: Llano GA (ed) Antarctic terrestrial biology, Am Geophys Union, Antarct Res Ser 20:83–95
Lindblom L, Søchting U (2008) Taxonomic revision of Xanthomendoza borealis and Xanthoria mawsonii (Lecanoromycetes, Ascomycota). Lichenologist 40:399–409
Longton RE (1988) Biology of polar bryophytes and lichens. Cambridge University Press, Cambridge, p 400
McIntosh WC, Kyle PR (1990) Hallett volcanic province. In: LeMasurier WE, Thompson JW (eds) Volcanoes of the Antarctic plate and southern oceans, Am Geophys Union, Antarct Res Ser, vol 48, pp 9–47
Murray J (1963) Lichens from Cape Hallett area, Antarctica. Bot Trans Roy Soc NZ 2:59–72
Ochyra R, Smith RIL, Bednarek-Ochyra H (2005) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Oksanen J (2004) Vegan: R functions for vegetation ecologists. Version: vegan_1.64, released on June 10, 2004. http://cc.oulu.fi/jarioksa/softhelp/vegan.htm
Oksanen J, Minchin PR (1997) Instability of ordination results under changes in input data order: explanation and remedies. J Veg Sci 8:447–454
Øvstedal DO, Smith RIL (2001) Lichens of Antarctica and South Georgia. A guide to their identification and ecology. Cambridge University Press, Cambridge
Pannewitz S, Green TGA, Scheidegger C, Schlensog M, Schroeter B (2003) Activity pattern of the moss Hennediella heimii (Hedw.) Zand., in the Dry Valleys, Southern Victoria Land, Antarctica, during the mid-austral summer. Polar Biol 26:454–551
Peat H, Clarke A, Convey P (2007) Diversity and biogeography of the Antarctic flora. J Biogeog 34:132–146
Perez-Ortega S, Ortiz-Álvarez R, Green TGA, de los Rios A (2012) Lichen myco-and photobiont diversity and their relationships at the edge of life (McMurdo Dry Valleys, Antarctica). FEMS Microbiol Ecol 82:429–448
Pisa S, Biersma EM, Convey P, Patiño J, Vanderpoorten AV, Werner O, Ros RM (2014) The cosmopolitan moss Bryum argenteum in Antarctica: recent colonisation or in situ survival? Polar Biol 37:1469–1477
Pryor ME (1962) Some environmental features of Hallett Station, Antarctica, with special reference to Arthropods. Pac Insects 4:681–728
Reid BE (1964) Cape Hallett Adélie penguin rookery—its size, composition, and structure. Rec Dom Mus 5:11–37
Rudolph ED (1963) Vegetation of Hallett station area. Ecology 44:585–586
Rudolph ED (1966a) Terrestrial vegetation of Antarctica: past and present studies. In: Tedrow JCF (ed) Antarctic soils and soil forming processes. Am Geophys Union Antarct Res Ser, vol 8, pp 109–124
Rudolph ED (1966b) Lichen ecology and microclimate studies at Cape Hallett, Antarctica. In: Thomp SW, Weihe WH (eds) Proceedings of the third biometeorological congress, Pau, France, 1963, 2:900–910
Ryan KG, Burne A, Seppelt RD (2009) Historical ozone concentrations and flavonoid levels in herbarium specimens of the Antarctic moss Bryum argenteum. Global Change Biol 15:1694–1702
Sancho LG, Green TGA, Pintado A (2007) Slowest to fastest: extreme range in lichen growth rates supports their use as an indicator of climate change in Antarctica. Flora 202:667–673
Sancho LG, Palacios D, Green TGA, Vivas M, Pintado A (2011) Extreme high lichen growth rates detected in recently deglaciated areas in Tierra del Fuego. Polar Biol 34:813–822
Schofield E, Ahmadjian V (1973) Field observations and laboratory studies of some Antarctic cold desert cryptogams. In: Llano GA (ed) Antarctic terrestrial biology, Am Geophys Union, Antarct Res Ser, vol 20, pp 97–142
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2010) Fourteen degrees of latitude and a continent apart: comparison of lichen activity over two years at continental and maritime Antarctic sites. Antarct Sci 22:681–690
Schwarz A-MJ, Green TGA, Seppelt RD (1992) Terrestrial vegetation at Canada Glacier, Southern Victoria land, Antarctica. Polar Biol 12:397–404
Seppelt RD, Green TGA, Schroeter B (1995) Lichens and mosses from the Kar Plateau, Southern Victoria Land, Antarctica. NZ J Bot 33:203–220
Seppelt RD, Nimis PL, Castello M (1998) The Genus Sarcogyne (Acarosporaceae) in Antarctica. Lichenologist 30:249–258
Seppelt RD, Green TGA, Skotnicki M (1999) Notes on the Flora, Vertebrate Fauna and Biological Significance of Beaufort Island, Ross Sea, Antarctica. Polarforsch 66:53–59
Seppelt RD, Türk R, Green TGA, Moser G, Pannewitz S, Sancho LG, Schroeter B (2010) Lichen and moss communities of Botany Bay, Granite Harbour, Ross Sea, Antarctica. Antarct Sci 22:691–702
Smykla J, Krzewicka B, Wilk K, Emslie S, Śliwa L (2011) Additions to the lichen flora of Victoria land, Antarctica. Pol Polar Res 32:123–138
Søchting U, Castello M (2012) The polar lichens Caloplaca darbishirei and C. soropelta highlight the direction of bipolar migration. Polar Biol 35:1143–1149
Søchting U, Olech M (1995) The lichen genus Caloplaca in polar regions. Lichenologist 27:463–471
Søchting U, Seppelt RD (2003) Caloplaca coeruleofrigida sp. nova, a species from continental Antarctica. Mycotaxon 86:163–168
Stevens M, Greenslade P, Hogg ID, Sunnucks P (2006) Southern Hemisphere Springtails: could any have survived glaciation of Antarctica? Mol Biol Evol 23:874–882
Theobald MR, Crittenden PD, Sim Tang Y, Sutton MA (2013) The application of inverse-dispersion and gradient methods to estimate ammonia emissions from a penguin colony. Atmos Environ 81:320–329
Torricelli G, Frati F, Convey P, Telford M, Carapelli A (2010) Population structure of Friesea grisea (Collembola, Neanuridae) in the Antarctic Peninsula and Victoria Land: evidence for local genetic differentiation of pre-Pleistocene origin. Antarct Sci 22:757–765
Yee TW, MacKenzie M (2002) Vector generalized additive models in plant ecology. Ecol Model 157:141–156
Yee TW, Wild CJ (1996) Vector generalized additive models. J R Stat Soc B 58:481–493
Acknowledgments
We are grateful to Antarctica New Zealand (AntNZ) for logistical support over several years as part of the Latitudinal Gradient Project coordinated by Shulamit Gordon. Logistics support was also provided by the Australian Antarctic Programme and the US Coastguard Reserve. These are all gratefully thanked. The New Zealand Foundation for Research, Science, and Technology (FRST), the University of Waikato Vice Chancellor’s Fund and the Department of Biological Sciences, University of Waikato provided financial support. During completion of the research, TGAG and LB were supported by the FRST grant, “Understanding, valuing and protecting Antarctica’s unique terrestrial ecosystems: predicting biocomplexity in Dry Valley ecosystems”. Field research in 1966 of OLL was financed by the US National Science Foundation under the US Antarctic Research Programme (USARP) within the frame of a research project of the late Prof. Dr. V. Ahmadjian, Clarc University, Worcester, Massachusetts. We are indebted to Dr. R.L. Stuckey who carefully archived Professor E. D. Rudolph’s research material in the Herbarium of Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Green, T.G.A., Seppelt, R.D., Brabyn, L.R. et al. Flora and vegetation of Cape Hallett and vicinity, northern Victoria Land, Antarctica. Polar Biol 38, 1825–1845 (2015). https://doi.org/10.1007/s00300-015-1744-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-015-1744-6