Abstract
This study seeks to determine the effects of local hydrography on the distribution, abundance and feeding of chaetognaths in the Lazarev Sea, an area strongly controlled by physical processes which has been held responsible for initiating the Weddell Polynya. Zooplankton samples were taken at 39 stations on four transects located between 6°W and 3°E and from 60°S to 70°S between surface and 350 m. The dominant species, Eukrohnia hamata, accounted for 86.5% of all chaetognaths, followed by Sagitta gazellae (8.1%) and Sagitta marri (5.4%). These three species showed distinct vertical and horizontal distribution patterns. While E. hamata and S. marri had maximum abundances below 250 m depth, S. gazellae showed a narrow distribution band in the upper 150 m depth. The distribution pattern was strongly modified at the Greenwich meridian with an upward transport of a high abundance of deep dwelling organisms (S. marri and E. hamata) and a displacement of S. gazellae to the surface, likely coupled with the rise of the warm, saline halo around the Maud Rise. Small copepods were the main prey of all three chaetognath species. Feeding rates (FR) varied among species and depth. Sagitta marri showed the highest FR with 0.38 prey d−1, followed by S. gazellae and E. hamata (0.22 and 0.07 prey d−1). Feeding rates were usually highest in the 25–80-m stratum. Size distribution and maturity of E. hamata revealed a dominance of small and immature organisms along all depths and stations, suggesting that this area might be acting as an important source of recently spawned organisms to the surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chaetognaths are among the most abundant predators in the Southern Ocean (SO) (Øresland 1990; Pakhomov et al. 1999). They contribute between 30 and 90% of the total biomass of carnivorous zooplankton and achieve up to 50% of the total predation impact of the carnivorous zooplankton (Pakhomov et al. 1999). As main predators of the omnivorous copepod community, they are able to remove up to 5.2% of the standing stock per day (Øresland 1990) and thus play a central role in the SO planktonic food web (Pakhomov et al. 1999). Chaetognaths may not only act as a link between small zooplankton and top predators, and they can also contribute to carbon sequestration (up to 12% of the total particulate organic carbon export) from the surface to the deep ocean by producing large, fast-sinking fecal pellets (Giesecke et al. 2010), playing an essential role in the biogeochemical cycling in the Southern Ocean.
Chaetognatha are one of the few phyla of marcozooplankton distributed throughout the SO (David 1958). Highest abundances are generally located in the upper 500-m water column where Eukrohnia hamata dominates with up to 92% of the total chaetognath abundance followed by Sagitta gazellae (6%) and Sagitta marri (2%) (David 1958; Bielecka and Zmijewska 1993). Abundance is drastically reduced below 500 m where other species such as Eukrohnia bathypelgica, Eukrohnia bathyantarctica and Heterokrohnia fragilis are part of the chaetognath community (Kruse et al. 2009).
The study on spatial and vertical distribution of chaetognaths in the Southern Ocean is influenced by the local hydrography; thus, they can serve as good indicators of water masses (David 1965). Despite great efforts in determining the spatial and vertical distribution of chaetognaths in the Southern Ocean, information is still fragmentary and mainly focused on the Antarctic Peninsula (Bielecka and Zmijewska 1993; Duró et al. 1999; Øresland 1995) and transects along latitudinal gradients across the SO (David 1958; Timonin 1968; Terazaki 1989; Johnson and Terazaki 2004). Information of a finer scale is still sparse, especially in areas between the ice shelf and the Antarctic Polar Front.
The eastern Weddell Sea is one of the most interesting regions in the SO due to complex physical processes associated with the local topography. Much of the mixing between the Weddell Sea Warm Deep Water (WDW) and the Circumpolar Deep Water (CDW) that flows southwestward into the Weddell Sea from the Antarctic Circumpolar Current (ACC) occurs in the Weddell Sea, near the Maud Rise (Muench et al. 2001).
The aims of this study are to determine vertical and horizontal distribution patterns of chaetognaths and to estimate the feeding rate and diet of the most important chaetognath species in the Lazarev Sea.
Materials and methods
Collection of samples
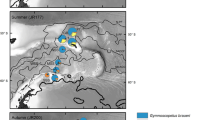
Zooplankton samples were collected during the LAKRIS ANT XXIII/2 cruise on board the R/V ‘‘Polarstern’’ between December 6, 2005, and January 1, 2006, at 39 stations on four transects located between 6°W and 3°E and from 60 to 70°S (Fig. 1). Chaetognaths were collected on vertical hauls in five depth strata (0–25, 25–80, 80–150, 150–250 and 250–350 m) using a Multinet with a mesh size of 200 μm (opening 0.25 m2). Samples were immediately preserved in formaldehyde (4% final concentration, buffered with hexamine).
Taxonomic identification was conducted on species level under a stereomicroscope, using specialized literature (Alvariño 1969; Casanova 1999). The most abundant species E. hamata and S. gazellae were classified into maturity stages according to Kramp (1939) and David (1955) respectively, while individuals of S. marri were grouped into four stages, viz stage I or juveniles (specimen with no visible ovaries); stage II (those with small developing ova); stage III, (individuals with some large ova); and stage IV (individuals with large ova).
Feeding rate
Organisms were collected for experiments at several stations using a WP-2 net (200 μm mesh size), equipped with a large (25 L volume) non-filtering transparent cod end. The net was towed vertically up to a depth of 150 m at low speed (~0.3 m s−1) in order to avoid damage to the organisms. After collection, the cod end was immediately transferred to a thermo-regulated laboratory at 0 ± 1°C. Organisms in good condition (intact fins, normal swimming activity and transparent body) were separated from the cod end, transferred into 2-L containers and fed with live copepods ad libitum. Chaetognaths were kept under observation every hour for at least 3 days. Those chaetognaths swallowing prey were individually transferred to 200-mL (small chaetognaths) and 600-mL (S. gazellae) incubation flasks and checked every hour to verify the position of the prey in the gut. Once the prey reached the last quarter of the gut, observations were carried out every 30 min until defecation. Time was measured from the moment of ingestion until defecation in order to estimate digestion time (DT), which is used to calculate the feeding rate.
The feeding rate (FR) was calculated using the equation proposed by Bajkov (1935):
where NPC is the number of prey ingested per chaetognath and DT is the digestion time in hours during a 24-h period. NPC was later estimated by dissecting the gut from the fixed chaetognaths collected by the Multinet. All chaetognaths were sorted, counted, checked for gut content and classified into maturity stages. For E. hamata, we also measured body length, excluding caudal fin, to the nearest 0.5 mm. To account for possible cod-end feeding, prey items found in the front of the ventral ganglion were not included in the analysis (Øresland 2000).
Chaetognath gut contents were assessed by dissection under a stereomicroscope and were further analyzed under an inverted microscope (at 400× magnification) following the methodology used by Øresland (1987) and Giesecke and González (2004). Partially digested copepods were identified up to genus level based on their mandible blades following the procedure used by Øresland (1987). Mandible blades of Antarctic copepods were already characterized by Michels and Schnack-Schiel (2005) and used in this study for copepod identification. Chaetognaths and polychaets found in gut contents were recognized by grasping spines and chaeta, respectively. The presence or absence of lipid droplets in guts was also checked under stereomicroscope. Gut content and the presence of lipids were analyzed on samples collected at all stations and in all depth ranges along 3°E, 0° and 3°W transects.
Statistical analyses
To resolve pairwise differences in size, lipid content and NPC among depths, non-parametric Wilcoxon/Kruskal–Wallis tests were used in lieu of ANOVA, in the absence of a transformation that achieved normality. Then, a non-parametric multiple comparison test was conducted (Sokal and Rohlf 1981). The surface stratum was usually omitted from the analysis due to the low number of organisms collected.
Results
Abundance and spatial distribution of chaetognaths
Eukrohnia hamata was the most abundant chaetognath at all stations contributing with 86.5% to total chaetognath abundance, followed by S. gazellae and S. marri with 8.1 and 5.4%, respectively. Eukrohnia hamata was present at almost all stations and depths sampled, with a low abundance at the surface (above 80 m depth) at all four transects (Fig. 2). Eukrohnia hamata showed a sharp decline in abundance south of 66.5°S up to the ice shelf (<1 ind m−3), from the surface down to 250-m-depth strata, between 3°E and 3°W (Fig. 2a, b, c). At lower latitudes, there was a general increase in abundance below 80 m depth (>2 ind m−2) along all transects. At the 0° transect and partially at 3°W, we observed a high abundance patch of E. hamata which seems to have been transported from the deepest strata at 66°S progressively toward the north, reaching 80 m depth at around 64°S (Fig. 2b).
The number of S. marri also rose (~2 ind m−3) at deeper layers (below 150 m depth) between 66° and 64°S (Fig. 3a, b, c). Additional high abundance patches of S. marri were found along four transects in the 250–350-m-depth strata: one close to the ice shelf (~69°S), which is observed at 3°E, 3°W and 6°W; a second patch further north between 64°S and 66°S, which is noticeable between 3°E and 3°W; and one close to 60°S (observed between 0° and 6°W). Unfortunately, at 6°W (Fig. 3d), the spatial sampling density is sparse and hence does not allow the recognition of the complete latitudinal structure, which is displayed on other three transects. At the surface (0–25 m depth) and in the 25–80-m-depth strata, almost no S. marri were collected along all transects (Fig. 3).
Sagitta gazellae, in turn, displayed a narrow distribution band between depths of 25 and 150 m with few or no individuals at surface or below 250 m depth (Fig. 4). A disruption of the narrow distribution band of this species occurred along 0° transect (Fig. 4b), between 64° and 66°S, with a displacement of organisms toward the surface. At 3°W to 6°W, the vertical distribution pattern displayed by this species gets gradually lost and abundances decrease to <0.25 ind m−3 at 6°W (Fig. 4d). We observed high abundances at the surface between 60.5°S and 61.5°S at 3°W, close to the southern boundary of the Antarctic Polar Front (Fig. 4c).
Size distribution and lipids in E. hamata
At the surface, we observed a high variance in the size of E. hamata associated with low number of organisms collected (n = 21) (Fig. 5a). However, below 25 m, the mean body length decreased significantly with increasing depth (K–W P < 0.05), with average organism lengths of 9.9 mm (25–80-m strata), 7.6 mm (80–150-m strata) and 6.3 mm (150–250-m strata). Below 150 m, the size remained constant, with no significant difference between two deepest layers (150–250 and 250–350 m, K–W P > 0.05). As size decreased with depth, there was also a slight increase in the percentage of lipids in the guts of E. hamata; however, no significant differences could be observed due to the high variance of data except between 25- and 80- and 250- and 350-m-depth strata (K–W P < 0.01) (Fig. 5b).
Some significant latitudinal differences in length could be observed among stations (Fig. 6). Along the 3°E transect (Fig. 6a), body length showed two maxima at 64°S and at the margin of the ice shelf at 70°S. No relationship between body length and abundance or lipid content was observed at this transect (abundance, r = 0.28, P = 0.4; lipids, r = 0.266, P = 0.4). Further west at 0° (Fig. 6b), the pattern was more consistent, with a gradual increase in body length from 60°S to 65°S followed by an abrupt decrease in length close to the ice shelf between 66° and 68°S. The length distribution along this transect followed the same pattern as the abundance, being both positively correlated (r = 0.737, P = 0.02) which is linked to the rise of deep dwelling organisms up to the surface strata.
The mean length of organisms and the percentage of the population with lipid droplets did not show any significant correlation in any transect (length r = 0.62, P = 0.07; abundance r = 0.28, P = 0.5). Close to 60.5°S at the 3°W transect (Fig. 6c), we recorded the largest mean body size of E. hamata, (13.2 mm) at stations where maximum abundances were recorded (2 ind m−3) in the deeper strata. Further south, there was a drastic decrease in size (mean length <7 mm); however, no significant relationship between length and abundance (abundance r = 0.38, P = 0.3) or length and lipids could be observed (lipid, r = 0.44, P = 0.35).
Maturity stage
All individuals of E. hamata and S. gazellae belonged stage I, with unripe male and female gonads, whereas all maturity stages of S. marri were present (Fig. 7). Along 0°, we observed the highest proportion of mature organisms with 21% individuals of stage II, 21% of stage III and 14% of stage IV, while in general at three transects the highest percentages of mature organisms were mainly associated with the presence of patches of high abundance. The vertical structure of maturity stage composition showed a dominance of immature organisms in the 25–80-m strata (66.6%). At intermediate depths (80–150 m and 150–250 m), the number of stages II and III organisms increased, while at deeper strata, the population was again dominated by immature organisms (Table 1). Fully mature organisms (stage IV) were only collected in high abundances in the 150–250-m stratum, accounting for 10.3% of the total population, while at other depths, their relative abundance remained constant between 3 and 4%.
Gut content and feeding rate
In general, a low proportion of E. hamata found with food in their guts (NPC 0.03, Table 1), with no significant difference among depths, stations or transects (K–W P > 0.05). As seen in Table 1, there is a slight decrease in NPC, below the 0–25-m strata; however, due to the high variance of the data, no significant difference could be found. Sagitta marri showed the highest proportion of food in their gut with a maximum of 0.27 in the 80–150 m, decreasing with depth to 0.21 and 0.13 at 150–250- and 250–350-m-depth strata. At the surface, only three specimens of S. marri were collected with one prey in their gut, which leads to a NPC of 0.33. Due to the low number of organisms, however, it was not considered for further analysis. As in E. hamata, no differences were observed among depths, stations and transects (K–W P > 0.05). In S. gazellae, NPC varied slightly among strata from 0.11 to 0.13 between 25–150 and 150–250 m, respectively.
The most common prey found in E. hamata were small unidentified copepods, accounting for 47% of total prey, followed by jellyfish remains with 25%. (Table 1) Almost all prey were located in the posterior part of the gut, thus in an advanced stage of digestion. Total copepods including unidentified copepod remains and the copepods Oithona spp. Metridia gerlachei, Microcalanus spp. Paraeuchaeta spp. and Calanus propinquus accounted for 68% of total prey. Chaetognath remains, radiolarians, eggs and decapod larvae were less common prey. All E. hamata with lipid droplets in their gut content was analyzed under an inverted microscope to verify, if they contained prey; however, in almost all cases, no prey were found associated with the lipid droplets. Therefore, the presence of lipid droplets was not classified as ingested prey.
Gut content analysis of S. gazellae showed a similar prey diversity as in E. hamata, mainly dominated by non-identifiable copepods (37%), followed by M. gerlachei (21%) and polychaetes (16%). Other copepods such as C. propinquus and Oithona spp. were also found in low percentages in the gut.
In S. marri, we found the highest percentage of copepods in the guts accounting for 92% of the total prey, dominated by unidentified copepod prey (40%), M. gerlachei (16%), copepod nauplii (16%), Oithona spp. (8%), C. propinquus (8%) and one Paraeuchaeta sp. (4%). Other non-copepod preys were unidentifiable jellyfish remains (10%).
Feeding rate and digestion time
Digestion time could be measured in just one E. hamata, captured with the modified large cod end WP-2 net. The specimen was collected in perfect condition with one prey item (small copepod ~700 μm prosome length) trapped on the grasping spines. Digestion process was followed under a cold light stereomicroscope in a temperature controlled environment (in situ temperature, 0 ± 1°C). The digestive process was easy to follow due to the transparency of the body. The period from the ingestion of the prey until it reached the ventral ganglion took ca. 24 min and continued at a constant rate until it reached the gut near the anus in ca. 1 h, where the food bolus remained until defecation (~10 h). The entire process lasted 11 h.
The same process was observed on S. marri whose DT could be followed for three specimens with a mean DT of 10.2 ± 0.8 h (mean ± SD). The digestive process of S. gazellae could be recorded for five specimens. The ingestion of prey and its slow, steady movement through the gut could be observed. The prey usually reached the ventral ganglion after 1.5–3 h. Transport continued until the prey reached mid-gut. At this point back and forth movement commenced within the gut; which took up 70% of the total digestive process time (see Giesecke et al. 2010). The estimated digestion time ranged from 9 to 15.8 h, with a mean of 11.5 ± 1.3 h (mean ± SD, Giesecke et al. 2010). Larger prey tended to increase the DT and a DT of 15.8 h was obtained for a 6.7-cm long S. gazellae that had preyed on a 1.2-cm long euphausiid, while relatively constant DTs (8.8–10.5 h) were obtained from 5.8 to 6.7-cm long chaetognaths preying upon copepods between 2.0 and 4.2-mm prosome length (Giesecke et al. 2010).
Discussion
In the Southern Ocean, three dominant chaetognath species in the epipelagic realm are E. hamata, S. gazellae and S. marri. (David 1955, 1958; Froneman et al. 1998; Johnson and Terazaki 2004), while in meso- and bathypelagic regions there is an increase in diversity with a presence of nine species (Kruse et al. 2009) and where S. marri and E. hamata usually dominate. The latter is by far the most abundant species in Antarctic waters, accounting for more than 90% of the total chaetognath abundance (Bielecka and Zmijewska 1993; Duró et al. 1999) and is almost the only species of macrozooplankton, together with the polychaet Tomopteris sp., which has a ubiquitous distribution south of the Antarctic Polar Front (Baker and De 1954). Eukrohnia hamata usually reaches their highest abundances close to the Antarctic Convergence above a depth of 500 m (David 1958; Timonin 1968; Terazaki 1989; Johnson and Terazaki 2004). The vertical distribution of this species (Fig. 2) suggests highest abundances below a depth of 350 m and most likely restricted to a thin layer, since below a depth of 500 m abundance tends to decrease to less than 1 ind. m−3 (Øresland 1995; Kruse et al. 2009).
The high abundance recorded in the Greenwich meridian between 66° and 64°S and similar patches at 3°E and 3°W suggest a rise of deep dwelling organisms to the surface. The latter agrees with the local circulation pattern forced by local topography mainly due to the presence of the Maud Rise. The Maud Rise is a seamount of ca. 100 km in diameter that extends upward from the 5,000 m abyssal plain to ~1,600 m below the sea surface. This topographic feature initiates a number of dynamic events such as the formation of a Taylor column over the rise and an upward flow of the relatively warm, saline halo around the Maud Rise of combined Weddell Sea Warm Deep Water (WDW) and the Circumpolar Deep Water (CDW) (De Steur et al. 2007). These processes influence the sea-ice concentration (De Veaux et al. 1993) and allow the formation of the Weddell polynya (Holland 2001) and persistent phytoplankton blooms (Moore and Abbott 2002), making this a particularly productive area (Spiridonov et al. 1996). As observed in this study, the upwelling of warm deep water observed at the Greenwich meridian also transported S. marri populations to surface and created a disruption of the narrow distribution band of S. gazellae.
S. marri has a vertical distribution, mainly associated with warm deep waters (David 1965), showing highest densities around depths of 200 and 600 m (David 1958), and almost an absence at the surface (David 1958; Timonin 1968; Johnson and Terazaki 2004). This agrees with our observations. Due to the transport of specimens further north of the Antarctic Convergence by the Antarctic Intermediate Water, the northern distribution may be quite variable (David 1958), while the bulk of the population is mainly restricted the south of the Antarctic convergence up to the Antarctic continental slope.
Unlike E. hamata and S. marri, which display maximum abundances at depth, S. gazellae does display a very narrow distribution located mainly above 150 m, with some patches at the surface close to the APF. In the upper 200 m, S. gazellae is known to reach maximum abundances (David 1955, 1958), especially in areas close to the Antarctic convergence (up to 100 ind m−3, Terazaki 1989), while this species gradually decreases in number toward the ice shelf (Hosie et al. 2000; Giesecke et al. 2010). The high abundances observed close to the surface at 3°W, i.e. for E. hamata and S. marri, are most likely related to local mesoscale physical structures such as meander-induced upwelling and increased eddy mixing, frequently observed close to the APF (Abbott et al. 2001). These processes may cause a transport of organisms to the surface. However, the extent of the process may be short (a couple of weeks; Moore and Abbott 2002) and spatially variable, causing a patchy distribution of organisms around major frontal zone systems.
Size distribution of Eukrohnia hamata
Individuals showed a positive skew to the small-sized spectrum, with only a few larger organisms across the entire water column. It is a well-known feature that smaller organisms (<15 mm) of E. hamata inhabiting oceanic regions in the Southern Ocean tend to distribute in the upper 300 m depth (Terazaki 1989; Johnson and Terazaki 2004) while larger organisms tend to live in depths below 500 m (Kruse et al. 2009); this pattern, however, tends to disappear in coastal shallow regions, where a more heterogeneous and less skewed size distribution is observed (Øresland 1990). The use of different sampling gears has demonstrated to have a considerable impact on the capturing efficiency of different size segments and thus might alter the size distribution structure of chaetognaths (Kehayias and Ntakou 2008). Detailed studies comparing different net efficiencies carried out by Hagen (1985) showed that nets with mesh size of 200 μm are more accurate in the quantitative sampling chaetognaths between 2 and 25 mm and are therefore ideal for catching Antarctic chaetognats S. marri and E. hamata. For larger chaetognaths such as S. gazellae nets, RMT 1 and RMT 8 are best suited.
The dominance of small E. hamata in this study leads to presume that a recent breeding event may have occurred at depth. However, in order to unveil changes in reproduction, a seasonal study with higher sampling frequency and covering greater depths (>3,000 m) is required.
Eukrohnia hamata in the same manner as S. marri and S. gazellae displays ontogenetic migration, characterized with the aggregation of mature organisms at depth (Øresland 1995; Timofeev 1998; Kruse et al. 2009) where they reproduce. After hatching at depth, juveniles migrate to the surface where they feed and grow. Highest growth rates are usually achieved during the first stages of development (stages I and II) (Sameoto 1987) during the first year (Sands 1980). During the second year, energy is allocated in reproduction, followed by a decrease in growth (Sands 1980). The gradual increase in size E. hamata during this study can be followed in the vertical size distribution pattern, as a result of growth during vertical migration to the surface (Fig. 5). The strategy behind the rapid growth rate at the first stages of development would facilitate the escape from the prey spectrum of small-sized predators and increases the own prey size spectrum simultaneously (Øresland 1995). The high amount of small organisms in the study area is probably favored by the passive transport of deep, recently hatched organisms to the surface by the advection of deep waters. The latter process would favor the development of the population of E. hamata by adverting them into highly productive regions such as the Weddell polynya (Spiridonov et al. 1996).
Contrary to herbivorous and some omnivorous zooplankton, chaetognaths remain active and even reproduce during winter (Øresland 1995) and thus can take advantage of the early opening of the sea ice in the polynya. West of Maud Rise, where the highest abundance of chaetognaths was recorded during this study, is also an area with relatively high levels of chlorophyll (0.1–0.15 mg chl-a m−3), which are maintained during all seasons (Spiridonov et al. 1996). An elevated amount of copepods at this location (i.e., Calanoides acutus, Rhincalanus gigas, copepodites of C. propinquus (Bathmann et al. 1993) serves as prey for chaetognaths and might act as an important recruitment area of several planktonic species and providing a feeding ground for juvenile forage fish and ultimately piscivorous fish, marine birds and mammals (Karnovsky et al. 2007).
Distribution of maturity stages in S. gazellae, E. hamata and S. marri
Ontogeny plays a crucial role in the distribution of Antarctic chaetognaths. Mature organisms, especially E. hamata and S. gazellae, migrate to depths below 750 m. However, the vertical distribution of mature E. hamata is not completely understood. Kruse et al. (2009) sampled between 500- and 2,000-m-depth strata at the same region as this study and found only a small abundance of mature organisms, which leads to the idea that maturity stages may be located deeper or that the sampling schedule may have mismatched the periods of high reproductive activity. Basically, due to the low depths reached in this study, the probability of catching mature organisms is low, even taking into account that deep convection process transported organisms to the surface. It also indicates that the rise of organisms is probably linked with upwelling from mid-depths rather than deeper layers.
The same occurs for S. gazellae, whose mature population is very rare above a depth of 750 m and are most common between 1,000 and 1,500 m (David 1955). In addition, the largest organisms of S. gazellae collected during this study were ~40 mm in length, while maturity is usually reached at size classes above 55 mm (David 1955).
The reproductive cycle of S. marri seems to be completely different, with a high abundance of developing stages in every depth sampled, with a dominance of stages I and II. Observations on the life cycle of S. marri suggest that this species matures in the 750–1,000 m depth range (David 1965), while some stages I and II individuals might be frequent below 500 m (Kruse et al. 2009). Despite the presence of late development stages (stages III and IV) across the complete sampling range, the low numbers of organisms and the restricted sampling depth do not allow to draw a conclusion of the reproductive and ontogenetic cycle of this specie. There are still only fragmentary information on the life cycle and growth of several chaetognath species, especially those from extreme latitudes which are usually more difficult to sample due to the great depth at which they breed. Further investigation into these aspects is needed in order to eliminate the gap in knowledge of these important predators.
Feeding
Chaetognaths have been shown to be among the most important predators in the Antarctic and Subantartic realm (Froneman et al. 1998; Pakhomov et al. 1999) and play a key role in its pelagic subsystem and in the export of organic carbon via the carbon pump (Giesecke et al. 2010). As expected, copepods made up the bulk of prey found in chaetognath guts, accounting for more than 68% of all prey ingested. Some differences among species were observed. While E. hamata preys mainly on small copepods and other unidentified prey remains, they also have the lowest proportion of copepod prey and the highest diversity of prey with a high proportion of unidentified prey (jellyfish remains). Due to the advanced stage of digestion of almost all prey, they had to be identified based on their hard part remains (i.e., mandible blades for copepods). The small size of the E. hamata collected made the identification of prey even more difficult. As observed by (Kruse et al. 2010) in deep dwelling E. hamata, the presence of jellyfish remains can also be a important fraction of the preys found in Eukrohnia spp. guts. Unlike our study, they were able to recognize these remains as part of cnidarians, due to the presence of nematocysts. Unfortunately, due to the advanced stage of digestion, it was not possible to identify the origin of the jelly remains in our samples. On the basis of our observations, maximum abundances of siphonophores (0.6 ind m−3 between 80 and 150 m, mainly Diphyes antarctica) match with the highest proportions of jellyfish remains in E. hamata guts (unpublished results). Thus, it is possible that some tentacles, mainly from siphonophores and small hydromedusa, could be a part of the E. hamata diet; however, the feeding process behind these findings needs to be cleared up, since it is well known that chaetognath usually do not prey upon larger preys than their own head width (Pearre 1980). The potential effect of chaetognaths on other carnivorous zooplankton has never been estimated and could be an important issue, since by feeding on tentacles they reduce the predatory capacity of siphonophores and reduce competition for food sources.
The feeding activity of E. hamata is usually low, with a low proportion of prey in the guts 0.02 to 0.11, which agreed with the previous observations made by Froneman and Pakhomov (1998) (range 0.01–0.21, mean 0.05), during the late austral summer of the Prince Edward Islands, Øresland (1995) 0.1–0.26 at Gerlache strait during winter and Kruse et al. (2010), 0.01 to 0.11 at depths below 500 m during summer and winter. All these studies cover a wide spatial and temporal range with a pronounced gradient of prey abundance; however, restricted feeding activity persists among all environments, with feeding activity below 0.2 preys chaetognath−1, leading to the conclusion that this species might have unlimited food sources and reinforces the idea that chaetognaths in general are not superfluous feeders (Reeve 1964).
One of the main drawbacks in estimating feeding rates and predation impact on the zooplankton population has been the difficulty in keeping organisms alive for sufficient time due to their fragile nature. Even if healthy organisms are collected (especially of Eukrohnia species), they do not feed under laboratory conditions. In order to solve this problem, several approaches have been used in order to determine feeding rates using indirect methods. One of the most used is that proposed by Bajkov (1935). This method is based on the observation of food in the guts of preserved animals and estimates of digestion time (DT) to calculate feeding rate in nature. The approach is based on the assumption that all animals in the population are equally likely to feed and that the food items are cleared from the gut by digestion and defecation “def” at a constant rate (units/hour). On a steady-state approach, “def” is equivalent to the maximum possible feeding rate. If animals feed at a rate of “f” (units/hour) (with the steady-state restriction that f ≤ def), food will be found in a fraction of time equal to f/def, this being the probability that a given animal has food in its gut when captured or the same as the fraction “p” of the population with food in the gut. Therefore, f = def*p, and the feeding rate may be estimated from the digestion time and the fraction containing food (Szyper 1976).
Hence, the estimation of digestion time in chaetognaths has become so crucial, when trying to determine feeding rates. In this study, we were able for the first time to measure digestion time of three dominant chaetognath species in the SO. It is interesting that the digestion time was similar among three species, with some slight differences in S. gazellae. For the latter species, we have obtained more measurements of DT (5) rather than only three for S. marri and one estimate for E. hamata. Digestion time increases with prey size (Øresland 1987, Giesecke et al. 2010); therefore, more estimates are needed on the effect of size on DT for other two species. We are more confident with the S. gazallae estimation and suggest that those estimated for S. marri and E. hamata be treated with caution.
On the basis of our observations, DT of Antarctic species takes longer than other species at low temperatures. For instance, S. elegans display DT of 10.2 h at 0°C at northeast cost of the USA (Feigenbaum 1982) and 10 h Gullmarsfjorden, Sweden, at 6°C (Øresland 1995).
Digestion time is close to the estimated using the empirical equation of Stuart and Verheye (1991) for Sagitta, where DT = 10.96e−0.086*T resulting in a DT between 10 and 12 h at 0 ± 1°C. This, however, assumes that DT is only dependent on temperature which is a clear oversimplification, as DT might itself be affected by predators (species, development stage, metabolism, etc.) as well as prey size and type. Our results validate the use of this equation, when no information is available. However, more information must be generated on the DT of distinct species under a range of temperatures in order to get a more consistent empirical estimation of DT. Using our in situ estimates on DT on Bajkov (1935) equation, we obtain feeding rates of E. hamata between 0.24 and 0.04 preys d−1, which is lower than observations made by Øresland (1995) (0.3–0.5 preys d−1) and Froneman and Pakhomov (1998) (0.05 and 0.5 preys d−1). In case of Froneman and Pakhomov (1998), oil droplets were also included as gut content (0.05 and 0.5 preys d−1) and the use of shorter DT in their estimations (10 h) may have caused this difference. The mean feeding rate of Sagitta was always higher than that of Eukrohnia species (Øresland 1990). In this study, S. marri displayed feeding rates between 0.3 and 0.63 prey d−1 (excluding 0–25-m stratum). By recalculating the NPC results of Øresland (1990), with the DT obtained in this study, we obtain a FR of 0.54 which supports our data. If we do the same for S. gazellae, we obtain a feeding activity which is lower than the one estimated by Øresland (1990), 0.57 prey d−1 but which is close to the mean values recorded by Froneman and Pakhomov (1998) of 0.22 prey d−1.
Lipid content in E. hamata
The presence of lipid droplets in E. hamata has been reported by several authors (Sameoto 1987, Øresland 1990, Froneman et al. 1998, Kruse et al. 2010); however, their benefit or ecological value is still unknown. It is suggested that they may be used as an energy resource (Kapp 1991), as buoyancy or both (Øresland 1990).
During our experiments, we made observations every 6 h on the size of the lipid droplets of two starved, healthy E. hamata kept under laboratory conditions for five days (in filtered sea water). During this period, we did not observe any changes in the size of lipid droplets, suggesting that the droplets were no use as food reserves. Metabolic budgets of E. hamata suggest that this species have relatively low metabolic demands, with a body carbon respiration between 0.5 and 0.7% d−1 (Kruse et al. 2010), similar to that measured in S. gazellae (Ikeda and Kirkwood 1989) with 0.5% body carbon d−1. The low metabolism of Antarctic chaetognaths combined with high buoyancy and low muscle development allows them to sustain long periods of starvation, which, in turn, is reflected in the relatively low ingestion rates measured in this and other studies. The exception is S. marri, which have a higher development of the muscular tissue and less buoyant, making this species less transparent (Giesecke personal observations). This species is able to swim in incubation flasks by rapid and continuous movement of its tail, which also implies higher metabolic demands and thus higher feeding rates as observed in this study. S. gazellae and E. hamata, in turn, only swim short distances due to single brief bursts, with long periods of inactivity.
The low energetic demand of chaetognaths in comparison with other predators, constant feeding rate and their usually high abundance makes them a successful phylum in almost any environment. As seen food does not be limited, metabolic demands are low (Kruse et al. 2010), they have probably a low, but constant breeding throughout the year (Øresland 1995), with a generation time expected of up to 2 years (for E. hamata in Western Norway; Sands 1980), thus in order to maintain its high abundances mortality must be remained low, especially in the first development stages. The presence of hydrographic processes, as the rise of deep “warm” water mass (Maud Rise polynya), holding a large number of juveniles, as observed to the northwest of Maud Rise, may play a key role in transporting organisms to areas of high biological productivity, thereby ensuring the survival and rapid growth of juveniles. Further observations on the fate of chaetognath production in marine environments should be addressed in order to evaluate their role not only as predators, but also as link to higher trophic levels.
References
Abbott MR, Richman JG, Nahorniak JS, Barksdale BS (2001) Meanders in the antarctic polar frontal zone and their impact on phytoplankton. Deep-Sea Res II 48:3891–3912
Alvariño A (1969) Los Quetognatos del Atlántico. Distribución y notas esenciales de sistemática. Trab Inst Esp Oceanogr 37:1–290
Bajkov AD (1935) How to estimate the daily food consumption of fish under natural conditions. Trans Am Fish Soc 65:288–289
Baker A, De C (1954) The circumpolar continuity of Antarctic plankton species. Discov Rep 29:201–218
Bathmann U, Makarov RR, Spiridonov VA, Rohardt G (1993) Winter distribution and overwintering strategies of the Antarctic copepod species Calanoides acutus, Rhincalanus gigas, and Calanus propinquus (Crustacea, Calanoida) in the Weddell Sea. Polar Biol 13:333–346
Bielecka L, Zmijewska MI (1993) Chaetognatha of Drake Passage and Bransfield Strait (December 1983–January 1984, BIOMASS–SIBEX). Pol Polar Res 14:65–74
Casanova JP (1999) Chaetognatha. In: Boltovsky D (ed) South Atlantic Zooplankton. Backhuys, Leiden, pp 1354–1374
David PM (1955) The distribution of Sagitta gazellae Ritter-Záhony. Discov Rep 27:235–278
David PM (1958) The distribution of the Chaetognatha of the Southern Ocean. Discov Rep 29:199–228
David PM (1965) The Chaetognatha of the Southern Ocean. In: Mieghen JV, Oye PV (eds) Biogeography and ecology in Antarctica. Junk Press, Hague, pp 296–323
De Steur L, Holland DM, Muench RD, McPhee MG (2007) The warm-water “Halo” around Maud Rise: properties, dynamics and impact. Deep-Sea Res I 54:871–896
De Veaux RD, Gordon AL, Comiso JC, Bacherer NE (1993) Modeling of topographic effects on Antarctic sea-ice using multivariate adaptive regression splines. J Geophys Res 98:20307–20319
Duró A, Sabates A, Gili J-M (1999) Mesoscale spatial distribution of chaetognaths along hydrographic gradients in the South Scotia Sea (Antarctica). Polar Biol 22:195–206
Feigenbaum DK (1982) Feeding by the chaetognath, Sagitta elegans, at low temperatures in Vineyard Sound, Massachusetts. Limnol Oceanogr 27:699–706
Froneman PW, Pakhomov EA (1998) Trophic importance of the chaetognaths Eukrohnia hamata and Sagitta gazellae in the pelagic system of the Prince Edward Islands (Southern Ocean). Polar Biol 19:242–249
Froneman PW, Pakhomov EA, Perissinotto R, Meaton V (1998) Community structure and predation impact of two chaetognath species Sagitta gazellae and Eukrohnia hamata in the vicinity of the Prince Edward Archipelago (Southern Ocean). Mar Biol 131:95–101
Giesecke R, González HE (2004) Feeding of Sagitta enflata and vertical distribution of chaetognaths in relation to low oxygen concentrations. J Plank Res 26:475–486
Giesecke R, González HE, Bathmann U (2010) The role of the chaetognath Sagitta gazellae in the vertical carbon flux of the Southern Ocean. Polar Biol 33:293–304
Hagen W (1985) On the distribution and population structure of Antarctic Chaetognatha. Meeresforschung 30:280–291
Holland DM (2001) Explaining the Weddell Polynya—a large ocean eddy shed at Maud Rise. Science 292:1697–1700
Hosie GW, Schultz MB, Kitchener JA, Cochran TG, Richards K (2000) Macrozooplankton community structure off East Antarctica (80–150◦E) during the austral summer of 1995/1996. Deep-Sea Res II 47:2437–2463
Ikeda T, Kirkwood R (1989) Metabolism and elemental composition of a giant chaetognath Sagitta gazellae from the Southern Ocean. Mar Biol 100:261–267
Johnson TB, Terazaki M (2004) Chaetognath ecology in relation to hydrographic conditions in the Australian sector of the Antarctic Ocean. Polar Biosci 17:1–15
Kapp H (1991) Some aspects of buoyancy adaptations of chaetognaths. Helgolander Meeresunters 45:263–267
Karnovsky NJ, Ainley DG, Lee P (2007) The impact and importance of production in polynyas to top-trophic predators: two case histories. In: Smith WO Jr, Barber DG (eds) Polynyas: windows to the world. Oceanography series 74. Elsevier, Amsterdam, pp 391–410
Kehayias G, Ntakou E (2008) Abundance, vertical distribution and feeding of chaetognaths in the upper 50 m layer of the eastern Aegean Sea. J Nat Hist 42:633–648
Kramp PL (1939) The Godthaab Expedition 1928. Chaetognatha. Medd Gronl 80:3–40
Kruse S, Bathmann U, Brey T (2009) Meso- and bathypelagic distribution and abundance of chaetognaths in the Atlantic sector of the Southern Ocean. Polar Biol 32:1359–1376
Kruse S, Hagen W, Bathmann U (2010) Feeding ecology and energetics of the Antarctic chaetognaths Eukrohnia hamata, E. bathypelagica and E. bathyantarctica. Mar Biol 157:2289–2302
Michels J, Schnack-Schiel SB (2005) Feeding in dominant Antarctic copepods—does the morphology of the mandibular gnathobases relate to diet? Mar Biol 146:483–495
Moore JK, Abbott MR (2002) Surface chlorophyll concentrations in relation to the Antarctic Polar Front: seasonal and spatial patterns from satellite observations. J Mar Syst 37:69–86
Muench RD, Morison JH, Padman L, Martinson D, Schlosser P, Huber B, Hohmann R (2001) Maud Rise revisited. J Geophys Res 106:2423–2440
Øresland V (1987) Feeding of the chaetognaths Sagitta elegans and S. setosa at different seasons in Gullmarsfjorden, Sweden. Mar Ecol Prog Ser 39:69–79
Øresland V (1990) Feeding and predation impact of the chaetognath, Eukrohnia hamata in Gerlache Strait, Antarctic Peninsula. Mar Ecol Prog Ser 63:201–209
Øresland V (1995) Winter population structure and feeding of the chaetognath, Eukrohnia hamata and the copepod, Euchaeta antarctica in Gerlache Strait, Antarctic Peninsula. Mar Ecol Prog Ser 119:77–86
Øresland V (2000) Diel feeding of the chaetognath Sagitta enflata in the Zanzibar Channel, western Indian Ocean. Mar Ecol Prog Ser 193:117–123
Pakhomov EA, Perissinotto R, Froneman PW (1999) Predation impact of carnivorous macrozooplankton and micronekton in the Atlantic sector of the Southern Ocean. J Mar Syst 19:47–64
Pearre S (1980) Feeding by Chaetognatha: the relation of prey size to predator size in several species. Mar Ecol Prog Ser 3:125–134
Reeve MR (1964) Feeding of zooplankton with special reference to some experiments with Sagitta. Nature 201:211–213
Sameoto DD (1987) Vertical distribution and ecological significance of chaetognaths in the Arctic environment of Baffin Bay. Polar Biol 7:317–328
Sands NJ (1980) Ecological studies on the deep-water community of Korsfjorden, western Norway. Population dynamics of the chaetognaths from 1971–1974. Sarsia 65:1–12
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics in biological research, 2nd edn. W.H. Freeman and Company, San Francisco
Spiridonov VA, Nöthig E-M, Schröder M, Wisotzki A (1996) The onset of biological winter in the eastern Weddell Gyre (Antarctica) planktonic community. J Mar Syst 9:211–230
Stuart V, Verheye HM (1991) Diel migration and feeding patterns of the chaetognath, Sagitta friderici, off the west coast of South Africa. J Mar Res 49:493–515
Szyper JP (1976) The role of Sagitta enflata in the southern Kaneohe Bay ecosystem. Phd Dissertation, University of Hawaii
Terazaki M (1989) Distribution of chaetognaths in the Australian sector of the Southern Ocean during the Biomass Sibex Cruise (KH-83–4). Proc NIPR Symp Polar Biol 2:51–60
Timofeev SF (1998) Reproduction of the chaetognath Eukrohnia hamata (Möbius 1875) in the Arctic Ocean. Rep Polar Mar Res 287:70–73
Timonin AG (1968) Distribution of chaetognaths in the Southern Ocean. Oceanology (Mosc) 8:702–709
Acknowledgments
The authors would like to thank the crew and scientists of R/V ‘Polarstern’ for their help and support during the Lazarev Sea Krill Study (LAKRIS) project. Helpful comments of Svenja Kruse and two anonymous reviewers are highly appreciated. The participation of RG was funded by a CONICYT doctoral fellowship, a DAAD fellowship and a POGO-IOC-SCOR travel-fellowship. Additional support from FONDAP-COPAS grant no. 15010007 and the Instituto Antártico Chileno made possible the post-cruise analysis of zooplankton samples grant G12-09.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giesecke, R., González, H.E. Distribution and feeding of chaetognaths in the epipelagic zone of the Lazarev Sea (Antarctica) during austral summer. Polar Biol 35, 689–703 (2012). https://doi.org/10.1007/s00300-011-1114-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1114-y