Abstract
Microphytoplankton populations were studied in shallow coastal water (<60 m) near the Brazilian Antarctic Station Comandante Ferraz (EACF) and three reference areas in Admiralty Bay in early and late summer (2002–2003). Phytoplankton was diverse (113 taxa), but not abundant (103 cells l−1). The highest abundances (>104 cells l−1) were caused by pennate benthic diatoms (Fragilaria striatula Lyngbye) that occurred mainly in early summer, associated with the presence of ice. In late summer, when the water temperature (−0.4 to 1.5°C), salinity (34 to 35), and phosphate (2.6 to 4.5 μmol l−1) were highest and the dissolved oxygen was lowest (6.4 to 2.9 ml l−1), centric diatoms (Thalassiosira spp.) were more abundant, suggesting an influence of oceanic waters. Phytoplankton abundance (≤102 cells l−1) and chlorophyll a concentrations (0.22 μg l−1) were lowest close to EACF. Pennate diatoms were dominant close to shore and in surface waters elsewhere, probably because of ice melting or sediment resuspension caused by water mixing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies of nearshore antarctic phytoplankton are relatively scarce because of its apparent low contribution to the total primary production of the Southern Ocean (El-Sayed and Fryxell 1993). High phytoplankton abundance and biomass in coastal areas are usually related to resuspension of benthic microalgae (Brandini and Rebello 1994).
The first phytoplankton sample from Admiralty Bay was collected during the Pourquoi-Pas? Expedition in 1909 and the research on phytoplankton began in the 1970s with the establishment of research stations within the bay (Ligowski and Kopczÿnska 1993). After that, other studies related to the Admiralty Bay phytoplankton have been made mainly during the 1980s by Polish scientists (Rakusa-Suszczewski 1980, Rakusa-Suszczewski et al. 1993), as well as during the 1990s by Polish and Brazilian scientists (Brandini and Rebello 1994).
Most anthropogenic activities in the bay have been related to four research stations: Henryk Arctowski (Poland) at Thomaz Point, the Comandante Ferraz Station—EACF (Brazil) at Keller Peninsula, Machu Picchu (Peru) at Crepin Point, and Copacabana (USA) at Llano Point (ATCM XXVIII).
The objective of this work was to evaluate the temporal and spatial distribution of microphytoplankton (cells > 20 μm) during the austral summer of 2002–03, through the study of abundance and specific composition associated with hydrological features near EACF and three other reference areas at Admiralty Bay.
Study area
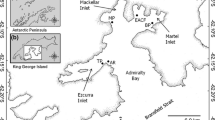
Admiralty Bay (62°03′−12′S, 58°18′−38′W) is located at King George Island, (area 122 km2) and has an estimated water volume of 24 km3. It is a deep fjord-like embayment with 500 m maximum depth at its center (Rakusa-Suszczewski et al. 1993).
The water from the bay meets the oceanic deep waters from Bellingshausen and Weddell Seas at its southern opening, which connects to the Bransfield Strait (Rakusa-Suszczewski 1980; Lipski 1987).
According to Rakusa-Suszczewski (1980), the freshwater that flows to the sea at Admiralty Bay originates from the glaciers, covering more than 90% of King George Island. The melt water carries nutrients and organic matter to the sea, and lowers the seawater salinity. High values of sediment content and total phosphorus were found near the main terrestrial input areas. Strong winds and gusts are common in the coastal areas of the Antarctic Peninsula and South Shetland Islands. These winds, together with the tides and freshwater drainage, play an important role in the transport of organic matter, nutrients and trace metals to the sea, which influences primary production.
Materials and methods
Admiralty Bay was surveyed during the early and late austral summer of 2002–03 (25 November–10 December, 2002 and 3–7 February, 2003). Samples were taken near EACF and three other reference areas distant from any anthropogenic activity using a Beyond BACI approach (Underwood 1994; Stark et al. 2003). The three other reference areas were Hennequin Point, Botany Point and an area about 300 m away from Thomaz Point within the Ezcurra Inlet (Fig. 1a). Three sampling stations were established in each area following the bathymetries of 20 m (St. A), 30 m (St. B) and 60 m (St. C) (Fig. 1b). At each station, samples were collected at surface, mid-water and at 1 m above the bottom.
Physical and chemical analyses
The temperature was obtained using protected reversion thermometers, and the salinity was measured using a Backman inductive salinometer. Dissolved oxygen was determined by the Winkler method using a Mettler DL 21 automatic titrator, following the Grasshoff et al. (1983) protocol.
The nutrient samples were collected using Go-flo bottles (General Oceanic®), filtered in Whatman GF/F membrane and stored in polyethylene flasks pre-washed with HCl 1:1, rinsed with distilled water. Then, the filtered water samples were frozen (−20°C) for further analysis. The analyses of nitrate and nitrite were performed using an automatic system—AutoAnalyzer II—Bran-Luebbe®. The silicate and phosphate analyses were processed by a spectrophotometric method and measured in a Genesys II spectrophotometer, Bausch & Lomb®. All these analyses followed the recommendations of Grasshoff et al. (1983).
Phytoplankton and chlorophyll a analyses
For these analyses, the water samples were collected using Nansen bottles. Chlorophyll a was extracted from 1 l filtered samples (Whatman GF/F membrane), frozen (−20°C), and determined by fluorimetry after extraction in 90% acetone, using a Turner TD-7000 fluorometer (Parsons et al. 1984).
Phytoplankton samples were stored in 250 ml dark bottles and fixed with 2% borax-buffered formaldehyde, and analyses were taken according to the Utermöhl (1958) method.
The cells were allowed to settle in 50–100 ml chambers for 48–72 h. Using an inverted microscope (Olympus®IX70) equipped with phase contrast at a magnification of 200× (ocular 10×, objective 20×), organisms larger than 20 μm were analyzed throughout the whole counting chamber. A higher magnification (400×) was used to identify the smallest taxa, such as some diatoms.
Species composition was determined based on the following works: Peragallo and Peragallo (1921), Cupp (1943), Medlin and Priddle (1990), Round et al. (1990) and Hasle and Syverten (1997).
The diatom taxa that could not be identified were grouped according to their morphotypes in: Group 1 (pennate diatoms, free cells), Group 2 (pennate diatoms, chain-forming), Group 3 (centric diatoms, free cells).
The occurrence of taxa was considered frequent (more than 40% of samples) or constant (more than 80% of samples). In terms of abundance, they were categorized according to Lobo and Leighton (1986) as abundant (one particular species being higher than the mean abundance of all species that appear in the sample) or dominant (one species constituting more than 50% of the abundance of the sample).
Statistics
In order to normalize distributions and eliminate zero values, the biological data were transformed using the log factor: log10 (x + 1). The Correspondence Analysis considered the biological data to distinguish patterns of distribution of phytoplankton populations. Results were compared using a One-Way ANOVA with a Kruskal–Wallis test (P < 0.05) for multiple comparisons. All these analyses were performed using Statistica® version 6.0 (Statsoft).
Results
The values of hydrological and biological data for the austral summer of 2002–03 are listed in Table 1. During the early summer, the water was relatively cold (−0.4 ± 0.2°C) compared to late summer (1.5 ± 0.3°C, P < 0.01), while the salinity showed little variation (34.3 to 34.8) (Fig. 2a).
Dissolved oxygen showed highest concentrations (6.4 ± 1.2 ml l−1) during early summer (Fig. 2b). Phosphate, as well as temperature, increased in late summer.
The estimated ratios N:P showed three groups of samples: (1) Early summer, N:P ratio between 10 and 15, associated with the lowest temperatures; (2) Late summer, N:P ratio < 10 influenced by highest phosphate concentrations; (3) Late summer, N:P ratio > 10 because of a high inorganic nitrogen availability associated with low phosphate concentrations (Fig. 2c).
Chlorophyll a concentrations were constantly low (0.44 ± 0.29 μg l−1), except for two peaks (>1 μg l−1) from the surface waters at Hennequin Point (St. A). The silicate values did not vary.
Microphytoplankton
The microphytoplankton assemblage was diverse, mostly represented by diatoms (87 taxa), dinoflagellates (12 taxa), cyanobacteria (8 taxa—mainly Nostocales and Oscilatoriales) and flagellates (6 taxa—mainly unidentified cryptophyceans).
In general, the microphytoplankton abundance was low (1.5 × 103 cells l−1 ± 2.7 × 103 cells l−1) with a high standard deviation because of the temporal and spatial variations. Diatoms were dominant in terms of the number of taxa (77%) and abundance (90%).
Temporal variation
Composition, number of taxa (Table 2) and abundance of microphytoplankton differed during the study periods. In early summer, the highest number of taxa (P > 0.05) occurred due to the contribution of pennate tychoplanktonic diatoms in the water column. However, the abundance (6.2 × 102 ± 6.9 × 102 cells l−1) was lower than in late summer (1.5 × 103 ± 1.5 × 103 cells l−1) (Fig. 3), except for the surface waters at station A, at Hennequin Point, where, the chain-forming pennate benthic diatoms (cell linear length ≅60 μm) were responsible for the high abundance (>1.5 × 104 cells l−1). Pennate diatoms reached 85% of abundance during early summer.
In late summer, centric (55%) and pennate diatoms (42%) dominated the phytoplankton assemblage, and the highest abundance was caused by centric diatoms.
Dinoflagellates showed highest abundance (P < 0.01) in late summer, and the other groups (cyanobacteria and flagellates) were not well represented during early or late summer.
Spatial variation
The phytoplankton abundance (Fig. 4a) was different between sampling areas (P = 0.03). The EACF (5.2 × 102 ± 3.7 × 102 cells l−1) and Ezcurra Inlet (1.2 × 103 ± 0.8 × 103 cells l−1) sample areas had the lowest (<1.5 × 103 cells l−1) and most homogeneous (SD < 103 cells l−1) values. Conversely, the highest (>1.5 × 103 cells l−1) and most heterogeneous (SD ≥ 103 cells l−1) values were found at Botany Point (1.8 × 103 ± 2.1 × 103 cells l−1) and Hennequin Point (2.3 × 103 ± 4.8 × 103 cells l−1) (Fig. 4a) due to the high abundance (>104 cells l−1) of chain-forming pennate diatoms (Fragilaria striatula Lyngbye, Fragilariopsis Hustedt) (Fig. 4b).
There were no significant differences between the sampling stations in regard to the microphytoplankton abundance (Fig. 5a). However, there were differences (P = 0.02) in the taxonomic groups’ representation. The highest contribution was from the pennate diatoms in shallow water (80%) at St. A, whereas the centric diatoms were more abundant (65%) at St. C. The cyanobacteria and flagellates were sparse at St. A, while at the deepest stations they represented more than 5% of the total abundance (Fig. 5b).
Different sampling areas had distinct vertical distribution patterns (Fig. 6), with highest abundance in surface water at Hennequin Point in early summer (>5 × 103 cells l−1) and Botany Point in late summer (>4 × 103 cells l−1). There was a decreasing vertical distribution gradient in early summer, but a homogeneous gradient in late summer at Ezcurra Inlet. No vertical distribution variation was observed at EACF.
Species composition and Correspondence Analysis
Considering the 113 taxa identified in Admiralty Bay’s flora, and according to the criteria of abundance and occurrence, 31 taxa were selected for the Correspondence Analysis, which shows the temporal and spatial distribution of the taxa.
The I × II factorial plan (Fig. 7a, b) represented 42% of the total variance and isolated the samples from late summer in the positive portion of Axis I (23%). These samples were characterized by the dominance of centric diatoms, such as Corethron pennatum (Grunow) Ostenfeld (39%), Thalassiosira anguste-lineata (Schmidt) Fryxell & Hasle, Thalassiosira cf. frenguellii Kozlova, Thalassiosira spp., and some species identified as “Group 3”, besides the pennate diatom Pseudo-nitzschia “delicatissima” (Cleve) Heiden & Kolbe complex.
Admiralty Bay (summer 2002/03): correspondence analysis of microphytoplankton species: sampling areas (a); He Hennequin Point, BP Botany Point; taxa (b). see Table 3
The surface samples from shallow stations (St. A and B) appear in the negative portion of Axis I. These were characterized by high concentrations (>1.5 × 103 cells l−1) of pennate diatoms, mainly: Group 1, benthic species such as the chain-forming Fragilariopsis ritscheri,—especially at Hennekin Point (>30%)—Fragilaria striatula, Achnanthes brevipes (Kützing) Cleve and others; along with Group 2, the single-celled Licmophora gracilis (Ehrenberg) Grunow and unidentified single pennate diatoms.
Axis II (19%) established a separation between early and late summer of the Botany Point sampling area. The early summer samples (positive portion) were characterized by the highest abundance (>20%) of species included in Pleurosigma/Gyrosigma and Cylindrotheca closterium (Ehrenberg) Lewin & Reimann/Nitzschia longissima (Brébisson) Ralfs complexes. In samples from late summer (negative portion) the pennate diatom Fragilaria striatula was dominant (55% of abundance) (Fig. 7b).
The centric Corethron pennatum was the only species that was present in all four sampling areas and during both sampling periods (>88% of the samples). Moreover, this species was considered abundant in 68% of the samples and dominant in 31% (mostly in late summer).
The pennate diatoms, such as Licmophora gracilis, Pseudogomphonema kamtshaticum (Grunow) Medlin, Cocconeis cf. costata (Gregory) Cleve and the Cylindrotheca closterium/Nitzschia longissima complex, had an important role in Admiralty Bay’s phytoplankton population. They were considered frequent (>50% of the samples) and abundant (Table 3).
Discussion
In the context of water column production, the Admiralty Bay nearshore water can be considered a high nutrient—low chlorophyll (HNLC) area (Platt et al. 2003). The pattern of temporal variation of the inorganic nutrients distribution is correlated with the biogeochemical processes and physical dynamics of the Bay (light incidence, melting ice, oceanic input, resuspended sediments, and wind stress generating advective processes). This can be seen in the N:P ratios, which showed the lowest values (8.5 ± 4.5) in late summer as a result of the highest phosphate concentrations (4.5 ± 2.5 μmol l−1). During the 2002–03 summer, the microphytoplankton abundance was low (103 cells l−1) when compared to values reported in other Antarctic areas like the Weddell Sea (Kang et al. 2001, Moisan and Fryxell 1993, Estrada and Delgado 1990) and Prydz Bay (Kang and Fryxell 1991), but similar to Maxwell Bay (Ahn et al. 1997), Bransfield Strait and Bellingshausen Sea (Bidigare et al. 1996) (Table 4).
The abundance values shown by different authors should be compared with caution, as different methods may have been used. In this work, cells smaller than 20 μm were disregarded. Conversely, the authors cited above took into account micro (>20 μm) and nanoplankton (2–20 μm) (Brandini et al. 1997). This may be the cause of the higher values found by these authors compared to those found in this work. Another reason may be that the sampling was carried out before and after but not within the high primary productivity season in the mid-summer...
The absence of strong water currents that resuspend the sediment allow the microalgae to sink to the bottom of the euphotic zone, causing low phytoplankton abundance (≤103 cells l−1) in the water column at Admiralty Bay (Rakusa-Suszczewski 1980). Conversely, strong turbulence may have been present only at the shallowest station (St. A) at Hennequin Point during the 2002–03 early summer, and probably caused the highest phytoplankton abundance (104 cells l−1) and chlorophyll a concentrations (>1 μg l−1). These high phytoplankton abundance and chlorophyll a concentrations have also been reported previously by Brandini and Rebello (1994) at the same area.
With the exception of Hennequin Point, Admiralty Bay showed the highest phytoplankton abundance in late summer with a direct relation to the water temperature. El-Sayed and Fryxell (1993), Kang et al. (1997), Kopcsynska et al. (1998) and Kang et al. (2002) reported similar results from other areas in the Southern Ocean. The lowest phytoplankton abundance (<102 cells l−1) and chlorophyll a concentrations (0.22 ± 0.05 μg l−1) associated with the highest ammonia values (5.7 ± 2.9 μmol l−1) near the EACF area suggest a response from the environment to either water turbulence (Brandini and Rebello 1994) or human presence at the Brazilian station along with mammals and penguins that often use the Keller Peninsula throughout the summer. Further study is necessary to distinguish between these two influences.
Dominant species
The centric diatom Corethron pennatum and several species of the pennate diatom genus Fragilariopsis were dominant in Admiralty Bay in the summer of 2002/03, as found previously by Kopczynska (1993) in Admiralty Bay, by Kang and Fryxell (1991), and Kopczynska et al. (1995) in Prydz Bay.
The similarity between this flora and the one found in Bellingshausen Sea (Bidigare et al. 1996) and Bransfield Strait (Burkholder and Sieburth 1961) strengthens the hypothesis that the offshore waters from Bellingshausen Sea inflow through Bransfield Strait, as suggested by Lipski (1987) and Madejski and Rakusa-Suszczewski (1990). The Weddell Sea presents some similarities to Admiralty Bay as well, where diatoms such as Nitzschia lecointei Van Heurck and several species of the genera Fragilariopsis, Thalassiosira and Pseudo-nitzschia are most frequently found (Kang et al. 2001).
Some areas inside Admiralty Bay such as Hennequin Point and Botany Point have similar flora to those found in Maxwell Bay (King George Island) which is highly influenced by ice and benthic diatoms (Fragilaria striatula, Achnanthes brevipes, Fragilariopsis ritscheri) (Ahn et al. 1997). However, the processes that allow benthic diatoms in the water column in Admiralty Bay may be different. Instead of being detached from the sea ice only, these algae also may be carried from the bottom to the water column by upwelling water currents (Brandini and Rebello 1994; Gilbert 1991), or by low saline meltwater-sediment suspension (Pichlmaier et al 2004).
The influence of temporal variation on sea ice cover and phytoplankton populations was identified by the alternate dominance of pennate and centric diatoms. After a heavy winter during which most of Admiralty Bay froze (INPE 2002), the sea ice within the bay melted in approximately 2 months during the spring (November and December). This fast melting may have caused an increase of benthic and ice diatoms, such as Achnanthes brevipes, Cocconeis spp., Navicula directa (Smith) Ralfs, Fragilaria striatula in the water column. However, during late summer, there was an increase in the abundance of the centric diatoms Corethron pennatum, Actinocyclus actinochilus (Ehrenberg) Simonsen, and Thalassiosira ritscheri (Hustedt) Hasle in Hasle & Heimdal derived from open waters, possibly inflowing from Bellingshausen Sea (Rakusa-Suszczewski 1980).
This temporal variation of phytoplankton composition was very clear at Hennequin Point. During early summer, there was the dominance of the chain-forming pennate diatom Fragilariopsis ritscheri, suggesting the influence of sea ice melting or sediment resuspension, as this diatom is abundant at marginal ice zones (Kang et al. 2001), into the sea ice (Crosta et al. 2004), and in surface sediments (Mohan et al. 2006). Conversely, during late summer, Corethron pennatum was the most abundant species, as found at the other areas of the bay.
At Botany Point, the dominance of benthic pennate diatoms during early (Pleurosigma/Gyrosigma complex) and late summer (Fragilaria striatula) reflected the high influence of resuspended sediments (Pichlmaier et al 2004).
The dominance of centric diatoms (60%) at EACF and Ezcurra Inlet was caused basically by Corethron pennatum, which reached abundances up to 103 cells l−1, mainly in late summer.
C. pennatum is cosmopolitan and planktonic (Fryxell 1989), and it is dominant during low-productivity seasons in this area, especially in Bransfield (von Bodungen 1986), suggesting the influence of Bransfield waters in the bay. Also, C. pennatum usually shows high abundances (107 cells l−1) under ice cover and at ice edges (Moisan and Fryxell 1993; Kang and Fryxell 1991; Garrison 1991).
The dominance of C. pennatum, which was present at the four sampling areas (88% of all samples) with relatively high abundance (abundant in 68% of all samples), was previously reported in Admiralty Bay (Kopcsynska 1993) and areas adjacent to King George Island, such as Marian Cove at Maxwell Bay (Kang et al. 1997).
For the reasons mentioned above, it is suggested that C. pennatum is an important component for primary production and maintenance of the food web at Admiralty Bay. Key organisms like krill (Euphausia superba) preferably feed on this microalga (Schultes et al. 2006; Granéli et al. 1993). Some diatom taxa identified in this study of Admiralty Bay, such as Fragilariopsis, Licmophora, and Thalassiosira, are commonly grazed by krill as well (Schultes et al. 2006; Ligowski 2000).
Admiralty Bay’s phytoplankton is dominated by diatoms, and highly influenced by benthic species, derived from the sediment resuspension or from the sea ice melting.
References
Ahn I-Y, Chung H, Kang J-S (1997) Diatom composition and biomass variability in nearshore waters of Maxwell Bay, Antarctica, during the 1992/1993 austral summer. Polar Biol 17:123–130
ATCM XXVIII Working Paper: review of the Admiralty Bay Antarctic Specially Managed Area management plan (ASMA no 1). Brazil and Poland, p 32
Bidigare RR, Iriarte JL, Kang S-H, Karentz D, Ondrusek ME, Fryxell GA (1996) Phytoplankton: quantitative and qualitative assessments. In: Foundations for ecological research west of the Antarctic Peninsula. Antarctic Research Series 70:173–198
Bodungen B (1986) Phytoplankton growth and krill grazing during spring in the Bransfield Strait, Antarctica—implications from sediment trap collections. Polar Biol 6(3):153–160
Brandini FP, Rebello J (1994) Wind effect on hydrography and chlorophyll dynamics in the coastal pelagial of Admiralty Bay, King George Island, Antarctica. Antarct Sci 6(4):433–442
Brandini FP, Lopes RM, Gutseit KS, Spach HL, Sassi R (1997) Planctonologia na plataforma continental brasileira—Diagnose e revisão bibliográfica. MMA/CIRIM/FEMAR
Burkholder PR, Sieburth JM (1961) Phytoplankton and Chlorophyll in the Gerlache and Bransfield Straits of Antarctica. Limnol Oceanogr 6(1):45–52
Crosta X, Sturm A, Armand L, Pichon J-J (2004) Late Quaternary sea ice history in the Indian sector of the Southern Ocean as recorded by diatom assemblages. Mar Micropaleontol 50:209–223
Cupp EE (1943) Marine plankton diatoms of the West of North America. University of California Press, California, p 235
El-Sayed SZ, Fryxell GA (1993) Phytoplankton. In: Antarctic microbiology, pp 65–122
Estrada M, Delgado M (1990) Summer phytoplankton distributions in the Weddell Sea. Polar Biol 10:441–449
Fryxell GA (1989) Marine phytoplankton at the Weddell Sea ice edge: seasonal changes at the specific level. Polar Biol 10:1–18
Garrison DL (1991) Antarctic sea ice biota. Am Zool 31(1):17–34
Gilbert NS (1991) Microphytobenthic seasonality in near-shore marine sediments at Signy Island, South Orkney Islands, Antarctica. Estuarine. Coast Shelf Sci 33(1):89–104
Granéli E, Granéli W, Rabbani MM, Daugbjerg N, Fransz G, Cuzin-Roudy J, Alder VA (1993) The influence of copepod and krill grazing on the species composition of phytoplankton communities from the Scotia-Weddell Sea. Polar Biol 13:201–213
Grasshoff K, Ehrhardt M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Verlag Chemie, Weinheim, p 419
Hasle GR, Syverten EE (1997) Marine diatoms, Chap 2 In: Tomas CR (ed) Identifying marine phytoplankton. Academic Press, St. Petersburg, pp 5–385
INPE (2002) http://www.cptec.inpe.br/antartica/antartica.shtml
Kang S-H, Fryxell GA (1991) Most abundant diatom species in water column assemblages from five leg 119 drill sites in Prydz Bay, Antarctica: distributional patterns. In: Barron J et al (eds) Proceedings of the ocean drilling program, scientific results 119:645–666
Kang S-H, Kang J-S, Chung K-H, Lee M-Y, Lee B-Y, Chung H, Kim Y, Kim D-Y (1997) Seasonal variation of nearshore antarctic microalgae and environmental factors in Marian Cove, King George Island, 1996. Korean J Polar Res 8(1,2):9–27
Kang S-H, Kang J-S, Lee S, Chung KH, Kim D, Park MG (2001) Antarctic phytoplankton assemblages in the marginal ice zone of the northwestern Weddell Sea. J Plankton Res 23(4):333–352
Kang J-S, Kang S-H, Lee JH, Lee S (2002) Seasonal variation of microalgal assemblages at a fixed station in King George Island, Antarctica, 1996. Mar Ecol Prog Ser 229:19–32
Kopcsynska EE, Fiala M, Jeandel C (1998) Annual and interannual variability in phytoplankton at a permanent station off Kerguelen Islands, Southern Ocean. Polar Biol 20:342–351
Kopczynska EE (1993) Net phytoplankton annual cycle (February 1990–January 1991) in Admiralty Bay, King George Island, West Antarctic. Pol Polar Res 14(4):383–392
Kopczynska EE, Goeyens L, Semeneh M, Dehairs F (1995) Phytoplankton composition and cell carbon distribution in Prydz Bay, Antarctica: relation to organic particulate matter and its δ13C values. J Plankton Res 17(4):685–707
Ligowski R (2000) Benthic feeding by krill, Euphausia superba Dana, in coastal waters off West Antarctica and in Admiralty Bay, South Shetland Islands. Polar Biol 23:619–625
Ligowski R, Kopczÿnska EE (1993) Phytoplankton. In: The marine antarctic coastal ecosystem of Admiralty Bay, pp 45–48
Lipski M (1987) Variations of physical conditions, nutrients and chlorophyll a contents in Admiralty Bay (King George Island, South Shetland Islands). Pol Polar Res 8:307–332
Lobo L, Leighton G (1986) Estructuras comunitarias de las fitocenosis planctonicas de los sistemas de desembocaduras de rios y esteros de la zona central de Chile. Revista Biología Marina 1(22):1–29
Madejski P, Rakusa-Suszczewski S (1990) Icebergs as tracers of water movement in the Bransfield Strait. Antarct Sci 2(3):259–263
Medlin LK, Priddle J (1990) Polar marine diatoms. British Antarctic Survey, Natural Environment Research Council, p 214
Mohan R, Shanvas S, Thamban M, Sudhakar M (2006) Spatial distribution of diatoms in surface sediments from the Indian sector of Southern Ocean. Curr Sci 91(11):1495–1502
Moisan TA, Fryxell GA (1993) The distribution of antarctic diatoms in the Weddell Sea during austral winter. Botanica Marina 36:489–497
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford, p 173
Peragallo MH, Peragallo M (1921) Diatomées d’eau douce et diatomées d’eau salée. In: Charcot J (ed) Deuxième Expedition Antarctique Française, Masson et Éditeurs, Saint-Germain, Paris (VI):41–102
Pichlmaier M, Aquino FE, Da-Silva CS, Braun M (2004) Suspended sediments in Admiralty Bay, King George Island (Antarctica). Braz Antarct Res 4:77–85
Platt T, Broomhead DS, Sathyendranath S, Edwards AM, Murphy EJ (2003) Phytoplankton biomass and residual nitrate in the pelagic ecosystem. Proc R Soc Lond A 459:1063–1073
Rakusa-Suszczewski S (1980) Environmental conditions and the functioning of Admiralty Bay (South Shetland Islands) as part of the near shore Antarctic ecosystem. Pol Polar Res 1(1):11–27
Rakusa-Suszczewski S, Mietus M, Piasecki J (1993) Weather and climate. In: Rakusa-Suszczewski S (ed) The maritime coastal ecosystem of Admiralty Bay. Dept. Antarctic Biol, Polish Academy of Science, Warsaw, pp 19–25
Round FE, Crawford RW, Mann DG (1990) The diatoms biology & morphology of the genera. Cambridge University Press, Cambridge, p 747
Schultes S, Verity PG, Bathmann U (2006) Copepod grazing during an iron-induced diatom bloom in the Antarctic Circumpolar Current (EisenEx): I. Feeding patterns and grazing impact on prey populations. J Exp Mar Biol Ecol 338:16–34
Stark JS, Riddle MJ, Simpson RD (2003) Human impacts in soft-sediment assemblages at Casey Station, East Antarctica: spatial variation, taxonomic resolution and data transformation. Aust Ecol 28:287–304
Underwood AJ (1994) On Beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol Appl 4:3–15
Utermöhl H (1958) Perfeccionamento del metodo cuantitativo del fitoplancton. Comum Assoc Int Limnol Teor Apl 9:1–89
Acknowledgments
This project is part of a Research Network dedicated to local environmental impact studies in Admiralty Bay, funded primarily by the Brazilian Ministry of Environment (MMA), Ministry of Science and Technology (MCT), and National Research Council (CNPq). We are thankful to the logistical support provided by Interministerial Secretary for the Sea Resources (SECIRM). We thank Dr. Rosalinda Montone (IO-USP) for the oxygen analysis, Vitor Chiozzini and Glaucia Berbel (IOUS-USP) for the salinity and nutrients analysis, Dr. Rodolfo Paranhos (UFRJ) for the chlorophyll a analysis, and Rafael B de Moura for drawing the map of Admiralty Bay. We are also very grateful to Dr. Silvia Nascimento (UENF), Dr. Frederico Brandini (UFPR), Dr. Elzbieta Kopczýnska and two other unidentified reviewers for their useful suggestions to this manuscript, and to Alberto Bezerril and Martha Villac for the English review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, P.K., Tenenbaum, D.R., de Santis Braga, E. et al. Microphytoplankton assemblages in shallow waters at Admiralty Bay (King George Island, Antarctica) during the summer 2002–2003. Polar Biol 30, 1483–1492 (2007). https://doi.org/10.1007/s00300-007-0309-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0309-8