Abstract
Key message
CRISPR/Cas9-mediated introduction of a single base mutation in SOC1, a transcription factor that regulates flowering time, results in late-bolting phenotypes in lettuce.
Lettuce is a widely consumed leafy vegetable crop. One of the molecular approaches that can increase leaf yield of lettuce is to delay the onset of flowering. Flowering time or time-to-bolting is not only a valuable trait for lettuce, but also a sought-after phenotype for other leafy vegetable crops. This is because delayed flowering enables more extensive vegetative growth, which leads to higher leaf numbers, and possibly larger leaves. Here, we deployed the most recent gene-editing technique to reduce the expression of SOC1, which is a gene that encodes one of several transcription factors that regulate the onset of flowering in plants. By inducing a single base mutation in SOC1 through Cas9 protein-gRNA ribonucleoproteins (RNPs) system, we showed that the time to first flower bud formation in lettuce is longer than that of wild type. In addition, expression of the floral regulatory genes including LsLFY, LsFUL, LsAPL1, and LsAPL2, was lower in the SOC1 gene edited plants than that of the wild type. The gene-editing technique established in this study could be directly applied for diverse quality improvement of lettuce by direct RNP transfer from protoplasts. Furthermore, it is expected that direct RNP transfer from protoplasts can be used as a useful mean for developing various gene edited crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
New breeding technologies have been used to generate improved crop cultivars (Chen et al. 2019). One example is protoplast-based gene editing, which employs somatic hybridization, cytoplasmic recombination, micronucleus transfer, direct DNA uptake, and mutation selection (Schaart et al. 2016). In the past decade, genome editing has rapidly gained prominence as a method of choice for molecular crop breeding. Zinc-finger nucleases (ZFNs) and activator-like effector nucleases (TALENs) were the first gene-editing tools to have been successfully applied for new crop cultivar development. The use of these tools has since been surpassed by a more recent genome-editing tool that employs RNA-guided endonucleases (RGENs) (Nekrasov et al. 2013), which consist of a guide RNA (gRNA) and clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (Cas9) derived from Streptococcus pyogenes. The gRNA hybridizes with a 20-base pair target DNA sequence. Cas9 is the most popular and widely used RGEN and recognizes the NGG trinucleotide sequence known as the protospacer-adjacent motif (PAM). The introduction of these endonucleases into plant cells enables rapid and accurate editing of target genes by cleaving DNA that is complementary to that of the gRNA, resulting in sequence-specific double-strand breaks (DSBs), whose repair by endogenous repair systems leads to sequence-specific changes in the targeted gene. DSBs are repaired by homologous recombination (HR) and non-homologous end joining (NHEJ). Various modifications of the targeted sequence such as small deletions or insertions occur during repair of DSBs by the NHEJ pathway. When a DNA fragment with a homologous sequence to the target gene exists, gene replacement or correction is possible through HR.
Lettuce (Lactuca sativa) is an important vegetable crop that is cultivated for its edible leaves. In recent years, lettuce has gained increased attention because of its high commercial value and mechanized cultivation through indoor farming. Among the desired horticultural traits of lettuce, late bolting or flowering is one that is directly related to productivity and quality. Early flowering not only causes the deterioration of leaf quality but also shortens the harvest period. Therefore, late flowering is an important horticultural trait for improving the quality of lettuce.
Previous studies reported that SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), which is a MADS-box transcription factor, plays a crucial role in regulating multiple flowering pathways (Lee and Lee 2010; Li et al. 2008). SOC1 controls flowering time by integrating signals from four flowering pathways, including the photoperiod pathway, the vernalization pathway, autonomous floral induction, and gibberellin-related pathways. The main floral integrators, FLOWERING LOCUS T (FT)-FLOWERING LOCUS D (FD) dimer and SOC1 activate central meristem identity genes, such as FRUITIFUL (FUL), APETALA1 (AP1), and LEAFY (LFY) to initiate the floral transition (Kim 2020). SOC1 also induces heat-promoted bolting in lettuce (Chen et al. 2018). Therefore, genetic modification of SOC1 could be a suitable strategy for the creation of late-bolting commercial cultivars of lettuce.
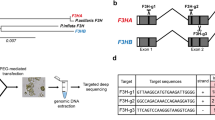
Here, we used the RGEN system to disrupt the L. sativa (Ls) homolog of the Arabidopsis thaliana SOC1 gene in lettuce protoplasts (Fig. 1A and Fig. S1). Two target sites of LsSOC1, corresponding to 20 bp sequences of LsSOC1 exon1 (target 1) and exon3 (target 2), were designed using the CRISPR RGEN tools (http://www.rgenome.net/cas-designer) (Park et al. 2015). Before sgRNA synthesis, the target LsSOC1 gene sequence of cultivar Cheongchima was confirmed because different cultivars within the same species have different genomes. The annotated sequences (Fig. S2) at the target regions revealed that the sequencing data from genomic DNA PCR analysis were identical. The two designed gRNAs of LsSOC1 were then synthesized and tested using an in vitro cleavage assay (Fig. S3). After showing that sgRNAs worked properly, the Cas9 protein and each of the two gRNAs were transfected into lettuce mesophyll protoplasts using the polyethylene glycol (PEG) method. When only Cas9 was transfected into protoplasts, no mutations were found in target 1 and target 2. Mutation frequency in target 1 and target 2 reached 7.5% and 1.5%, respectively, when the entire RNP complex was transfected into protoplasts (Fig. 1B and Fig. S4). Based on the recorded mutation frequencies, target 1 gRNA was selected and used to generate gene-edited lettuce plants. The micro-calli derived from RNP-transfected protoplasts were transferred into a shoot induction medium (Supplementary Methods). Adventitious shoots that formed from the calli were carefully excised and transferred onto half-strength Murashige and Skoog (MS) basal medium for root induction (Fig. S1). Sixty-six independent T0 plants were obtained and genotyped by direct sequencing. Primers were designed around the LsSOC1 target 1 region. The DNA fragments from each line were amplified by PCR and sequenced. Sequencing revealed mutations in 9 of the 66 independent transgenic plants. This result indicated a mutation efficiency of 13.64% at the whole plant level. All sequencing chromatograms of PCR products from the nine mutants (i.e., CR-lssoc1 #3, #36, #37, #38, #43, #51, #60, #64, #65) showed double-peaks starting at the target region, which suggested that these were heterozygotes. The PCR amplicon obtained from each line was cloned, and six to ten individual clones were sequenced (Fig. S5). A single mutation was detected in all nine plants, which indicated a heterogeneous pattern. The mutation was a single nucleotide (T, thymine) insertion that led to a premature stop codon in the LsSOC1 amino acid sequence.
Development of late-flowering plants from mesophyll protoplast of lettuce by CRISPR/Cas9-mediated knockout of the LsSOC1 gene. A Schematic illustration of LsSOC1 and the corresponding target sequences. Each target region is shown in green letters followed by the protospacer adjacent motif (PAM) (NGG; red). B Insertion/deletion (Indel) frequencies at the LsSOC1 target sites upon delivery of Cas9 and each gRNA (gRNA 1 or gRNA 2) as RNP complexes into lettuce protoplasts. The protoplast samples were collected 48 h after PEG transfection. C: Mutant DNA sequences induced by RGEN RNPs in a T1 plant, a homozygous biallelic mutant called CR-lssoc1 #37. The target region is shown in green letters followed by the PAM (NGG; red) and the inserted thymine (T) nucleotide in blue. D Sanger-sequencing electropherograms showing a 1 bp addition at the LsSOC1 target1 site of the T1 plant, CR-lssoc1 #37. E Phenotypes of wild-type (WT) and CR-lssoc1 T2 plants. Bar = 2 cm. F Days to first flower bud of WT and CR-lssoc1 T2 plants. G: The relative gene expression level of flower regulatory genes, LsLFY, LsAPL1, LsAPL2, and LsFUL. Quantitative RT-PCR on total RNA from WT and CR-lssoc1 T2 plants. Values are means ± standard deviation (SD) of three biological replicates. Significant differences are determined by Welch's t test (*p < 0.05, **p < 0.01)
Putative off-target sites of LsSOC1 target 1 in T0 plants were sequenced (Table S2). Four potential off-target sites were identified using the Cas-OFFinder online tool (http://www.rgenome.net/cas-offinder/) (Bae et al. 2014). One mismatch and two mismatches of LsSOC1 target 1 did not exist in the lettuce genome, and four with three mismatches were found on chromosome 3 (position: 244,338,284–244,338,262), chromosome 4 (position: 190,700,634–190,700,656), chromosome 5 (position: 193,037,393–193,037,415), and chromosome 7 (position: 140,167,052–140,167,030). There were no mutations in any of these four off-targets (Fig. S6). These results indicated that LsSOC1 target 1 was a suitable region that has greatly reduced off-target effects.
T1 seeds were obtained from a fully grown heterozygous mutant. The T1 generation was screened to identify homozygous lines harboring LsSOC1 mutations. Segregation of homozygotes, heterozygotes, and wild type was observed in the T1 generation. The mutant allele was transmitted to the next generation, which led to the identification of homozygote mutants (CR-lssoc1 #37, Fig. 1C). The mutation gave rise to a premature stop codon in the LsSOC1 amino acid sequence (Fig. 1D). Because only plants with the same type of mutation were obtained, phenotypes of ten independent T2 lines (i.e., ten progeny from CR-lssoc1 #37) were examined. These mutants had a late-bolting phenotype (Fig. 1E and Fig. S7). The phenotype of the CR-lssoc1 mutants at the early growth stage was not significantly different from that of the wild type. However, the length of CR-lssoc1 mutant stems was shorter than that of the wild type at the flowering stage. Previous studies have shown that SOC1 mutants exhibit delayed flowering and short primary stems due to the delay in floral transition (Chen et al. 2018; Lee and Lee 2010). The appearance of the first flower bud took 113.7 days in the mutants, whereas it took 108 days in the wild type. The times to flowering of CR-lssoc1 mutants were significantly longer than that of the wild type (p < 0.01) (Fig. 1F). LFY, FUL, and AP1 are well-known downstream genes of SOC1 to regulate the time of flower formation (Kim 2020). Expression of the flower regulatory genes, LsLFY and LsFUL (Kim 2020), was lower in the mutant than in the wild type (Fig. 1G). Furthermore, the expression of other genes involved in flowering, such as LsAP-LIKE (APL)1 and LsAPL2, was lower than that of the control group. These results are consistent with the report that LsLFY gene expression is lower in LsSOC1-RNA interference (RNAi) lines than in the control lines (Chen et al. 2018). The data presented here confirm that the LsSOC1 gene is a positive regulator of flowering time in lettuce. SOC1 integrates flowering signals induced by sunlight, temperature, hormones, and aging, and regulates the activities of various flowering promoters and inhibitors. Thus, mutation of SOC1 could be used to develop late-flowering phenotypes of other vegetable crops. In the case of Chinese cabbage, its marketability is greatly decreased if the number of leaves are reduced by early flowering. In particular, since spring and winter cabbages are highly likely to be exposed to low temperatures, late-flowering characteristics are essential to increase productivity. In addition, the harvest time of horticultural crops could be controlled by manipulating flowering time, independently of changes in the surrounding climate. Therefore, the introduction of the SOC1 mutation into other crops could increase their production.
The regulatory requirements of some countries for handling genome-edited crops are not as stringent as those for genetically modified organisms (GMOs) because there is no external foreign DNA in genome-edited plants. Therefore, RNP-transformed lettuce seeds from the genome-edited lettuce lines described here are expected to be of commercial value. Here, we used a genome-editing method to breed new lettuce cultivars with mutation of SOC1, a transcription factor that regulates flowering time. The introduction of a single base mutation into SOC1 resulted in late-bolting phenotypes. As reported previously for other crops, genome editing in protoplasts is advantageous for the development of genetically improved crops. Tomatoes with high amounts of γ-aminobutyric acid (GABA) developed with CRISPR–Cas9 technology are being commercially marketed for the first time (Waltz 2022). There are many desirable plant phenotypes resulting from RNAi- or transfer (T)-DNA insertion-induced mutations to specific genes. Similar to the LsSOC1 gene described here, these genes present ideal targets for developing genome-edited crop cultivars free of foreign DNA using CRISPR/Cas9.
References
Bae S, Park J, Kim J-S (2014) Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475
Chen Z, Zhao W, Ge D, Han Y, Ning K, Luo C, Wang S, Liu R, Zhang X, Wang Q (2018) LCM-seq reveals the crucial role of LsSOC1 in heat-promoted bolting of lettuce (Lactuca sativa L.). Plant J 95:516–528
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70:667–697
Kim D-H (2020) Current understanding of flowering pathways in plants: focusing on the vernalization pathway in Arabidopsis and several vegetable crop plants. Hortic Environ Biotechnol 61:209–227
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 15:110–120
Nekrasov V, Staskawicz B, Weigel D, Jones JD, Kamoun S (2013) Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31:691–693
Park J, Bae S, Kim J-S (2015) Cas-designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 31:4014–4016
Schaart JG, van de Wiel CCM, Lotz LAP, Smulders MJM (2016) Opportunities for products of new plant breeding techniques. Trends Plant Sci 21:438–449
Waltz E (2022) GABA-enriched tomato is first CRISPR-edited food to enter market. Nat Biotechnol 40:9–11
Funding
This work was supported by the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5282223) and the New Breeding Technologies Development Program (Project No. PJ016530), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
SHC, EYJ, HC and SWK designed the experiments and performed data analyses; SHC and WSA performed the experiments; SHC and SWK wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, S.H., Ahn, W.S., Jie, E.Y. et al. Development of late-bolting plants by CRISPR/Cas9-mediated genome editing from mesophyll protoplasts of lettuce. Plant Cell Rep 41, 1627–1630 (2022). https://doi.org/10.1007/s00299-022-02875-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-022-02875-w