Abstract
The CRISPR system enables us to induce precisely targeted mutations in a plant genome. The widely used CRISPR system is composed of a Cas9 protein derived from Streptococcus pyogenes (SpCas9) and a target site-specific guide RNA. In this study, we successfully generated the early-flowering Chinese cabbage (Brassica rapa spp. pekinensis), which is one of the most important vegetables in the world. To generate early-flowering B. rapa without requiring vernalization, we designed seven guide RNAs which target B. rapa homologous genes to the Arabidopsis thaliana FLOWERING LOCUS C (FLC). We first examined the indel mutation efficacy of the designed guide RNAs in protoplasts isolated from young leaves of Kenshin (an inbred line of B. rapa). After selecting four guide RNAs, genome-edited plants were established by delivering the plant binary vectors harboring SpCas9 along with respective guide RNAs into B. rapa hypocotyl explants. In the T0 generation, we found BraFLC2 and BraFLC3 double knockout lines with the indel efficiency of 97.7% and 100%, respectively. The simultaneous mutations of both BraFLC2 and BraFLC3 were inherited in T1 generations with 100% of indel efficiency. The edited lines obtained showed an early-flowering phenotype that did not depend on vernalization. This study provides a practical gene-editing protocol for Chinese cabbage and verifies the function of its multi-copy BraFLC genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The efficacy of the CRISPR system can be examined by generating gene-edited animals and plants. Hundreds of guide RNAs are easily designed for Streptococcus pyogenes Cas9 protein (SpCas9) even when only one gene is knocked out, but gene-editing efficacy differs depending on the loci of guide RNA-binding sites (Doench et al. 2014; Hinz et al. 2015; Isaac et al. 2016; Wilson et al. 2018). For plant genome editing, highly efficient guide RNAs should be selected, because the whole plant transformation process may take from several months to a year. Recently, a deep-learning algorism has been developed to predict the gene-editing efficacy of guide RNAs for Cas12a (Cpf1) system (Kim et al. 2018). However, it is still hard to predict the efficacy of guide RNAs in silico for SpCas9, which is extensively used for plant genome editing. Therefore, it would be better to validate the gene-editing efficacy of guide RNAs in interested plants, before plant transformation and regeneration occur.
Chinese cabbage (Brassica rapa spp. pekinensis), an important vegetable crop, has been cultivated for many centuries. Late-flowering cultivars have been selected to increase crop productivity as in other leafy vegetables (Jung and Müller 2009; Blümel et al. 2015). In addition, many late-flowering B. rapa require cold treatment for several weeks to induce flowering and ultimately to harvest seeds (Osborn et al. 1997; Schranz et al. 2002). However, the late-flowering trait has several disadvantages for researchers and breeders who want to do forward and reverse genetics with B. rapa. Therefore, an early-flowering B. rapa that does not require vernalization is highly desirable as it allows scientist to analyze gene function for plant development, physiology, and ecology in Chinese cabbage (Williams and Hill 1986).
The molecular basis of vernalization-induce flowering has been well studied in the model plant, Arabidopsis thaliana (Blümel et al. 2015). Long-term cold treatment reduces the level of FLC transcripts, which accelerates the flowering of Arabidopsis (Michaels and Amasino 1999). Natural Arabidopsis mutants in the FLC gene show early-flowering phenotypes that do not require vernalization. Therefore, we hypothesized that mutations in B. rapa homologous genes to Arabidopsis FLC were supposed to generate an early flowering B. rapa (Kim et al. 2007). There are four B. rapa FLC genes (BraFLC1, BraFLC2, BraFLC3, and BraFLC5) with high sequence similarity to the Arabidopsis FLC gene (Schranz et al. 2002b; Kim et al. 2007). However, little is known about which genes are important in vernalization or whether there is functional redundancy among the multiple BraFLC genes (Kim et al. 2007). Hence, the purpose of this study was to introduce the targeted mutation into FLC genes using the CRISPR system and to generate an early flowering in Chinese cabbage without requiring vernalization.
Materials and methods
Plant materials and growth conditions
Chinese cabbage inbred line, Kenshin (Brassica rapa L. ssp. pekinensis) (Lee et al. 2013) was used in this study. The mature seeds were sterilized in 2% active chloride for 10 min, and rinsed eight times with sterilized distilled water. The seeds were placed in glass bottles (height 15 cm, diameter 7 cm) containing half-strength MS medium (Murashige and Skoog 1962, Duchefa Biochemie, Netherlands) containing vitamins, 30 g/L sucrose (Duchefa Biochemie) and 8 g/L phytoagar (Duchefa Biochemie). Glass bottles with seeds were maintained under long day (16 h:8 h, light:dark) conditions in a growth room at 25 ± 1 °C for 3 days, and seed-containing bottles were transferred to a growth room under dark conditions for 7 days.
Chinese cabbage transformation
After removing cotyledons and root parts, dark-grown hypocotyls were cut into 0.7–1.0 cm segments, and then kept under long day (16 h:8 h, light:dark) conditions in a growth room at 25 ± 1 °C for 3 days. We used our plant binary vector system (pHAtC) (Kim et al. 2016) harboring 35S::SpCas9 and U6::guide RNA for Agrobacterium-mediated transformation. The following compositions of YEP medium (all from Duchefa Biochemie) were used for the growth of Agrobacterium (LBA4404); 50 mg/L streptomycin, 50 mg/L spectinomycin, 50 mg/L rifampicin in 50 mL of YEP medium. Cell cultures were grown overnight at 250 rpm on a rotary shaker at 28 °C until the culture reached the final concentration of OD600nm = 0.6. Before transformation, Agrobacterium was washed three times with 30 mL of YEP medium. The following conditions were used for centrifugation: 22 °C, 5000 rpm for 15 min.
Agrobacterium was finally resuspended with 25 mL of YEP medium and poured into a Petri dish. The chopped hypocotyl explants were added to the Petri dish containing Agrobacterium and gently shaken for 10 min. The following composition of co-culture medium was used to incubate explants for 2 days under dark conditions at 25 ± 1 °C; 4.4 g/L of MS including vitamins (Duchefa Biochemie), 30 g/L of sucrose, 1 mg/L of NAA, 4 mg/L of BA, 4 mg/L of AgNO3, 10 mg/L of acetosyringone, and 7.5 g/L of agar, pH 5.8. After 2 days, explants were washed with washing medium (4.4 g/L of MS including vitamins, 30 g/L of sucrose, 1 mg/L of NAA, 4 mg/L of BA, 4 mg/L of AgNO3, 250 mg/L of cefotaxime and 10 mg/L of acetosyringone, pH 5.8) for at least 5 times. Washed explants were again placed on sterilized filter paper and dried for a minute to reduce media contamination and transferred on callus induction media (4.4 g/L of MS including vitamins, 30 g/L of sucrose, 1 mg/L of NAA, 4 mg/L of BA, 4 mg/L of AgNO3, 250 mg/L of cefotaxime, 10 mg/L of acetosyringone, 10 mg/L of hygromycin and 8 g/L of agar, pH 5.8). The cultures were subcultured once per 1–2 weeks until the explants exhibited a leaf-like organ. The regenerated shoot parts were transferred into maturation medium (4.4 g/L of MS including vitamins, 30 g/L of sucrose, 250 mg/L of cefotaxime, 10 mg/L of hygromycin and 8 g/L of agar, pH 5.8). The cultures were maintained on the same maturation medium every 2 weeks until several leaves were regenerated. When multiple shoots were regenerated, this shoot part was transferred to rooting media (2.2 g/L of MS including vitamins, 30 g/L of sucrose and 10 g/L of agar, pH 5.8).
In vitro guide RNA synthesis
IVT primers (F: T7 promoter + ‘G’ + 18-20 bp of target-binding sites + universal, R: universal + guide RNA scaffold, see Supplemental Table 1) were designed for in vitro guide RNA synthesis. Polymerase chain reaction (PCR) was performed to amplify a template DNA for guide RNA synthesis in total 50 µL of reaction mixture containing 1 µL of 100 µM forward primer, 1 µL of 100 µM reverse primer, 10 µL of 5X buffer, 1 µL of 10 mmol dNTPs, and 0.5 µL Phusion™ DNA polymerase (Thermo Scientific, USA). The following PCR conditions were used to amplify template DNA: 98 °C for 30 s, followed by 40 cycles of 98 °C for 10 s, 54 °C for 20 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR product was analyzed using 1.5% agarose gel, and PCR purification was done using Expin™ PCR SV kit (Gene All, Korea).
After PCR purification, guide RNAs were transcribed in vitro using T7 RNA polymerase (New England Biolabs, USA) with the following conditions: in total 100 µL of reaction mixture containing 1.5 µg of PCR products, 4 µL of 100 mM ATP, 4 µL of 100 mM UTP, 4 µL of 100 mM CTP, 4 µL of 100 mM GTP, 28 µL of MgCl2 (50 mM), 10 µL of 10X buffer, 10 µL of 100 mM DTT, 2.5 µL of RNase inhibitor, and 10 µL of T7 RNA polymerase. This mixture was incubated overnight at 37 °C to maximize RNA transcription efficiency. After overnight synthesis, 10 µL of DNase I (New England Biolabs) was added to the mixture and kept at 37 °C for 30 min to remove template DNA in the mixture. The synthesized RNA concentration was quantified by nano-drop (Thermo Scientific, USA).
Protoplast isolation and transfection
Protoplast isolation was performed using 7-day-old seedlings as previously described (Woo et al. 2015; Kim et al. 2016, 2017). The ribonucleoprotein (RNP) premixture with 30 µg of SpCas9 protein and 80 µg of sgRNA was prepared in a Falcon tube (14 mL) and stabilized for 30 min at room temperature. Approximately, 2 × 105 protoplast cells were mixed with RNP premixture and suspended slowly with 300 µL of MMG solution (0.4 mM of mannitol, 15 mM of MgCl2 and 4 mM of MES, pH 5.7) followed by the addition of the equal volume of PEG solution (40% of PEG 4000, 0.2 M of mannitol and 0.1 M of CaCl2). In addition, the mixture was then incubated at 22 °C for 24 h. After incubation, protoplasts were washed at least three times with an equal volume of W5 solution (154 mM of NaCl, 125 mM of CaCl2, 5 mM of KCl, 5 mM of glucose and 2 mM of MES).
Genomic DNA extraction and in vitro cleavage assay
The genomic DNA of Chinese cabbage was extracted with DNeasy Plant Mini Kit (Qiagen, USA) and used as a template for amplifying DNA fragments containing guide RNA-binding sites. The PCR reaction was performed with the following parameters: 98 °C for 30 s, followed by 35 cycles of 98 °C for 10 s, 65 °C for 15 s, 72 °C for 15 s, and a final extension at 72 °C for 5 min. The primers used for amplifying guide RNA-binding products (BraFLC1-F, TCTCTGCCCTATACATGTTCCA; BraFLC1-R, ACATGTGTTTTACCCACTCCT; BraFLC2-F, TCTCCGGCGAGAGTTGAAAC; BraFLC2-R, ACCGAGAAATCCACATGCGA; BraFLC3-F, CCTTGTGTCGAGAGCCTCAA; BraFLC3-R, CCAAAATGCCCTAATCTCGACA; BraFLC5-F, ACCTCTCGGAGACAGAAGCT; BraFLC5-R, GCTCATCACAACATTGTTCTTCCT) were designed by GENEIOUS program (https://www.geneious.com). An in vitro cleavage assay was performed in total 20 µL of reaction mixture containing 240 ng of DNA template, 2 µg of the SpCas9 protein (Toolgen, Korea), 1.5 µg of guide RNA and 2 µL of 10X NEB 3.1 buffer (NEB). The mixture was incubated at 37 °C for 1 h, and 4 µL of 20 mg RNase A (NEB) was added and incubated at 37 °C for 30 min. Finally, 6 µL of 5X STOP solution (1.2% of SDS, 12.5 mmol of EDTA) was added. DNA fragments were purified by Expin™ PCR SV kit (Gene All) and analyzed by 1.5% agarose gel electrophoresis.
Next-generation sequencing (NGS) analysis
Targeting regions of SpCas9 and guide RNA complex were amplified by three rounds of PCR. First, the genomic region containing guide RNA-binding sites was amplified with the size of approximately 1 kb. First-time PCR products were diluted at 1:100 or 1:1000 and used as a template for second-time PCR. Illumina adaptors and bar-code sequences were added to second- and third-time PCR products. Final PCR products were quantified by nano-drop (Thermo Scientific, USA) and purified with Expin™ PCR SV kit (Gene All). High-throughput sequencing was performed using Illumina MiSeq. Indel frequencies and mutation patterns were analyzed by CAS-Analyzer (Park et al. 2017).
Results
Protein alignment of putative FLC homologous genes in Chinese cabbage
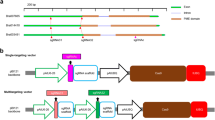
The full lengths of four putative FLC homologous genes in Chinese cabbage (Kim et al. 2007; Schranz et al. 2002) were isolated by BLAST search against the genome sequence of cv. Kenshin cultivar using the Arabidopsis FLC (At5g10140) protein sequence (Fig. 1). Gene names are based on the sequence similarity between B. rapa and Brassica napus FLCs. As previously reported (Schranz et al. 2002), we did not find the homologous gene of B. napus FLC4 in B. rapa. The protein sequence of BraFLC1, BraFLC2, and BraFLC3 showed high similarity to the protein sequence of Arabidopsis FLC (BraFLC1, Identifies = 81%, Positives = 87%, Gaps = 5%; BraFLC2, Identifies = 84%, Positives = 91%, Gaps = 2%; BraFLC3, Identifies = 83%, Positives = 90%, Gaps = 1%). BraFLC5 had 58% identical amino acid sequence (Positives = 62%, Gaps = 27%) and no 50 amino acids in the middle region when compared with Arabidopsis FLC. Since the BraFLC proteins showed high protein similarity to Arabidopsis FLC, we expected functional redundancy among BraFLC proteins (Kim et al. 2007). Therefore, we decided to generate single- or double-knockout mutants of BraFLC genes simultaneously via conserved guide RNAs.
Protein alignment of putative Arabidopsis FLC orthologs in Brassica rapa. Full-length amino acid sequences were aligned using the Geneious software V (https://www.geneious.com). The accession number of Arabidopsis thaliana FLC is At5g10140. FLC, flowering locus C
Testing guide RNA efficiency in protoplasts isolated from Chinese cabbage
Among various CRISPR systems (Hsu et al. 2014; Sander and Joung 2014; Sternberg and Doudna 2015; Puchta 2017), engineered Cas9 and guide RNA originated from S. pyogenes (Jinek et al. 2012) has been widely used for plant genome editing. To introduce insertion and deletion (indel) mutations in the Chinese cabbage FLC genes, we designed a total of seven guide RNAs (Fig. 2a): guide RNA1 (gRNA1) targets the first exon of the BraFLC1 and BraFLC2 genes; both gRNA2 and 3 target the second exon of the BraFLC1 and BraFLC2 genes; gRNA4 targets the first exon of BraFLC2 and BraFLC3 genes; gRNA5 targets the fifth exon of BraFLC2 and BraFLC5 genes; and both gRNA6 and gRNA7 target the BraFLC5 gene. We first performed the in vitro cleavage assay to examine the activity of the pre-assembled ribonucleoprotein (RNP) complex, consisting of in vitro synthesized guide RNAs and recombinant SpCas9 proteins purified from E. coli. This CRISPR RNP complex cleaved PCR products containing the guide RNA target site (Fig. 2b).
In vivo activity of guide RNAs targeting BraFLCs. a Seven guide RNAs were designed to target one or two BraFLC genes. b An in vitro cleavage assay was performed to check the activity of the SpCas9 protein and synthesized guide RNA complex. c Indel (insertion and deletion) ratio (%) in B. rapa protoplasts induced by the SpCas9 and guide RNA complex. d Indel mutation patterns and ratio induced by the SpCas9 and gRNA4 complex. The mutation ratio (indel %) was calculated by dividing the number of reads containing indels at the target site (Read #) by the number of total sequencing reads (Total #). WT, wild-type sequence of the gRNA4-binding site; blue, the protospacer adjacent motif sequence; red, inserted or deleted sequence; + 1 and − 1 indicates one-base insertion and deletion, respectively, in the guide RNA-binding sequence
We then verified the genome-editing efficiency of the guide RNAs in vivo in Chinese cabbage protoplasts. Each CRISPR RNP complex was delivered into the protoplasts by PEG transfection, and 2 days later, the target regions were amplified by PCR. The indel frequency and mutation patterns of each guide RNA were analyzed by targeted deep sequencing (Fig. 2c). To consider the naturally occurring mutations and sequencing errors, the same guide RNA-targeted region was amplified and sequenced in the protoplasts harboring only the SpCas9 protein (control protoplasts). As expected, there were almost no mutations (less than 0.1% mutation) in the control protoplasts. Each RNP complex induced indel mutations at various efficacies ranging from 3 to 39%: 7.4% for BraFLC1 and 8.9% for BraFLC2 in gRNA1-transfected protoplasts; 3.4% for BraFLC1 and 10.0% for BraFLC2 in gRNA2-transfected protoplasts; 23.0% for BraFLC1 and 38.5% for BraFLC2 in gRNA3-transfected protoplasts; 6.6% for BraFLC2 and 6.0% for BraFLC3 in gRNA4-transfected protoplasts; 13.3% for BraFLC2 and 23.1% for BraFLC5 in gRNA5-transfected protoplasts; 3.6% for BraFLC5 in gRNA6-transfected protoplasts; and 12.0% for BraFLC5 in gRNA7-transfected protoplasts (Fig. 2c). Analysis of the mutation pattern revealed that a single-nucleotide insertion or deletion mainly occurred in the 3 bp upstream region of a PAM sequence (Fig. 2d).
Generation of BraFLC knockout mutants
To generate the knockout plants of BraFLC genes, we selected four guide RNAs (gRNA3, gRNA4, gRNA5, and gRNA7), all of which showed high efficiency in the protoplast assay, and cloned each guide RNA into a plant binary vector (pHAtC) (Kim et al. 2016) harboring SpCas9. We next delivered the binary vectors into B. rapa hypocotyl explants via Agrobacterium-mediated transformation. B. rapa seeds were germinated in a high-height glass bottle to get seedlings with a long hypocotyl (Fig. 3a). After cutting the hypocotyl into small pieces, the explants were inoculated with the binary vector-containing Agrobacterium (Fig. 3b), and antibiotic-resistant calli were selected on callus-inducing medium (Fig. 3c). Then, we regenerated shoot and root from the selected calli (Fig. 3d–f). The proportion of calli showing hygromycin resistance was about 1.3–5.6%. Six T0 regenerated plants were obtained from each gRNA2- and gRNA4-transformed calli, and a single T0 plant was obtained from each gRNA5- and gRNA7-transformed calli (Fig. 3g).
Transformation and regeneration of gene-edited Brassica rapa. a–f Regeneration procedure of B. rapa after delivering the plant binary vector harboring the SpCas9 and guide RNA by Agrobacterium-mediated transformation. g The number of explants used for Agrobacterium-mediated transformation and the number of T0-regenerated plants
Next, we analyzed the indel mutation frequencies in T0 plants. Unfortunately, we found no mutation on the target sites of gRNA3-, gRNA5-, and gRNA7-transformed T0 plants (data not shown). However, all gRNA4-transformed lines contained 50% or 100% mutations on the target sites (Fig. 4a). The SpCas9 and gRNA4 complex induced one-base insertion or deletion mutation at the target sites of BraFLC2 and BraFLC3 genes as observed in the protoplasts (Fig. 2d). For instance, Braflc2flc3-3 lines mainly had one-base (T) deletion (87%) at the cleavage site on BraFLC2 and one-base (T) deletion (49.8%) and one-base (T) insertion (41.6%) on BraFLC3. Braflc2flc3-4 lines mainly had one-base (T) deletion (46.7%) at the cleavage site on BraFLC2, and one-base (T) deletion (49.8%) and one-base (A) insertion (40.6%) on BraFLC3. The Braflc2flc3-9 line mainly had one-base (T) deletion (48.1%) and one-base (T) insertion (38.2%) on BraFLC2, and interestingly showed a large deletion of 23 bases (66.9%) and one-base (T) insertion on BraFLC3 (Fig. 4b).
Generation of T0BraFLC2 and BraFLC3 double-knockout plants. a The mutation ratios (indel frequency, %) in gRNA4-transformed T0 plants. b Indel mutation patterns and ratios in gRNA4-transformed T0 plants. The mutation ratio (indel %) was calculated by dividing the number of reads containing indel at the target site (Mutation Read #) by the number of total sequencing reads (Total #). WT, wild-type sequence of the gRNA4-binding site; blue, the protospacer adjacent motif sequence; red, inserted or deleted sequence; + 1 and − 1 indicates one-base insertion and deletion, respectively, in the guide RNA-binding sequence. c Early-flowering phenotype in Braflc2flc3-10 line without vernalization
The B. rapa cv. Kenshin we used in this study requires long-term cold treatment (vernalization) to produce a flower. Whereas a wild-type B. rapa produced only leaves and no flower without vernalization, double-knockout mutants in BraFLC2 and BraFLC3 genes produced flowers without vernalization (Fig. 4c).
Heritability of Cas9-induced mutations in B. rapa
To check the heritability of the mutations and the edited trait, we germinated T1 seeds collected from all Braflc2flc3 T0 lines and performed the targeted sequencing. The progeny of Braflc2flc3-3 mutant follows the Mendelian segregation pattern: on the BraFLC2 target site, all Braflc2flc3-3 progeny (T1 plants) had one-base deletion (T), and on the BraFLC3 target site, the Braflc2flc3-3-1 mutant showed 92.9% of one-base deletion (T), the Braflc2flc3-3-2 mutant showed 92.7% of one-base insertion (T), and the Braflc2flc3-3-3 showed 54.1% of one-base deletion (T) and 39.4% of one-base insertion (T) (Fig. 5a). Those T1 double-knockout plants kept an early-flowering phenotype unlike wild-type plants (Fig. 5b).
Inheritability of CRISPR-induced mutations in Brassica rapa. a Indel mutation patterns and ratios in T1 progenies of T0Braflc2flc3-3 plant. b Early-flowering phenotype in Braflc2flc3-1 line without vernalization. c Phenotypes of wild-type and T1Braflc2flc3-4-3 lines without requiring vernalization. The monoallelic wild-type allele of BraFLC2 in biallelic BraFLC3 mutation background is enough to suppress flowering of B. rapa without requiring vernalization. d Indel mutation patterns and ratios in T1 progeny of T0Braflc2flc3-4 plant
Interestingly, we found phenotypic segregation in the progeny of Braflc2flc3-4. Some progeny of Braflc2flc3-4 showed a similar flowering phenotype with a wild-type B. rapa (Fig. 5c) and some progeny showed the early-flowering phenotype that did not require vernalization. The T1 mutant plants with similar phenotypes to the wild type have the 49.3% indel frequency on BraFLC2 and 92.5% indel frequency on BraFLC3 (Fig. 5d), suggesting that the expression of one copy of BraFLC2 gene is enough to suppress flowering.
Discussion
Homologous gene editing of B. rapa (Chinese cabbage)
In this study, we showed that the CRISPR system enables us to induce targeted mutation in B. rapa FLC genes and that the mutation pattern observed in parental lines is passed on to the next generation. To our knowledge, these Braflc2flc3 double-knockout lines are the first genome-edited Chinese cabbage. Before generating gene-edited B. rapa, we tested the efficacy of designed guide RNA by delivering the SpCas9 and guide RNA complex into the protoplasts isolated from B. rapa leaves. Based on the gene-editing efficacy of each guide RNA, four guide RNAs (gRNA3, gRNA4, gRNA5, and gRNA7) with high efficiency were selected and delivered into B. rapa hypocotyl explants by Agrobacterium-mediated transformation. Although from one to six T0 plants were regenerated from each guide RNA-transformed line, the targeted editing was identified only in regenerated plants containing gRNA4. The indel efficacy of gRNA3 in protoplasts was better than that of gRNA4, but we found no mutation in any of the six gRNA3-transformed plants. This phenomenon might be due to the difference of editing efficacy between leaf protoplasts and hypocotyl callus or the difference in the positions of T-DNA insertion affecting SpCas9 and guide RNA expression.
Vernalization and four FLC genes in B. rapa
The Braflc2flc3 double-knockout lines we made in this study produced flowers without requiring long-term cold treatment. In addition, monoallelic wild-type BrFLC2 in biallelic BraFLC3 mutation background was enough to suppress the flowering of B. rapa, such as wild-type B. rapa (Fig. 5c). This result is consistent with the previous study in which overproducing a single BraFLC gene in Arabidopsis resulted in late flowering (Kim et al. 2007). Our results clearly show that there is the functional redundancy between BraFLC2 and BraFLC3 for B. rapa’s response to vernalization treatments. However, we know little about whether BraFLC1 and BraFLC5 play overlapping roles with BraFLC2 and BraFLC3 in the response to vernalization. This question remains to be tested.
Mutation pattern of tissue culture-based genome editing
Arabidopsis researchers have used a floral dipping method to deliver a plant binary vector harboring SpCas9 and guide RNA during floral development. SpCas9 proteins are normally expressed under strong promoters, such as 35S or ubiquitin. The 35S promoter-derived SpCas9 system works quite efficiently to induce targeted mutations in reproductive cells, but this system also produces many somatic mutations in leaves. To produce heritable mutations in plants, egg cell-specific promoters have been implemented to express the SpCas9 protein only in reproductive cells (Mao et al. 2015; Tsutsui and Higashiyama 2017).
However, the problem of non-inheritable mutations has not been well documented in attempts to edit plant genomes, except for that of Arabidopsis. We think that the differences in the transformation method are responsible for the inheritability of mutations. In most plants, tissue culture is required to deliver a plant binary vector into plant cells, and the single-transformed cell regenerates into a whole plant. If SpCas9 and guide RNA expression are fast and high enough to induce a mutation in this single cell, we can observe the simple mutation patterns in the whole regenerated plant, such as monoallelic (50% mutation) or biallelic (100% mutation) in T0 plants (Xu et al. 2014; Kim et al. 2016; Wang et al. 2016; Sun et al. 2017; Zhang et al. 2017; Jiang et al. 2019).
Generation of a rapid cycling B. rapa
We generated a rapid cycling B. rapa by editing two functionally redundant BraFLC genes. The short life cycle of the edited Chinese cabbage is suitable for forward and reverse genetic study and allows us to examine the function of genes in B. rapa (Williams and Hill 1986). In many previous studies, to understand the function of B. rapa genes, the genes are typically over-expressed in A. thaliana and the altered phenotype is analyzed (Kim et al. 2007). However, the development of CRISPR technology has allowed us to develop a crop as a model plant; this in turn allows us to study the function of genes in the crop directly.
This study clearly shows how to examine the efficacy of guide RNAs in B. rapa protoplasts, how to regenerate gene-edited B. rapa, and how to calculate the mutation patterns and frequency in the transformed plant. By producing an early-flowering B. rapa that do not require vernalization, we have obtained materials that can be used to study how a gene functions in B. rapa. Shortly, CRISPR will be widely used to develop species-specific model systems for precisely targeted molecular and breeding studies.
References
Blümel M, Dally N, Jung C (2015) Flowering time regulation in crops-what did we learn from Arabidopsis? Curr Opin Biotechnol 32:121–129
Doench JG, Hartenian E, Graham DB et al (2014) Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat Biotechnol 32:1262–1267
Hinz JM, Laughery MF, Wyrick JJ (2015) Nucleosomes inhibit Cas9 endonuclease activity in vitro. Biochemistry 54:7063–7066
Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell 157:1262–1278
Isaac RS, Jiang F, Doudna JA et al (2016) Nucleosome breathing and remodeling constrain CRISPR-Cas9 function. Elife 5:e13450
Jiang M, Hu H, Kai J et al (2019) Different knockout genotypes of OsIAA23 in rice using CRISPR/Cas9 generating different phenotypes. Plant Mol Biol 100:467–479
Jinek M, Chylinski K, Fonfara I et al (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821
Jung C, Müller AE (2009) Flowering time control and applications in plant breeding. Trends Plant Sci 14:563–573
Kim SY, Park BS, Kwon SJ et al (2007) Delayed flowering time in Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Cell Rep 26:327–336
Kim H, Kim ST, Ryu J et al (2016) A simple, flexible and high-throughput cloning system for plant genome editing via CRISPR-Cas system. J Integr Plant Biol 58(8):705–712
Kim H, Kim S-T, Ryu J et al (2017) CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat Commun 8:14406
Kim HK, Min S, Song M et al (2018) Deep learning improves prediction of CRISPR-Cpf1 guide RNA activity. Nat Biotechnol 36:239–241
Lee J, Lim YP, Han CT et al (2013) Genome-wide expression profiles of contrasting inbred lines of Chinese cabbage, Chiifu and Kenshin, under temperature stress. Genes Genom 35:273–288
Mao Y, Zhang Z, Feng Z et al (2015) Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol J 14(2):519–532
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Osborn TC, Kole C, Parkin IAP et al (1997) Comparison of flowering time genes in Brassica rapaB napus and Arabidopsis thaliana. Genetics 146:1123–1129
Park J, Lim K, Kim J-S, Bae S (2017) Cas-analyzer: an online tool for assessing genome editing results using NGS data. Bioinformatics 33:286–288
Puchta H (2017) Applying CRISPR/Cas for genome engineering in plants: the best is yet to come. Curr Opin Plant Biol 36:1–8
Sander JD, Joung JK (2014) CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotech 32:347–355
Schranz ME, Quijada P, Sung S-B et al (2002) Characterization and effects of the replicated flowering time gene FLC in Brassica rapa. Genetics 162:1457–1468
Sternberg SH, Doudna JA (2015) Expanding the biologist’s toolkit with CRISPR-Cas9. Mol Cell 58:568–574
Sun Y, Jiao G, Liu Z et al (2017) Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front Plant Sci 8:1–15
Tsutsui H, Higashiyama T (2017) PKAMA-ITACHI vectors for highly efficient CRISPR/Cas9-mediated gene knockout in Arabidopsis thaliana. Plant Cell Physiol 58:46–56
Wang F, Wang C, Liu P et al (2016) Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 11:1–18
Williams PH, Hill CB (1986) Rapid-cycling populations of Brassicas. Science 232:1385–1389
Wilson LOW, O’Brien AR, Bauer DC (2018) The current state and future of CRISPR-Cas9 gRNA design tools. Front Pharmacol 9:749
Woo JW, Kim J, Il Kwon S et al (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33:1162–1164
Xu R, Li H, Qin R et al (2014) Gene targeting using the Agrobacterium tumefaciens-mediated CRISPR-Cas system in rice. Rice 7:7–10
Zhang Y, Bai Y, Wu G et al (2017) Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J 91:714–724
Acknowledgements
SYJ, HK, YPL, J-SK, and S-GK designed the experiments; SYJ, HA, and YDP performed the plant transformation and tissue culture; SYJ, K-HW, HK, and JR analyzed the indel mutations and maintained transformant lines; SYJ and S-GK designed the guide RNA and made vector constructs; SYJ and S-GK mainly wrote the manuscript; YO, GS, HK, YPL, and S-GK contributed to the revision of the manuscript. This work was supported by Institute for Basic Science (IBS-R021-D1), the Basic Science Research Program of the National Research Foundation of Korea, funded by the Korean government (MSIT) [2017R1C1B5076421/ 2018R1A2B6006233] to H.K, the Next-Generation BioGreen 21 Program (PJ01322603) provided by the Rural Development Administration to YO and S-GK. YPL, SYJ, and GS were supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Golden Seed Project, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (no. 213006-05-2-SB110). GS was partially supported by the Korea Research Fellowship Program (2017H1D3A1A01054325) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, South Korea. The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeong, S.Y., Ahn, H., Ryu, J. et al. Generation of early-flowering Chinese cabbage (Brassica rapa spp. pekinensis) through CRISPR/Cas9-mediated genome editing. Plant Biotechnol Rep 13, 491–499 (2019). https://doi.org/10.1007/s11816-019-00566-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-019-00566-9