Abstract
Key message
Fertile hybrids were produced with genetic material transferred from Th. intermedium into a wheat background and supply a source of genetic variation to wheat improvement.
Abstract
Both symmetric and asymmetric somatic hybrids have been obtained from the combination of wheatgrass (Thinopyrum intermedium) and bread wheat (Triticum aestivum). Two wheat protoplast populations, one derived from embryogenic calli and the other from a non-regenerable, rapidly dividing cell line, were fused with Th. intermedium protoplasts which had been (or not been) pre-irradiated with UV. Among the 124 regenerated calli, 64 could be categorized as being of hybrid origin on the basis of plant morphology, peroxidase isozyme, RAPD DNA profiling and karyological analysis. Numerous green plantlets were regenerated from 13 calli recovered from either the symmetric hybrid (no UV pre-treatment) or the asymmetric one (30 s UV irradiation). One of these hybrid plants proved to be vigorous and self-fertile. The regenerants were all closer in phenotype to wheat than to Th. intermedium. Genomic in situ hybridization analysis showed that the chromosomes in the hybrids were largely intact wheat ones, although a few Th. intermedium chromosome fragments had been incorporated within them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bread wheat (Triticum aestivum) is one of the world’s three leading cereal crop species. Intensive selection over many decades has eroded the level of genetic variation available to breeders (Law 1993; Reynolds et al. 2012). Its wild relatives have therefore become an important genetic reservoir, and it is becoming increasingly urgent to broaden wheat’s genetic base by alien introgression. A favored relative in this context is the species Thinopyrum intermedium (intermediate wheatgrass), which harbors genes enabling it to survive high levels of salinity, drought and low temperature, as well as genes ensuring resistance against various fungal and viral diseases (Hohmann et al. 1996; Li and Wang 2009). Sexual hybrids between wheat and Th. intermedium have provided the starting point for a number of attempts to introgress some of these genes into wheat (Hohmann et al. 1996; Ayala-Navarrete et al. 2009; Li and Wang 2009; Georgieva et al. 2011). Numerous partial amphiploid, chromosome addition and substitution lines were obtained and widely used for improvement of wheat against diseases via normal sexual hybridization and chromosome engineering (Friebe et al. 1993; Li and Wang 2009; Georgieva et al. 2011). However, yield or quality penalty often occurs in lines that carry large chromosome fragments from Thinopyrum (Brown 2002; Li and Wang 2009), which prevents the use of certain genes especially that rarely exist in wheat. The development of an alternative introgression method is therefore important, especially combined with the chromosome engineering techniques such as irradiation to shorten the alien chromosome segments.
The salient feature of asymmetric somatic hybridization is that it tends to transfer fragments of the donor genome while retaining the majority of the recipient’s. Furthermore, producing this sort of hybrid is both easier and more rapid than producing sexual hybrids. Over recent years, we have demonstrated the potential of asymmetric somatic hybridization, particularly using a combination of wheat with UV-irradiated tall wheatgrass (Th. elongatum). We have also fused wheat protoplasts with those of Russian wildrye, oat and maize (Zhou et al. 2001a; Xiang et al. 2003, 2010; Xu et al. 2003). One of the derivatives of the wheat/tall wheatgrass hybrid has been released in China as a cultivar (ShanRong No. 3), selected for its expression of an enhanced level of abiotic stress tolerance, presumably inherited from the donor parent (Xia et al. 2003; Liu et al. 2007, 2012; Shan et al. 2008). In this paper, we describe fusion products obtained from the combination of wheat cv. JN177 and the hexaploid grass Th. intermedium.
Materials and methods
Protoplast preparation and fusion product culture
Protoplasts of cv. JN177 were prepared from both embryogenic calli (#176, a regenerable line) and suspension cells (#Cha9, a vigorously dividing but non-regenerable line) (Cheng and Xia 2004; Li et al. 2004). #176 is maintained on a solid medium, while #Cha9 is maintained in a liquid medium. Both media are formulated according to Murashige and Skoog (1962), and supplemented with 2 mg/L 2, 4-dichlorophenoxyacetic acid (Xia and Chen 1996). In preparation for protoplast fusion, #176 protoplasts were recovered after a 6–7 day period of subculture on the solid medium, and the #Cha9 ones after a 3–4 day period of subculture in the liquid medium. Th. intermedium protoplasts were isolated from regenerable calli. Protoplast isolation was performed as described by Xu et al. (2003). #176 and #Cha9 protoplasts were mixed in a 1:1 ratio. The Th. intermedium protoplasts were spread as a monolayer on a 3 cm petri dish, irradiated with 300 uW/m2 UV light for either 0 s (treatment I), 30 s (treatment II) or 60 s (treatment III), mixed with the wheat protoplasts in a 1:1 ratio and fused as described by Xia and Chen (1996). When the regenerated calli had grown to a diameter of 2–5 mm, they were transferred to a proliferation medium, and later to a differentiation medium (Xia and Chen 1996). Regenerated plantlets were transplanted to soil and grown in a greenhouse until maturity.
Identification of peroxidase variation in the fusion products
0.5 g/mL fresh callus was homogenized in 1 M Tris–HCl (pH 8.3), centrifuged (12,000×g, 10 min) and the supernatant electrophoresed through a polyacrylamide gel (4 % stacking and a 10 % separating gel), which was stained following Hu and Wan (1985).
RAPD genotyping
Genomic DNA was extracted from calli following Doyle and Doyle (1990), and used in PCRs primed with one of the decamer primers OPA-01, -06, -08, -17, -19, OPF-03, -05, -12, OPH-04, -20, OPI-10 or OPM-04 (Operon Technology, Alameda, CA). The amplification regime and subsequent visualization of amplicons were as given by Cheng et al. (2004).
Mitotic chromosome spreads and genomic in situ hybridization (GISH)
Calli and the seedling root tips of hybrid and each of the parents were immersed in ice water for about 24 h, then fixed in 3:1 ethanol:acetic acid for about a week. Chromosome counts were obtained using the conventional Feulgen method. For the purposes of GISH, the probe was Th. intermedium genomic DNA labeled by DIG-nick translation (Roche catalog No. 11745816910), and blocking DNA was provided by DNA extracted from #Cha9 and #176. The probe to blocking DNA ratio was between 1:100 and 1:120. The GISH protocol used followed Xiang et al. (2003).
Results
Putative hybrid fusion products
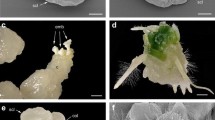
Granular calli were induced from each of the three combinations after about 4 weeks of culture in liquid P5 medium (Xia and Chen 1996) in the dark at 25 °C (Fig. 1b–e). In all, 124 small calli were recovered (Table 1). The calli increased in size once they had been transferred onto proliferating medium (Fig. 1f–i) and plantlets formed following the second transfer to the regeneration medium (Fig. 1j–m). The largest number of calli (64) was obtained from combination I (no exposure to UV). Of these, nine regenerated green plantlets, characterized by soft leaves and a fasciculate growth habit (Fig. 1j, k). Combination II (30 s UV irradiation) produced 49 calli, of which just four were regenerable. Upon transfer to the differentiation medium, one of these four calli (II-3) produced green plantlets with strong roots, and the resulting plants showed a wheat-like phenotype (Fig. 1l, m). A dozen putative hybrid plants recovered from calli I-34 and II-3 were potted into soil and grown in a greenhouse; only those regenerated from II-3 were self-fertile (Fig. 1n–p), and the morphology of its seeds was wheat-like (Fig. 1l–q). The 11 calli recovered from combination III (60 s UV irradiation) grew rapidly, but were non-regenerable. Fusion of either #176 or #Cha9 with Th. intermedium formed no calli, and stopped growing after a few rounds of cell divisions. Unfused parental protoplasts formed cell clusters, but no calli were generated (Fig. 1a).
Morphology of the somatic hybrid products, the recipient and the donor calli at 30 days after fusion. a Mixed #Cha9, #176 and Th. intermedium protoplasts, b, c combination I, d combination II, e combination III. Calli at 60 days after fusion. Regenerants f, j formed from callus I-34, g, k from I-54, h, l from II-3, i, m from II-6. n–p Spikelets formed by the regenerant from callus II-3. q Grain set by the regenerant from callus II-3 and its parents
Genotypic analysis
Peroxidase profiles
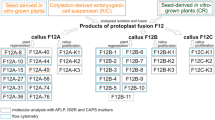
To characterize the hybrid nature of the regenerated calli, peroxidase assay was conducted. All the 124 calli from combination I, II and III showed the similar peroxidase patterns to that of parent wheat. Among them, 42, 28 and 4 calli of combination I, II and III still include specific bands of Th. intermedium, which were subsequently identified as hybrids. As shown in Fig. 2, the profiles of calli from combination I (e.g. I-2, -3, -10, -14, -24, -34 and -52), combination II (e.g. II-1, -14) and combination III (III-2, -6) included the parent bands. This result showed that the calli had retained most, if not all, of the wheat genome, and that parts of the donor genome had been introgressed in the fusion derivatives.
RAPD profiles
All the presumptive hybrid calli identified by the isozyme analysis were analyzed by the RAPD profile further to confirm their hybrid nature. Overall, 35 of the combination I, 24 of the combination II and 4 of the combination III calli contained DNA derived from Th. intermedium, and a few carried RAPD fragments not present in either parent (Table 1). The frequency of donor fragments decreased as the UV dosage increased (Table 1). The RAPD profiles of the regenerants included not only fragments derived from Th. intermedium and both #Cha9 and #176, but also some not present in the profiles of any of the parents (e.g., calli I-2, -10, -14, -24 and -34, see Fig. 3a, c). Template extracted from different #176 calli or samples of #Cha9 cells also produced variable RAPD profiles due to the somaclonal variations (Fig. 3). The OPA-06 amplicons of calli I-10 and -28 each included fragments specific to #Cha9, while the I-2, -14 and -24 amplicons included a fragment specific to #176; meanwhile the I-34 amplicon included both #Cha9 and #176 specific fragments (Fig. 3a). The results implied that all three protoplasts were involved in some of the fusion events. Overall, the proportion of the RAPD fragments assignable to the wheat parents varied from 75.0 to 89.5 %, to the Th. intermedium parent from 2.6 to 15.9 %, and the remainder related to fragments not present in any of the three parent’s profiles (Table 2). Th. intermedium and non-parental fragments were more frequent in combination I calli than in those from combinations II or III. Clone I-34 carried the largest number of Th. intermedium fragments: on the basis of its RAPD profiles, about 16 % of its genome was estimated to have been inherited from Th. intermedium.
RAPD analysis of putative hybrids and their parents. Profiles resulting from priming with a OPA-06, b OPA-08, c OPA-19, d OPF-05. I-2, -10, -14, -24, -28, -34, -54, and -62 derived from combination I, II-1, -3, -4, and -6 from combination II, III-1, -3, and -11 from combination III. M: lambda DNA digested with EcoRI and HindIII. The right-pointing arrows indicate fragments derived from each parent, arrowheads identify fragments not present in the parental profiles
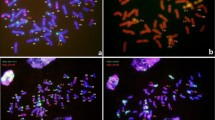
Chromosome number and GISH karyotype
The somatic chromosome number of #Cha9, #176 and Th. intermedium protoplasts was, respectively, 24, 34 and 42 (Fig. 4a–c), while that of the hybrids from combination I, II and III regenerants varied from 40 to 60 (Fig. 4d–i; Table 3). Chromosome fragments were common in the fusion products’ mitotic cells (Fig. 4d, h). One or two small chromosomes and/or chromosome fragments were present in 2.6 % cells of combination I, 7.01 % of combination II and 8.8 % of combination III. Multi-centromere chromosomes were seen in the cells of some of the regenerants (Fig. 4f). The GISH treated somatic chromosomes of regenerants from calli I-34 and II-3 were compared to those of the parents in Fig. 5. No intact Th. intermedium chromosomes were identified, but it was possible to detect many small segments incorporated into large wheat chromosomes (Fig. 5c–f), consistent with the notion that the somatic hybridization process was successful in introgressing only a limited amount of the Th. intermedium genome.
Karyotypic analysis. Arrow indicates chromosome fragments, arrowhead indicates polycentric chromosomes. a #176, 2n = 34, b #Cha9, 2n = 24, c Th. intermedium, 2n = 42, regenerant from d I-2, 2n = 48, e I-34, 2n = 52, f II-3, 2n = 50, g II-6, 2n = 54, and Calli from h III-1, 2n = 44, i III-11, 2n = 45
Discussion
Until now, plant regeneration from somatic hybridization calli has been difficult to achieve, and most regenerants have proven to be sterile and/or morphologically abnormal (Gamborg and Holl 1977; Bauer-Weston et al. 1993; Fahleson and Glimelius 1999; Xia 2009; Eeckhaut et al. 2013). The creation of asymmetric hybrids offers a potential way to avoid these problems. The extent of the asymmetry is a function of the severity of the irradiation dose, the phylogenetic distance between the parental species, the chromosome number of each parent, and differences in the cell cycle time of each of the parental materials (Dudits et al. 1987; Sears 1993; Nakanoa et al. 2006; Xia 2009). We have explored the use of a three cell system, based on two distinct types of recipient cells, both obtained from the same cultivar; one was a rapidly dividing but non-regenerable cell line (#cha9) and the other grew slowly but was regenerable (#176). Fusion of donor cells with #cha9 or #176 individually failed to regenerate green plants because of significant loss of chromosomes from the #Cha 9 (2n = 24) cell line, and the chromosome elimination of #176 (2n = 34) (Li et al. 2004; Xiang et al. 2010). Fusing these two different protoplasts with UV-irradiated donor protoplasts has been an effective strategy for the production of green regenerants from a range of donors, including oat (Xiang et al. 2003, 2010), maize (Xu et al. 2003), Russian wildrye (Li et al. 2004), Italian ryegrass (Cheng and Xia 2004) and foxtail millet (Cheng et al. 2004; Xiang et al. 2004), but no hybrid progenies were produced in these combinations. In the present study, regeneration was possible from about 10 % (13/124) of the calli, and the fertile hybrid plants were obtained between the fusion of wheat and Th. intermedium using this fusion system. The regenerants were genotypically largely wheat, with a small number of donor chromosome fragments incorporated into the largely wheat chromosomes (Fig. 5). Both the recipient cells lines were highly aneuploid (#Cha9 contained on average 24 chromosomes and #176 34 chromosomes, compared to the euploid number of 42, see Table 3), but in combination, they conferred both the ability to grow vigorously and to regenerate. The implication is that different chromosomes were missing in each of the two cell lines, so that together they provided a full complement.
Chromosome elimination frequently accompanies the fusion of highly differentiated genomes (Feldman et al. 1997; Kashkush et al. 2002; Xia 2009). Regenerants typically carry fewer chromosomes than predicted from the sum of each parent’s complement (Kisaka et al. 1997; Li et al. 2004; Xiang et al. 2010). The factors which probably govern the extent of chromosome elimination have been discussed by Xia (2009). In the present experiments, all the regenerants, whether derived from symmetric or asymmetric fusion, exhibited a degree of chromosome elimination: in three combinations, their somatic chromosome number was in the range 40–60, while the sum of the three parental complements was 100 (Table 3). GISH analysis demonstrated that the regenerants’ genomes were a mosaic of wheat and Th. intermedium chromosomes, in some cases involving small donor fragments inserted interstitially within a largely wheat chromosome, and in others involving the translocation of quite large donor fragments (Fig. 5). Whether or not the fusion was asymmetric, the overall chromosome complement was mostly inherited from one and/or the other recipient line. However, chromosome elimination was clearly not restricted to the donor genome, since the total wheat complement contributed by the two wheat parents was 58, a number far greater than the observed range of somatic chromosome number in the hybrid derivatives. UV irradiation of the donor cells encouraged the elimination of donor chromosomes. UV irradiation is known to fractionate chromosomes (Hall et al. 1992), and it is clear that increasing the UV dosage reduces the extent of donor material transferred into the recipient (Xia et al. 2003; Cheng et al. 2004; Cui et al. 2009). The overall somatic chromosome number was rather insensitive to the dosage of UV, but chromosome fragments were more frequently induced as the dosage was raised (Figs. 3, 5; Table 2). The suggestion is that a balanced genome is necessary to achieve regeneration and subsequent self-fertility.
Phylogenetic distance is known to influence regeneration capacity and fertility. In the combination wheat/oat, it was only possible to regenerate albino plants (Xiang et al. 2003). In other wide combinations (wheat with either maize (Xu et al. 2003), Italian ryegrass (Cheng and Xia 2004), foxtail millet (Chen et al. 2004; Xiang et al. 2004) and Russian wildrye (Li et al. 2004), green plants were regenerated, but the plants were all sterile. So far, fertile somatic hybrid plants have only been successfully produced from the combinations wheat/Haynaldia villosa (Zhou et al. 2001b) and wheat/Th. elongatum (Xia et al. 2003; Cheng et al. 2004; Cui et al. 2009), both of these donors being, like wheat, Triticeae species. In the wheat/Th. elongatum case, symmetric hybridization produced tall, perennial plants with an appearance quite similar to that of the donor. However, following the asymmetric route, some derivatives exhibited a wheat-like phenotype and others an intermediate one (Xia et al. 2003; Cheng et al. 2004). Cytological analysis of these regenerants has confirmed that the genome of the wheat-like lines had been predominantly inherited from the wheat parent (Xia et al. 2003), whereas in the wheatgrass-like ones, only a few wheat chromosome fragments were evident (Cui et al. 2009). The former type is similar, in terms of both phenotype and genome constitution, as pertained for the fertile derivative line II-3 described here (Fig. 5).
In conclusion, besides two wheat cell lines provided a full complement, the phenogenetic relationship of wheat with donor cereal was another key factor to influence the hybrid fertile. We provide a possible route to transfer segments of Th. intermedium chromosome(s) into wheat background and produce fertile derivatives using a three cell fusion system. The Th. intermedium genotype includes a number of potentially useful agronomic traits and has proven to be a valuable source for resistance to various diseases in wheat (Li and Wang 2009; Nevo and Chen 2010). The materials obtained from somatic hybridization are potentially interesting as sources of genetic variation of relevance to wheat improvement. The priority now will be to identify what traits have been successfully transferred and to monitor the expression of donor genes in the hybrid plants.
References
Ayala-Navarrete L, Tourton E, Mechanicos AA, Larkin PJ (2009) Comparison of Thinopyrum intermedium derivatives carrying barley yellow dwarf virus resistance in wheat. Genome 52(6):537–546
Bauer-Weston B, Keller W, Webb J, Gleddie S (1993) Production and characterization of asymmetric somatic hybrids between Arabidopsis thaliana and Brassica napus. Theor Appl Genet 86(2–3):150–158
Brown JK (2002) Yield penalties of disease resistance in crops. Curr Opin Plant Biol 5(4):339–344
Chen XL, Xia GM, Chen HM (2004) Nuclear and cytoplasmic genome analysis of somatic hybrid of Triticum aestivum L. and Leymus chinensis (Trin.) Tzvel. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 30(4):379–386
Cheng AX, Xia GM (2004) Somatic hybridisation between common wheat and Italian ryegrass. Plant Sci 166:1219–1226
Cheng AX, Xia GM, Zhi DY, Chen HM (2004) Intermediate fertile Triticum aestivum (+) Agropyron elongatum somatic hybrids are generated by low doses of UV irradiation. Cell Res 14(1):86–91
Cui HF, Yu ZY, Deng JY, Gao X, Sun Y, Xia GM (2009) Introgression of bread wheat chromatin into tall wheatgrass via somatic hybridization. Planta 229(2):323–330
Doyle JJ, Doyle JL (1990) A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13–15
Dudits D, Maroy E, Praznovszky T, Olah Z, Gyorgyey J, Cella R (1987) Transfer of resistance traits from carrot into tobacco by asymmetric somatic hybridization: regeneration of fertile plants 84(23):8434–8438
Eeckhaut T, Lakshmanan PS, Deryckere D, van Bockstaele E, van Huylenbroeck J (2013) Progress in plant protoplast research. Planta. doi:10.1007/s00425-00013-01936-00427
Fahleson J, Glimelius K (1999) Protoplast fusion for symmetric somatic hybrid production in Brassicaceae. Methods Mol Biol 111:195–209
Feldman M, Liu B, Segal G, Abbo S, Levy AA, Vega JM (1997) Rapid elimination of low-copy DNA sequences in polyploid wheat: a possible mechanism for differentiation of homoeologous chromosomes. Genetics 147(3):1381–1387
Friebe B, Jiang J, Gill BS, Dyck PL (1993) Radiation-induced nonhomoeologous wheat-Agropyron intermedium chromosomal translocations conferring resistance to leaf rust. Theor Appl Genet 86(2–3):141–149
Gamborg OL, Holl FB (1977) Plant protoplast fusion and hybridization. Basic Life Sci 9:299–316
Georgieva M, Sepsi A, Tyankova N, Molnar-Lang M (2011) Molecular cytogenetic characterization of two high protein wheat-Thinopyrum intermedium partial amphiploids. J Appl Genet 52(3):269–277
Hall RD, Rouwendal GJ, Krens FA (1992) Asymmetric somatic cell hybridization in plants. I. The early effects of (sub)lethal doses of UV and gamma radiation on the cell physiology and DNA integrity of cultured sugarbeet (Beta vulgaris L.) protoplasts. Mol Gen Genet 234(2):306–314
Hohmann U, Busch W, Badaeva K, Friebe B, Gill BS (1996) Molecular cytogenetic analysis of Agropyron chromatin specifying resistance to barley yellow dwarf virus in wheat. Genome 39(2):336–347
Hu NS, Wan XG (1985) Application of isozyme technique. Hunan Science and Technology Press, Changsha, China, pp 74–76
Kashkush K, Feldman M, Levy AA (2002) Gene loss, silencing and activation in a newly synthesized wheat allotetraploid. Genetics 160(4):1651–1659
Kisaka H, Kisaka M, Kanno A, Kameya T (1997) Production and analysis of plants that are somatic hybrids of barley (Hordeum vulgare L.) and carrot (Daucus carota L.). Theor Appl Genet 94(2):221–226
Law CN (1993) Wheat genetics–today and tomorrow. In: Li ZS, Xiu ZY (eds) Proceedings of the 8th International Wheat Genetics Symposium, China Scientech Press, Beijing, pp 3–10
Li H, Wang X (2009) Thinopyrum ponticum and Th. intermedium: the promising source of resistance to fungal and viral diseases of wheat. J Genet Genomics 36(9):557–565
Li C, Xia G, Xiang F, Zhou C, Cheng A (2004) Regeneration of asymmetric somatic hybrid plants from the fusion of two types of wheat with Russian wildrye. Plant Cell Rep 23(7):461–467
Liu S, Zhao S, Chen F, Xia G (2007) Generation of novel high quality HMW-GS genes in two introgression lines of Triticum aestivum/Agropyron elongatum. BMC Evol Biol 7:76
Liu C, Li S, Wang M, Xia G (2012) A transcriptomic analysis reveals the nature of salinity tolerance of a wheat introgression line. Plant Mol Biol 78(1–2):159–169
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Plant Physiol 15(3):473–497
Nakanoa M, Nomizub T, Mizunashia K, Suzukia M, Moria S, Kuwayamaa S, Hayashia M, Umeharaa H, Okaa E, Kobayashia H, Asanoa M, Sugawaraa S, Takagia H, Saitoc S, Nakatad M, Godod T, Haraa Y, Amanoa J (2006) Somaclonal variation in Tricyrtis hirta plants regenerated from 1-year-old embryogenic callus cultures. Sci Hortic 110(4):366–371
Nevo E, Chen G (2010) Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ 33(4):670–685
Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G (2012) Achieving yield gains in wheat. Plant Cell Environ 35(10):1799–1823
Sears ER (1993) Use of radiation to transfer alien chromosome segments to wheat. Crop Sci 33:897–901
Shan L, Li C, Chen F, Zhao S, Xia G (2008) A Bowman–Birk type protease inhibitor is involved in the tolerance to salt stress in wheat. Plant Cell Environ 31(8):1128–1137
Xia G (2009) Progress of chromosome engineering mediated by asymmetric somatic hybridization. J Genet Genomics 36(9):547–556
Xia GM, Chen HM (1996) Plant regeneration from intergeneric somatic hybridization between Triticum aestivum L. and Leymus chinensis (Trin.) Tzvel. Plant Sci 120:197–203
Xia G, Xiang F, Zhou A, Wang H, Chen H (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107(2):299–305
Xiang FN, Xia GM, Chen HM (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum) and Avena sativa L. Sci China C Life Sci 46(3):243–252
Xiang FN, Xia GM, Zhi DY, Wang J, Nie H, Chen HM (2004) Regeneration of somatic hybrids in relation to the nuclear and cytoplasmic genomes of wheat and Setaria italica. Genome 47(4):680–688
Xiang FN, Wang J, Xu CH, Xia GM (2010) The chromosome content and genotype of two wheat cell lines and of their somatic fusion product with oat. Planta 231(5):1201–1210
Xu CH, Xia GM, Zhi DY, Xiang FN, Chen HM (2003) Integration of maize nuclear and mitochondrial DNA into the wheat genome through somatic hybridization. Plant Sci 165:1001–1008
Zhou A, Xia G, Chen H, Hu H (2001a) Comparative study of symmetric and asymmetric somatic hybridization between common wheat and Haynaldia villosa. Sci China C Life Sci 44(3):294–304
Zhou A, Xia G, Zhang X, Chen H, Hu H (2001b) Analysis of chromosomal and organellar DNA of somatic hybrids between Triticum aestivum and Haynaldia villosa Schur. Mol Genet Genomics 265(3):387–393
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 31270385).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Chong.
Rights and permissions
About this article
Cite this article
Li, C., Cheng, A., Wang, M. et al. Fertile introgression products generated via somatic hybridization between wheat and Thinopyrum intermedium . Plant Cell Rep 33, 633–641 (2014). https://doi.org/10.1007/s00299-013-1553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-013-1553-8