Abstract
Key message
We developed an efficient protocol for chromosome scattering in Spathiphyllum microspores. The effects of plant material, developmental age, genotype and antimicrotubular toxin type, exposure and concentration were evaluated.
Abstract
Asymmetric hybridization through microprotoplast-mediated chromosome transfer (MMCT) is a known method for overcoming sexual breeding barriers between distantly related plant species. To obtain microprotoplasts, it is necessary to induce mass micronucleation either in somatic or gametic cells. We have tested the efficiency for micronuclei induction of five mitosis inhibitors, amiprophos-methyl (APM), butamiphos (BUT), chlorpropham (CIPC), oryzalin (ORY) and propyzamide (PRO), on developing microspores of diploid Spathiphyllum wallisii Regel. Besides the used toxins, also the effect of their concentrations and incubation period as well as plant genotypes and material was tested. We observed micronuclei (MNi) in pollen mother cells, dyads and tetrads as well as other abnormalities such as ball metaphases and chromosome bridges. The flower position on the spadix and the type of starting material (dissected anthers vs. complete spadices) did not significantly influence micronucleation frequencies. The highest micronucleation index of 86 % was obtained in microspores treated with 10 μM ORY during 72 h. All six genotypes tested formed micronuclei after this particular treatment, although the efficiency varied between cultivars. Next to ORY, CIPC was also a very efficient MNi inducer. The average number of MNi found in micronucleated cells varied between 1.67–6.44 for CIPC and 0.83–5.50 for ORY. The maximal number of MNi observed was 12 for CIPC and 9 for ORY. Our results demonstrate that CIPC and ORY can be applied for mass micronucleation on developing microspores of S. wallisii as a first step of MMCT in aroid interspecific or intergeneric breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The monocotyledonous species Spathiphyllum wallisii Regel (2n = 2x = 30), family Araceae, is known as an attractive indoor pot plant with pure white flowers. The inflorescences consist of a spadix enclosed by a spathe. The spadix is a fleshy spike covered with many small bisexual flowers. The species has a high in vitro regeneration capacity that has already been exploited for ploidy breeding (Eeckhaut et al. 2001, 2004). However, as in other ornamentals, interspecific or intergeneric breeding in aroids has the highest potential to create novel plants with ornamentally valuable traits. So far, within Araceae no intergeneric hybrids were produced, due to both pre-zygotic and post-zygotic sexual barriers. Duquenne et al. (2007) attempted asymmetric somatic hybridization between S. wallisii protoplasts and UV-irradiated Anthurium scherzerianum protoplasts as a method to overcome these barriers, and obtained hybrid cells that could not be regenerated. Irradiation can be used for fragmentation, but frequently causes random chromosome breakages, deletions, gene rearrangements and hybrid sterility (Gleba et al. 1988; Famelaer et al. 1989; Wijbrandi et al. 1990; Puite and Schaart 1993).
An alternative approach for gene transfer between sexually incongruent plant species might be microprotoplast fusion technique (Ramulu et al. 1995). In microprotoplast-mediated chromosome transfer (MMCT) somatic or gametic donor cells are treated with mitosis inhibitors to scatter the metaphase chromosomes in the cells. After decondensation of scattered chromosomes, a nuclear envelope is formed, resulting in so called micronucleated cells. For successful MMCT, mass induction and efficient isolation of micronucleated cells are very important. In many cases, cell suspensions are used as source, such as in Nicotiana plumbaginifolia (Verhoeven et al. 1990), Haemanthus katherinae Bak. (Morejohn et al. 1987) and Helianthus giganteus (Binsfeld et al. 2000). Alternatively, developing microspores were used (Matthews et al. 1999; Saito and Nakano 2002a). When using microspores instead of suspension cultures, there is no need of a synchronizing treatment, as meiotic cycles are usually synchronous within an anther (McCormick 1993).
The goal of this research was to develop an efficient protocol for mass induction of micronucleated cells in S. wallisii as a first necessary step toward MMCT among aroids. As no fast-growing S. wallisii cell suspension cultures were available, microspores were chosen as donor material. The effects of flower position, plant material type, spindle toxin type and concentration, exposure time and genotype were investigated.
Materials and methods
Plant materials
Six diploid S. wallisii Regel commercial genotypes ‘6054’, ‘6332’, ‘6341’, ‘6409’, ‘6526’ (provided and coded by Deroose Plants) and ‘Daniël’ (present in the ILVO collection) were used. All the plants were grown in standard greenhouses under natural light conditions. The day/night temperature was maintained at 20 ± 2 °C and the relative humidity around 70 %.
Spindle toxins
In the experiments, five spindle toxins were used: amiprophos-methyl PESTANAL® [APM, O-methyl-O–O-(4-methyl-6-nitrophenyl)-N-isopropyl phosphorothioamidate], chlorpropham PESTANAL® [CIPC, isopropyl N-(3-chlorophenyl)carbamate] and propyzamide VETRANAL™ [PRO, 3,5-dichloro-N-(1,1-dimethylpropyl)benzamide] were purchased from Sigma-Aldrich, Germany; oryzalin [ORY, 4-(dipropylamino)-3,5-dinitro-benzenesulfonamide] from Duchefa Biochemie, The Netherlands; butamiphos [BUT, O-ethyl O-6-nitro-m-tolyl sec-butylphosphoramidothioate] from Wako Pure Chemical Industries, Japan. All stock solutions (5 mM) were prepared in 100 % dimethylsulfoxide and stored at −20 °C.
Micronucleation
In introductory experiments, the ideal physiological stage of the spadix for micronuclei isolation was reached 8 days after its first appearance, at the very bottom of the plant, inside the leaf sheaths (Fig. 1b). For all experiments described in this manuscript, spadices at this particular stage were collected from the greenhouse, washed in 70 % ethanol for 3 min, sterilized in a 1 % NaOCl solution with two drops of Tween 20 for 10 min and thoroughly rinsed three times in sterile distilled water.
In Experiment 1, the effects of the flower position on the spadix, the type of plant material and exposure time to the spindle toxin were tested. Thereto both isolated anthers and whole spadices were collected from the upper, middle and lower part of a S. wallisii ‘6526’ inflorescence. The collected material was suspended in the micronucleation medium containing half-strength MS salts (Murashige and Skoog 1962), double-strength MS vitamins, 1 g L−1 casamino acids, and 100 g L−1 sucrose (pH 5.8). The medium was autoclaved at 121 °C for 30 min at a pressure of 500 hPa, and supplemented with 50 μM ORY afterwards. All treatments were done in Petri dishes (60 × 15 mm) containing 5 ml medium in four repetitions. Petri dishes were placed in a culture room at 23 ± 2 °C, 16-h photoperiod and 40 μmol m−2 s−1 photosynthetic active radiation, supplied by cool white fluorescent lamps (OSRAML36W/31). Observations were made on day 2, 4 and 8. The micronucleation index was defined as the ratio between the number of micronucleated cells and the total cell number and used to quantify the effects of the parameters. To calculate the index, pollen mother cells, dyads and tetrads were taken into account. Mature pollen was ignored because its exine layer was easily DAPI stained; this inhibited fluorescence observation of micronuclei inside the cell.
In Experiment 2, the efficiency of five different spindle toxins (APM, BUT, CIPC, ORY, PRO) for micronucleation was examined. Based on the results of Experiment 1, we used five complete ‘6526’ spadices and dissected all anthers. To minimize developmental stage effects, we collected all anthers in a single Petri dish in 20 ml basic micronucleation medium. Subsequently, anthers were transferred with a spatula to micronucleation medium supplemented with the various toxins at a concentration of 0, 10, 20, 50 or 100 μM. The anthers were analyzed after 24-, 48- and 72-h exposure. The rest of the procedure was as described for Experiment 1. After determination of the spindle toxins that resulted in the highest micronucleation indices, the exact number of MNi induced by these compounds was monitored. For this screening, a distinction was made between pollen mother cells (PMCs), dyads and tetrads.
In Experiment 3, five other cultivars, ‘6054’, ‘6332’, ‘6341’, ‘6409’ and ‘Daniël’ were used to control the applicability of the protocol for a range of genotypes. Isolated anthers of these five genotypes were, therefore, suspended in micronucleation medium supplemented with 10 μM ORY during 72 h, based on the micronucleation indices that were determined in Experiment 2. Depending on the genotype, we used three spadices (‘6409’) or one spadix (all other genotypes). The rest of the procedure was as described for Experiment 1.
Microscopic analysis
Cells were studied under a fluorescence microscope (Leica DM IRB). To this goal, they were stained with a mixture of 20 μL vectashield and 1 μg mL−1 4′,6-diamidino-2-phenylindole (DAPI). After squashing under a glass cover slip, samples were visualized by UV irradiation; the DAPI fluorescent cell nuclei were detected through a blue filter, whereas the cell walls were visualized under white light. Pictures were captured using a Leica DFC 320 camera with accompanying software (Leica Application Suite). The formation of abnormalities such as ball metaphases (complete destruction of spindle fibers followed by chromosome ‘clumping’) and chromosome bridges (chromatin bridges between centromeres) was also observed and registered.
Statistical analysis
For each treatment, 400 cells (4 repetitions of 100 cells per test) were analyzed. Analysis of variance (ANOVA) was performed using Statistica 10.0. A multifactorial analysis was done for experiment 1 and 2, while experiment 3 was treated as a one-factor experiment. Mean separation was accomplished with Duncan test (P = 0.05).
Results
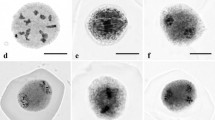
Micronuclei were seen in PMCs, dyads and tetrads (Fig. 2c–e). Though mature pollen could not be used to calculate micronucleation indices, micronuclei were occasionally observed inside. By observing cell wall formation, we distinguished micronuclei at dyad and early tetrad stage (Fig. 2d, e) and microcell formation at late tetrad stage (Fig. 2f). Other abnormalities were also seen, like ball metaphases and chromosome bridges (Fig. 2g, h).
Micronucleation in microspores of Spathiphyllum wallisii Regel ‘6526’, after treatment with various antimitotic toxins and visualization through DAPI staining. a, b Control treatment after 72 h of incubation: a under bright field; b under UV filter. c–f MNi in different pollen developmental stages: c pollen mother cell with MNi (CIPC, 20 μM, 48 h); d dyad with MNi (ORY, 10 μM, 72 h); e tetrad with MNi (ORY, 10 μM, 72 h); f microcell formation at late tetrad stage (CIPC, 50 μM, 72 h). g, h Chromosomal abnormalities: g ball metaphases (CIPC, 50 μM, 72 h); h chromosome bridges (ORY, 50 μM, 72 h). The bars represent 10 μm
Statistical analysis of data obtained in Experiment 1 showed that the position of the anthers on the spadix and the plant material type do not cause significantly different micronuclei indices. However, significant interactions between exposure time on the one hand and plant material or position on the spadix on the other hand were observed (Table 1). Therefore, these interactions were analyzed separately (Fig. 3). No significant differences between micronucleation indices were seen between 2 and 4 days of exposure to 50 μM ORY. This result was neither influenced by spadix position nor by the plant material type. After 8 days of exposure, a significant decrease was observed for samples at the lower and upper spadix (Fig. 3a) and in dissected anthers (Fig. 3b).
Interactions between exposure time and flower position on the spadix (a) and exposure time and plant material (b) on the micronucleation index in Spathiphyllum wallisii Regel ‘6526’ microspores after treatment with 50 μM ORY. Significantly different means (Duncan, P < 0.05) are marked by different symbols
Experiment 2 showed significant interactions between all tested parameters (spindle toxin, concentration and exposure time). Therefore, the results were presented separately for each spindle toxin (Fig. 4). Significantly higher micronucleation indices were obtained after treatment with ORY or CIPC compared to APM, BUT and PRO. The optimal concentration for all the products tested was situated between 10 and 50 μM. At a higher concentration the efficiency decreased. In general, exposure time had less influence.
More detailed information on the exact number of micronuclei per micronucleated cell generated by ORY and CIPC treatments is presented in Table 3. Micronucleation was more frequently observed in PMCs than in dyads or tetrads, especially when CIPC was used. The average number of MNi found in micronucleated cells varied between 6.44 and 1.67 for CIPC and 5.50 and 0.83 for ORY. The maximal number of MNi observed was 12 for CIPC and 9 for ORY.
All genotypes tested in Experiment 3 yielded micronucleated cells. Between the different genotypes micronucleation indices varied between 53 % for ‘6332’ and 2 % for ‘6409’. Because of high variations, genotype effects were not statistically significant (Table 4).
Discussion
Micronucleation (Fig. 2c–f) was observed when treating microspores of S. wallisii with different antimitotic agents. These agents are believed to block the growth of microtubules and to stop the movement of chromosomes to the poles, creating chromosome masses. In addition, Hertel et al. (1980) suggest that lower concentrations (5–15 μM) of ORY and APM deregulate and inhibit Ca2+ level uptake by plant mitochondria which subsequently lead to micronucleation by forming nuclear membranes around the fragmented genome.
In our experiments, micronuclei were present throughout all developmental phases from PMCs till mature pollen. Although the micronucleation efficiency could not be quantified in the latter phase, we expect that micronuclei are abundantly present in mature pollen, based on the efficient micronucleation in tetrads and the observation of micronuclei in pollen with poorly stained cell walls. For younger cells, the micronucleation frequency and quality observed depended mainly on the chemical used, the concentration and the exposure time.
In Experiment 1, the optimal exposure period was 4 days, but after 2 days the micronucleation index was not significantly lower. After 8 days ORY exposure, both flower position on the spadix and plant material used had a significant effect on micronucleation (Table 1; Fig. 3). A possible explanation might be the difference in developmental stage of the anthers from the bottom to the top of the inflorescence. Cells exposed at an ORY concentration as high as 50 μM can be assumed to have a higher risk of being completely arrested after longer incubation times, thus decreasing micronucleation indices. An interaction with the pollen developmental stage, as shown in Fig. 3, is in this case very probable. Likewise, in dissected anthers the developmental stage might be arrested compared to undetached ones. Significant effects of developmental stage were also reported in potato where successful micronucleation was only achieved by applying toxins on separated anthers but not on buds or inflorescences (Matthews et al. 1999).
After 4 days of incubation time, many shrunken cells appeared; this severely reduced the number of cells suitable for MNi counting. Furthermore, we can expect cell shrinking to be negatively correlated with chromosome scattering. For this reason, in following experiments, we exposed the cells to mitosis inhibitors for a maximal time frame of 72 h. Moreover, the results obtained by this experiment demonstrate that developmental stage effects are not statistically relevant after a short exposure time. We decided to use dissected anthers from the whole spadix as plant material source in subsequent testing.
Between the antimicrotubular toxins, significant differences were observed in Experiment 2. The most efficient ones were CIPC and ORY, while APM, BUT and PRO had only a limited or almost no effect. Not surprisingly, interactions with dose and exposure time were significant (Table 2). Similarly, Ramulu et al. (1991) found an increased percentage of micronucleated cells, as well as chromosome scattering, along with the increase in duration of the treatment in potato suspension cells when comparing APM and ORY with colchicine. They also reported that 30 μM ORY and 32 μM APM induced a higher frequency of micronucleated cells at 30 and 48 h, respectively.
After treatment with 10 μM ORY for 72 h, most cells with micronuclei were produced; the micronucleation index was 86.4 % (Fig. 4). Likewise, in L. longiflorum about 90 % of microspores produced more than 4 nuclei when treated with CIPC of 10 μM, while PRO (5 or 10 μM) and colchicine (120 or 240 μM) induced less than 10 % micronucleated cells, after an exposure time of 72–96 h (Saito and Nakano 2002a). An efficiency of 5–9 % of micronucleated cells was obtained using ORY at lower concentrations, 15 and 30 μM, in N. plumbaginifolia suspension cells (Verhoeven et al. 1990); 25 μM ORY also induces about 10 % microcell formation in microspores of Solanum tuberosum L (Matthews et al. 1999). In addition, both articles reported that APM had promising effects. However, in our study, this was not confirmed. Although APM, an amide herbicide, has approximately the same mode of action as ORY, its efficiency was significantly lower. ORY was also found to be effective in inducing micronucleation on H. katherinae Bak. and H. giganteus suspension cells (Binsfeld et al. 2000; Morejohn et al. 1987). Conversely, in Hemerocallis hybrida ‘Stella d’Oro’ suspension cultured cells, ORY induced less micronucleation when compared to other toxins such as APM, BUT, CIPC and PRO (Saito and Nakano 2001). Also in other publications, APM, BUT or PRO were relatively more successfully applied for micronucleation (Ramulu et al. 1994; Saito and Nakano 2001, 2002b). This disagreement with our results can be explained by differences in plant material, not only genotypic, but also physiological, such as a different division activity. For instance, in the latter articles, cell suspensions were used as donor material and mitosis inhibitors were often combined with synchronizing agents as hydroxyl urea and microfilament disrupting agents such as cytochalasin B.
A comparison of our results from Experiments 1 and 2 shows that after treatment with 50 μM ORY, 27–62 and 14–16 % micronucleated cells were formed in Experiments 2 (1–3 days incubation) and 1 (2–4 days incubation), respectively (Figs. 3, 4). We assume that this is caused by developmental stage differences, as different spadices were used for both experiments.
From a qualitative point of view, smaller micronuclei are more interesting for possible future applications such as microprotoplast isolation. We expect that the average DNA amount and the chromosome number per micronucleus are negatively correlated with chromosome scattering and the number of micronuclei per cell. For that reason, we checked the number of micronuclei generated by the two antimitotic compounds that yielded most cells with micronuclei, being ORY and CIPC (Table 3). Because exposure time had no significant effect on micronucleation in the previous experiment (Table 2), the mean result of the 24, 48 and 72 h treatments was evaluated. Compared to ORY, CIPC resulted in a higher number of MNi per cell suggesting a better fragmentation, although the micronucleation index was lower. Also in meiocytes of Lilium longiflorum, CIPC was found to be most efficient. The mean number of micronuclei induced was 7.5 with a maximum of 20 MNi which was higher than after treatment with APM, COL or PRO (Saito and Nakano 2002a). CIPC, a carbamate, alters the orientation of spindle microtubules which leads to multiple spindle formation. Thus, chromosomes move to many poles resulting in more scattering than ORY (Vaughn and Lehnen 1991). Micronuclei are more often found in PMCs than in dyads or tetrads, which can be explained by the application of the antitubular toxins in an early developmental stage.
Besides micronucleation, we observed abnormalities such as chromosome clumping, known as ball metaphases, and chromosome bridges. The same ball metaphases were observed by Damon (1957), Ramulu et al. (1987) and Verhoeven et al. (1990). However, in Citrus unshiu cultured cells, no such ball metaphases were observed (Zhang et al. 2006). Chromosome bridges were reported by Peng et al. (2003) in APM treated root tips of Triticum durum. They also occur during 2n pollen development in Begonia that could be induced by the dinitroaniline trifluralin (Dewitte et al. 2010a, b). In our experiment, ball metaphase was seen in all toxin treatments, in varying frequency (up to 0.42 % in PRO treated cells and 1.81 % in ORY treated cells), while chromosome bridges were only observed in ORY treated cells in a very low frequency of 0.17 %.
We tested the protocol that yielded the maximal micronucleation index in ‘6526’ (10 μM ORY during 72 h) on five other S. wallisii genotypes in Experiment 3. It provoked micronucleation in all genotypes, and in all but one at relatively high frequencies (Table 4). Only cultivar ‘6409’ produced only 2 % micronucleated cells. The lower micronucleation indices compared to 86 % obtained in Experiment 2 can be explained by the optimization of the protocol for ‘6526’; probably antimicrotubular toxin concentration and exposure are not optimal for other cultivars. However, Experiment 3 succeeded in demonstrating that micronuclei were formed in developing microspores of a range of genotypes. For future applications, we recommend that micronuclei-inducing protocols are optimized per genotype.
Significant differences between cultivars were not recorded, because the variation within a single cultivar was generally large (Table 4). Comparisons between different genotypes are also complicated, because the developmental stages of different spadices are not exactly equal. Furthermore, in ‘6409’, low amounts of immature pollen were present, accounting for the need to harvest material from three different spadices, even increasing variation. Also in Lilium, almost no genotypic effects were seen and in all cultivars tested efficient induction of micronucleation was possible (Saito and Nakano 2002a).
From our study, we conclude that mass micronucleation is possible in microspores of various S. wallisii Regel genotypes using either CIPC or ORY. The optimal treatment is probably genotype specific; moreover, the most efficient treatment for induction of micronucleated cells does not necessarily bring forth the largest number of micronuclei. We assume that minor developmental stage differences, that are uneasy to control, may affect micronucleation efficiency.
Isolation and purification of gametic microprotoplasts starting from micronucleated cells has been described previously in other crops (Matthews et al. 1999; Saito and Nakano 2002a). Our research can, therefore, be considered as a first step toward technology development for partial genome transfer through MMCT for intergeneric crosses within Araceae. Moreover, using microspores has some advantages compared to suspension cells as it avoids the establishment of a suspension culture, the need for a synchronizing treatment, and ultracentrifugation of isolated protoplasts of micronucleated cells as it readily produces microcells for microprotoplast isolation.
References
Binsfeld P, Wingender R, Schnabl H (2000) Characterization and molecular analysis of transgenic plants obtained by microprotoplast fusion in sunflower. Theor Appl Genet 101:1250–1258
Damon E (1957) Investigations into the cyto-morphogenetic effects of colchicine on varieties of Sorghum vulgare. Proc Okla Acad Sci 38:9–13
Dewitte A, Eeckhaut T, Van Huylenbroeck J, Van Bockstaele E (2010a) Induction of 2n pollen formation in Begonia by trifluralin and N2O treatments. Euphytica 171:283–293
Dewitte A, Eeckhaut T, Van Huylenbroeck J, Van Bockstaele E (2010b) Meiotic aberrations during 2n pollen formation in Begonia. Heredity 104:215–223
Duquenne B, Eeckhaut T, Werbrouck S, Van Huylenbroeck J (2007) Effect of enzyme concentrations on protoplast isolation and protoplast culture of Spathiphyllum and Anthurium. Plant Cell, Tissue Organ Cult 91:165–173
Eeckhaut T, Werbrouck S, Dendauw J, Van Bockstaele E, Debergh P (2001) Induction of homozygous Spathiphyllum wallisii genotypes through gynogenesis. Plant Cell, Tissue Organ Cult 67:181–189
Eeckhaut T, Werbrouck S, Leus L, Van Bockstaele E, Debergh P (2004) Chemically induced polyploidization of Spathiphyllum wallisii Regel through somatic embryogenesis. Plant Cell Tissue Organ Cult 78:241–246
Famelaer I, Gleba Y, Sidorov V, Kaleda V, Parokonny A, Boryshuk N, Cherep N, Negrutiu I, Jacobs M (1989) Intrageneric asymmetric hybrids between Nicotiana plumbaginifolia and Nicotiana sylvestris obtained by gamma-fusion. Plant Sci 61:105–117
Gleba Y, Hinnisdaels S, Sidorov V, Kaleda V, Parokonny A, Boryshuk N, Cherep N, Negrutiu I, Jacobs M (1988) Intergeneric asymmetric hybrids between Nicotiana plumbaginifolia and Atropa belladonna obtained by gamma-fusion. Theor Appl Genet 76:760–766
Hertel C, Quader H, Robinson D, Marme D (1980) Anti-Microtubular herbicides and fungicides affect Ca2+ transport in plant-mitochondria. Planta 149:336–340
Matthews D, Millam S, Wilkinson M (1999) Factors influencing the utility of gametic microprotoplasts for partial genome transfer in potato. Plant Cell Rep 18:786–790
McCormick S (1993) Male gametophyte development. Plant Cell 5:1265–1275
Morejohn L, Bureau T, Molebajer J, Bajer A, Fosket D (1987) Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264
Murashige T, Skoog F (1962) A revised medium for rapid growth and Bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Peng Y, Wang Z, Cheng L, Chen H (2003) Effect of phosphoric amide herbicide APM on the structure and protein composition of chromosome in Triticum durum. Plant Prod Sci 6:134–138
Puite K, Schaart J (1993) Nuclear genomic composition of asymmetric fusion products between irradiated transgenic Solanum brevidens and Solanum tuberosum: limited elimination of donor chromosomes and polyploidization of the recipient genome. Theor Appl Genet 86:237–244
Ramulu K, Verhoeven H, Dijkhuis P (1987) Mitotic dynamics of induced micronuclei and prospects for chromosome-mediated gene transfer in plants. Theor Appl Genet 75:575–584
Ramulu K, Verhoeven H, Dijkhuis P (1991) Mitotic Blocking, micronucleation, and chromosome doubling by oryzalin, amiprophos-methyl, and colchicine in potato. Protoplasma 160:65–71
Ramulu K, Dijkhuis P, Famelaer I, Cardi T, Verhoeven H (1994) Cremart: a new chemical for efficient induction of micronuclei in cells and protoplasts for partial genome transfer. Plant Cell Rep 13:687–691
Ramulu K, Dijkhuis P, Rutgers E, Blaas J, Verbeek W, Verhoeven H, Colijnhooymans C (1995) Microprotoplast fusion technique: a new tool for gene-transfer between sexually-incongruent plant-species. Euphytica 85:255–268
Saito F, Nakano M (2001) Partial synchronization of cell division and micronucleation in suspension-cultured cells of Hemerocallis hybrida: the effects of hydroxyurea and various spindle toxins. Breeding Sci 51:285–291
Saito H, Nakano M (2002a) Isolation and characterization of gametic microprotoplasts from developing microspores of Lilium longiflorum for partial genome transfer in the Liliaceous ornamentals. Sex Plant Reprod 15:179–185
Saito H, Nakano M (2002b) Isolation and characterization of microprotoplasts from propyzamide-treated cell suspension cultures of Hemerocallis hybrida. Breeding Sci 52:51–56
Vaughn K, Lehnen L (1991) Mitotic distrupter herbicides. Weed Sci Soc Am 39:450–457
Verhoeven H, Ramulu K, Dijkhuis P (1990) A comparison of the effects of various spindle toxins on metaphase arrest and formation of micronuclei in cell-suspension cultures of Nicotiana plumbaginifolia. Planta 182:408–414
Wijbrandi J, Zabel P, Koornneef M (1990) Restriction fragment length polymorphism analysis of somatic hybrids between Lycopersicon esculentum and irradiated L. peruvianum: evidence for limited donor genome elimination and extensive chromosome rearrangements. Mol Gen Genet 222:270–277
Zhang Q, Liu J, Deng X (2006) Isolation of microprotoplasts from a partially synchronized suspension culture of Citrus unshiu. J Plant Physiol 163:1185–1192
Acknowledgments
The authors wish to thank Deroose Plants for providing plant material and Ronald van den Oord for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A.-C. Schmit.
Rights and permissions
About this article
Cite this article
Lakshmanan, P.S., Eeckhaut, T., Van Huylenbroeck, J. et al. Micronucleation by mitosis inhibitors in developing microspores of Spathiphyllum wallisii Regel. Plant Cell Rep 32, 369–377 (2013). https://doi.org/10.1007/s00299-012-1370-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1370-5