Abstract
Artificially inducing 2n gametes through chromosome doubling is an effective way to obtain polyploids. In this study, Eucalyptus urophylla microsporogenesis and flower development were investigated to guide 2n pollen induction. We also investigated suitable conditions for colchicine treatment. Our results showed that E. urophylla 2n pollen was spherical and had a large volume (mean diameter 28.57 ± 0.46 μm), while normal untreated pollen (mean diameter 19.68 ± 0.11 μm) was tetrahedron. The highest rate of 2n pollen production was 28.71 % when the flower buds, which ranged in size from 3.5 to 4.0 mm, underwent treatment with 0.5 % colchicine solution for 6 h. Further studies suggested that diplotene to diakinesis and metaphase I to telophase I were suitable meiotic stages for chromosomes doubling, due to asynchronous development of microsporogenesis between the anthers in a single flower bud. These data help to illuminate research in other areas, such as triploid eucalyptus production by chromosome doubling of female gametes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus tree species, which belonging to the family Myrtaceae (Ladiges et al. 2003), are one of the most important hardwoods planted worldwide (Doughty 2000). In the latest taxonomic revision, over 700 species, belonging to 13 main evolutionary lineages, were recognized (Brooker 2000). Although some varieties are widely planted around the world because of their rapid growth, straight form, valuable wood properties, wide adaptability to soils and climates, and ease of management through coppicing, these trees are still in the early stages of domestication compared to crop species (Eldridge et al. 1993; Potts and Dungey 2004; Potts and Jackson 1986; Potts and Reid 1988).
Polyploids, particularly triploids induced by sexual polyploidization, are of great value to tree breeders, as they combine the merits of both genome dosage effects and heterosis (Adiwilaga and Brown 1991; Barba-Gonzalez et al. 2006; Becerra and Orjeda 2002; Dewitte et al. 2010). Some studies in Populus recently suggested that triploid trees had huge advantages in comparison with their diploid counterparts, such as faster growth rate, better timber quality, and higher stress resistance (Einspahr 1984; Weisgerber et al. 1980; Zhu et al. 1998). In general, the generation of 2n gametes through chromosome doubling is an effective way to obtain polyploids (Wang et al. 2010, 2012a, b; Lu et al. 2013, 2014). Since 2n gametes in a genus are rather exceptional, an efficient method of inducing 2n gametes is needed (Dewitte et al. 2009, 2010). While tetraploid Eucalyptus was artificially induced in vitro by colchicine treatment (Janaki-Ammal and Khosla 1969; Lin et al. 2010; Han et al. 2011), there are no reports of triploid Eucalyptus production induced by sexual polyploidzation (Myburg et al. 2007). Further, the production of 2n gametes by chromosome doubling during meiosis is conducive for genetic analysis and to accelerate the development of advanced germplasm in eucalyptus.
Colchicine has been applied to zygotes, seedlings and tissue as a polyploidizing agent in many plants (Pereira et al. 2014; Takamura and Miyajima 1996; van Tuyl et al. 1992; Arisumi 1964; Kang et al. 2004; Wang et al. 2010, 2012a, b; Li et al. 2008; Kobayashi et al. 2008; Johnsson and Eklundh 1940). Xi et al. (2011) reported 2n pollen induction in Populus × popularis by arresting meiosis via colchicine treatment as well as triploid formation from the offspring of Populus × euramericana. (Dode) Guinier pollinated with induced pollen from Populus × popularis. Kang et al. (1999, 2000) successfully induced over 88 % 2n pollen with colchicine treatment at pachytene in Populus, suggested that the efficiency of 2n gamete induction depended on whether the treatment period was suitable for chromosome doubling during microsporogenesis. However, these conditions are poorly understood in Eucalyptus, which could be a potential obstacle to Eucalyptus polyploidizing induction.
In this study, Eucalyptus urophylla microsporogenesis and flower development were investigated to guide the induction of 2n pollen. Suitable conditions for colchicine treatment were also investigated as a basis for further research, such as triploid eucalyptus production by chromosome doubling of female gametes.
Materials and methods
Plant materials
Floral branches used in this study were selected from of an Eucalyptus urophylla clone (2n = 2× = 22), which contains 10 individual trees. This clone was planted in a seed orchard at the Guangxi Dongmen Forest Farm (Guangxi Zhuang Autonomous Region, P R China), which was built in 1999.
Determination of the microsporogenesis development process
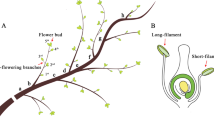
Eucalyptus flowers were observed in a cluster in an inflorescence, which was born in the axil of a leaf. The unit inflorescence is generally a simple umbel with 5–7 single flower buds (Doughty 2000). In Eucalyptus urophylla, flower development was asynchronous at different locations on a branch. The flower buds grew on the lower side of the branch showed greater increases in diameter. In addition, there is a relationship between microsporogenesis development and flower bud diameter growth (Yang and Kang 2015; Yang et al. 2016). In this study, when the microsporogenesis in the selected branches was observed for the first time, flower buds were sampled every 12 h before pollination in order to investigate the meiotic development of the pollen mother cell (PMC). All flower buds collected from the branch were fixed in Carnoy’s fixative (ethanol:acetic acid, 7:3) for 24 h at 4 °C, and were then preserved in 70 % ethanol at 4 °C for long-term storage until cytological observation. Because flower buds differed in size at the same time on the same branch, flower buds were measured before being fixed for further analysis. Different sizes of flower buds at different stages of development were collected and measured from June to August 2013.
To analyze microsporogenesis, anthers containing cells undergoing meiosis were extracted from flower buds, squashed, and stained in 2 % acetocarmine for approximately 5 min. Slides containing cell spreads were observed under a microscope (BX51; Olympus, Tokyo, Japan). Photomicrographs were acquired using the Olympus DP70 camera system.
Colchicine treatment
After associating the development of microsporogenesis with flower morphological characteristics, a total of 2956 flower buds of suitable size from 8 branches were treated with 0.5 % colchicine solution for 3 or 6 h. In order to analyze their meiosis period, another 528 flower buds which were in the same state from nearby branches were measured and collected in the meantime for further cytological observation. Colchicine treatment was performed according to Kang et al. (2000), colchicine in a centrifuge tube was slowly injected to the appropriate location (an incision near the floral shoot) on a flowering branch. Untreated flower buds served as the control group. To determine the treatment efficiency, treated flower buds were collected around anthesis for further cytological analysis; treatment was considered to be successful when at least 1 % large pollen was present (Dewitte et al. 2010).
Statistical analysis
To assess for differences among and between treatment durations, 2n pollen production efficiency data, including the proportion of successfully treated flower buds which at least 1 % large pollen was present, were analyzed using an analysis of variance (ANOVA). When treatments were significantly different, a least significant difference (LSD) multiple comparison test was used for pairwise comparison. Prior to analysis, percent 2n pollen rate data were transformed by the arcsine of the square root of p/100. Pearson’s correlation coefficient was calculated between the rate of 2n pollen production and the percentage of a certain male meiotic stage. All statistical analyses were performed using the SPSS software (version 19.0) or OriginPro software (version 8.5).
Results
Flower bud size and relationship with microsporogenesis
Most male meiotic stages in Eucalyptus urophylla were observed during the cytological analysis (Fig. 1). The most remarkable cytological finding was that multiple microsporogenesis developmental stages were observed in one section of a bud (Fig. 1l). After individual observations of each anther, asynchronous microsporogenesis development was observed between anthers in a single flower bud (Table 1). In general, an Eucalyptus urophylla flower bud contains two types of stamens: a longer one (close to the style in a bud) and a shorter one (away from the style in a bud). Microsporogenesis of the longer stamens was always at a later developmental stage than that of the shorter stamens. Based on the regular rule of asynchronous meiotic development between different stamens, the meiotic developmental stage of short stamens was predictable when the data of longer stamens was known. To facilitate discrimination, the meiotic stage was determined based on the primary meiotic stage of the anther on the longer stamens (close to the style in a bud).
We studied the relationship between flower bud size and male meiotic stage to guide colchicine treatments (Table 2). In this study, male meiosis did not occur when the flower bud size was smaller than 3.0 mm. When the flower bud diameters ranged from 3.0 to 3.5 mm, some PMCs began to undergo male meiosis and some microsporocytes were in the PMC to diakinesis stages (Fig. 1d). When the flower bud diameters ranged from 3.5 to 4.0 mm, we observed that most meiotic stages were in the PMC to tetrad stages (Fig. 1j). Although several meiotic stages, including leptotene (Fig. 1a), pachytene (Fig. 1b), and diplotene (Fig. 1c) were observed, microspores (Fig. 1k) were first observed when the flower bud size increased to 4.5 mm. When the flower bud diameters ranged from 4.5 to 5.0 mm, meiotic stages, including metaphase I (Fig. 1e), anaphase I (Fig. 1f), telophase I (Fig. 1g), metaphase II (Fig. 1h), and anaphase II (Fig. 1i), were observed. When flower bud diameters were greater than 5.0 mm, the meiotic development stage was at least in prophase II.
2n pollen induction via colchicine treatment
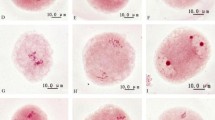
According to the relationship between flower bud size and male meiotic stage, a total of 2956 flower buds were divided into six groups for colchicine treatment, based on their diameters (Table 3). Cytological observation of treated flower buds showed that 2n pollen produced by colchicine-induced microspore chromosome doubling was successful (Fig. 2). Cytokinesis was simultaneous in the control group; the observation of abundant dyads in the treated group indicated the validity of colchicine treatment for chromosome doubling during microsporogenesis (Fig. 2a). In the control group, untreated normal pollen was tetrahedron, and the pollen diameter ranged from 18.56 to 21.16 μm (19.68 ± 0.11 μm). Compared with the control group, giant pollen was spherical with a much greater volume (Fig. 2b); 2n pollen diameter ranged from 27.16 to 33.06 μm (28.57 ± 0.46 μm). The ANOVA revealed a significant difference between normal and 2n pollen (F = 642.963, p = 0.010).
Effect of colchicine treatment on pollen mother cells (PMCs) in Eucalyptus urophylla. Scale bar = 10 μm. a Observation of dyad (arrows) indicated the validity of colchicine treatment for chromosome doubling during microsporogenesis. b Compared with normal tetrahedral pollen, 2n pollen (arrow) was spherical and had a much larger volume
Table 3 shows the effect of colchicine treatment on 2n pollen formation in different Eucalyptus urophylla flower bud sizes. Although only a few flower buds did not survive during the treatment because of the chemical or mechanical damage, our results showed that the highest rate of successful treatment was 28.71 % when the group that ranged in size from 3.5 to 4.0 mm underwent treatment with a 0.5 % colchicine solution for 6 h. Treatments were not valid in groups that were less than 3.0 mm or greater than 5.0 mm in size. According to the 2n pollen production efficiency data, the ANOVA revealed significant differences among groups of different flower bud sizes (F = 8.684, p = 0.010). The effects of treatment duration did not significantly differ (F = 0.645, p = 0.441). Further, the LSD multiple comparison tests showed that 2n pollen production efficiency was not significantly different between the groups that ranged from 3.5 to 4.0 and 4.0 to 4.5 mm; however, the 2n pollen production efficiency of these two groups was significantly better than that in groups with diameters ranging from 3.0 to 3.5 mm (α = 0.05). Therefore, our data suggest that flower bud diameters between 3.5 to 4.5 mm were suitable for microspore chromosome doubling in this species of eucalypt.
Stages in which colchicine treatment effectively induced pollen chromosome doubling
To determine the meiotic stages in which treatment was effective at microspore chromosome doubling, six groups comprised of 528 total flower buds of different sizes were analyzed by cytological observation (Table 4). Although each group of different-sized flower buds had asynchronous meiotic development, some meiotic stages were predominant. For example, in the group of flower buds that ranged in size from 3.5 to 4.0 mm, 29.08 and 31.21 % were in the leptotene to pachytene and diplotene to diakinesis meiotic stages, respectively, which was higher than other stages observed in the same group.
We used Pearson’s correlation analyses to determine the correlation between the 2n pollen rate and male meiotic stage percentage following treatment with 0.5 % colchicine solution for 6 h (i.e., one of the most suitable treatment conditions for microspore chromosome doubling). On the vertical coordinates in each graph (Fig. 3), meiotic stages proportion of treated flower buds in each size group were determined based on the relationship between microsporogenesis development and flower bud diameter growth (Table 4). The percentage of both meiotic stages (i.e., diplotene to diakinesis and metaphase I to telophase I) was significantly positively correlated with 2n pollen production in different flower bud size groups (r = 0.8955, p = 0.0558 and r = 0.8424, p = 0.00353, Fig. 3). However, the percentage value of the group diplotene to diakinesis cannot match the 2n pollen efficiency, neither did the group metaphase I to telophase I. Then, the sum of the percentage of these two groups was also significant positively correlated with 2n pollen production (r = 0.9704, p = 0.0052), suggesting that both of these two meiotic stage groups may be suitable treatment periods for chromosome doubling. In addition, the rate of flower buds successfully treated was gradually decreased with increasing the ratio of microspore at the end of the microsporogenesis (r = −0.3889, p = 0.4460).
Pearson’s correlation analyses of the 2n pollen production rate and male meiotic stages following treatment with 0.5 % colchicines solution for 6 h. Each point plotted by 2n pollen production rates and the proportion of meiotic stages. On the vertical coordinates in each graph, meiotic stage proportion of treated flower buds were determined based on the relationship between microsporogenesis development and flower bud diameter growth
Discussion
Previous research showed that the pollen ploidy level was related to their size (Adiwilaga and Brown 1991; Akutsu et al. 2007; Crespel et al. 2006; Dewitte et al. 2009, 2010; Okazaki et al. 2005): the diameter of 2n pollen was about 28–50 % larger than normal pollen depending on the species (Dewitte et al. 2009, 2010; Akutsu et al. 2007; Mao et al. 2013). Our results demonstrated that colchicine treatment could be an effective way to increase pollen size in Eucalyptus urophylla (Fig. 2); the mean diameter of treated pollen increased by 40 % compared with the control group. The pollen morphology was also different between the treated and control groups, which indicated that the increased DNA amount affected the shape of the pollen wall (Blackmore et al. 2007). These changes could be a simple way to distinguish giant pollen from normal pollen. Furthermore, cytokinesis during microsporogenesis in Eucalyptus was simultaneous (Davis 1968, 1969). Observation of dyads in our study indicated the validity of colchicine treatment for inducing chromosome doubling, which could be another way to identify the occurrence of 2n pollen.
It was known that flower bud size could be correlated with the pollen development stage during microsporogenesis in many plants (Wang et al. 2010, 2012a, b; Lu et al. 2013, 2014; Kang et al. 1999, 2000; Mao et al. 2013; Gao et al. 2004). Based on our study in Eucalyptus urophylla, pollen developmental stages among different flower buds were not synchronous. However six flower bud size groups, which were categorized by diameter, could be correlated to developmental stages ranging over several adjacent meiotic stages. According to previous research, the efficiency of 2n gametes induction depended on whether the treatment period was suitable for chromosome doubling during microsporogenesis (Kang et al. 1999, 2000). Gao et al. (2004) directly injected colchicine solution into flower buds at pachytene, and successfully induced 2n pollen in Eucommia ulmoides; the highest proportion was 49.5 %. Similar to Populus, over 80 % of 2n pollen was induced in the late laptotene to pachytene following colchicine treatment (Kang et al. 1999, 2000; Li et al. 2014). In this study, the highest rate of 2n pollen production was 28.71 %, when the diameter of flower buds ranged from 3.5 to 4.0 mm. Further research showed that the main meiotic stages were comprised of leptotene to diakinesis in this treatment group. In addition, it was notable that 13.13 % of 2n pollen was induced when the diameter of flower buds ranged from 4.5 to 5.0 mm, while the main meiotic stages were comprised of prophase II to microspore in this treatment group. This difference could be due to the asynchronous development of microsporogenesis between anthers in each eucalyptus flower bud. Davis (1968, 1969) reported the existence of two different length stamens in eucalyptus flower buds: the first-formed stamens (which were longer and closer to the style in a bud), and the shorter stamen away from the style. According to our study, the meiotic stages in the longer stamens were more advanced than that of the shorter stamens. To facilitate discrimination, the meiotic stage was determined based on the primary meiotic stage of the anther on the longer stamens (close to the style in a bud). In a single flower bud, some of the stamens were still in the early meiotic stages, which may be suitable for chromosome doubling, while later meiotic stages (e.g., tetrad) were observed in the bigger-size group of treated flower buds. This phenomenon may make it difficult for breeders to judge the optimal time for treatment, but also make the potential treatment period longer than expected. Therefore, determining the optimal treatment time should mainly based on flower bud size in order to increase the effective rate of treatment (Yang and Kang 2015; Yang et al. 2016). Further, a treatment duration of 6 h was better than 3 h to induce 2n pollen production. The most likely reason was that the colchicine solution was slowly injected into the appropriate location on flowering branch base, and it may take some time to be delivered to the anthers. Compared to previous work by other breeders (Kang et al. 1999, 2000; Li et al. 2014; Mao et al. 2013; Gao et al. 2004, Dover 1972; Wang et al. 2010; Xi et al. 2014), it could effectively reduce mechanical damage to the flower buds and improve the survival rates of treated flower buds.
2n gametes are an important source for innovation in plant breeding (Dewitte et al. 2010). Benefited from the successful induction of 2n pollen, triploids were obtained from offspring crossed with a diploid mother plant (Adiwilaga and Brown 1991; Dewitte et al. 2010; Okazaki et al. 2005; Hahn et al. 1990). In recently years, triploid breeding via 2n egg induction has become one of the most powerful approaches for the improving in Populus (Wang et al. 2010, 2012a, b; Lu et al. 2013, 2014; Li et al. 2008). Compared with microspore chromosome doubling, megasporogenesis and megagametogenesis chromosome doubling had some advantages in yield efficiency to produce triploids in Populus, because these could directly generate triploids after control or open pollination without distinguishing or removing normal pollen from 2n pollen. Our study on 2n pollen induction, particularly the determination of suitable treatment conditions for chromosome doubling, could provide illumination for further polyploidy breeding in Eucalyptus.
References
Adiwilaga KD, Brown CR (1991) Use of 2n pollen-producing triploid hybrids to introduce tetraploid Mexican wild species germ plasm to cultivated tetraploid potato gene pool. Theor Appl Genet 81:645–652
Akutsu M, Kitamura S, Toda R, Miyajima I, Okazaki K (2007) Production of 2n pollen of Asiatic hybrid lilies by nitrous oxide treatment. Euphytica 155:143–152
Arisumi T (1964) Colchicine-induced tetraploid and cytochimeral daylilies. J Hered 55:255–260
Barba-Gonzalez R, Miller CT, Ramanna MS, van Tuyl JM (2006) Nitrous oxide (N2O) induces 2n gametes in sterile F1 hybrids between oriental × asiatic lily (Lilium) hybrids and leads to intergenomic recombination. Euphytica 148:303–309
Becerra LL, Orjeda G (2002) Occurrence and cytological mechanism of 2n pollen formation in a tetraploid accession of Ipomoea batatas (sweet potato). J Hered 93:185–192
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Brooker MIH (2000) A new classification of the genus Eucalyptus L’Hér. (Myrtaceae). Aust Syst Bot 13:79–148
Crespel L, Ricci SC, Gudin S (2006) The production of 2n pollen in rose. Euphytica 151:155–164
Davis GL (1968) Floral morphology and the development of gametophytes in Eucalyptus melliodora A. Cunn. Aust J Bot 16:19–35
Davis GL (1969) Floral morphology and the development of the gametophytes in Eucalyptus stellulata Sieb. Aust J Bot 17:177–190
Dewitte A, Eeckhaut T, van Huylenbroeck J, van Bockstaele E (2009) Meiotic aberrations during 2n pollen formation in Begonia. Heredity 104:215–223
Dewitte A, Eeckhaut T, van Huylenbroeck J, van Bockstaele E (2010) Induction of 2n pollen formation in Begonia by trifluralin and N2O treatments. Euphytica 171:283–293
Doughty RW (2000) The Eucalyptus. A natural and commercial history of the gum tree. The Johns Hopkins Univ Press, Baltimore, p 237
Dover GA (1972) The organization and polarity of pollen mother cells of Triticum aestivum. J Cell Sci 11:699–711
Einspahr DW (1984) Production and utilization of triploid hybrid aspen. Iowa State J Res 58:401–409
Eldridge K, Davidson J, Harwood C, van Wyk G (1993) Eucalypt domestication and breeding. Oxford University Press, Oxford, p 288
Gao P, Lin W, Kang X (2004) Pollen chromosome doubling of Eucommia ulmoides induced by colchicine. J Beijing For Univ 26:39–42
Hahn SK, Bai KV, Asiedu R (1990) Tetraploids, triploids, and 2n pollen from diploid interspecific crosses with cassava. Theor Appl Genet 79:433–439
Han C, Xu JM, Du ZH, Li GY, Zeng BS, Wu SJ, Wang W (2011) Polyploidy induction of clone of Eucalyptus grandis with colchicine. Afr J Biotechnol 10:14711–14717
Janaki-Ammal EK, Khosla SN (1969) Breaking the barrier to polyploidy in the genus Eucalyptus. Proc Indian Acad Sci Sect B 70:248–249
Johnsson H, Eklundh C (1940) Colchicine treatment as a method in breeding hardwood species. Sven Papp Tidn 43:373–377
Kang X, Zhu Z, Lin H (1999) Study on the effective treating period for pollen chromosome doubling of Populus tomentosa × P. Bolleana. Sci Silave Sin 35:21–24
Kang X, Zhu Z, Zhang Z (2000) Suitable period of high temperature treatment for 2n pollen of Populus tomentosa × P. bolleana. J Beijing For Univ 22:1–4
Kang X, Zhang P, Gao P, Zhao F (2004) Discovery of a new way of poplar triploids induced with colchicine after pollination. J Beijing For Univ 26:1–4
Kobayashi N, Yamashita S, Ohta K, Hosoki T (2008) Morphological characteristics and their inheritance in colchicines-induced Salvia polyploids. J Jpn Soc Hortic Sci 77:186–191
Ladiges PY, Udovicic F, Nelson G (2003) Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. J Biogeogr 30:989–998
Li YH, Kang XY, Wang SD, Zhang ZH, Chen HW (2008) Triploid induction in Populus alba × P. glandulosa by chromosome doubling of female gametes. Silvae Genet 57:37–40
Li Y, Guo Q, Wang J, Tian J, Kang X (2014) Colchicine-induced pollen chromosome doubling and its cytological effects in Populus alba L. J Nucl Agric Sci 28:749–756
Lin H, Jian M, Liang LY, Pei WJ, Liu XZ, Zhang HY (2010) Production of polyploids from cultured shoot tips of Eucalyptus globulus Labill by treatment with colchicine. Afr J Biotechnol 9:2252–2255
Lu M, Zhang P, Kang X (2013) Induction of 2n female gametes in Populus adenopoda Maxim by high temperature exposure during female gametophyte development. Breed Sci 63:96–103
Lu M, Zhang P, Wang J, Kang X, Wu J, Wang X, Chen Y (2014) Induction of tetraploidy using high temperature exposure during the first zygote division in Populus adenopoda Maxim. Plant Growth Regul 72:279–287
Mao YK, Zhang PD, Shi L, Dong CB, Suo YJ, Kang XY (2013) The optimum conditions for inducing 2n pollen chromosome doubling by high temperature in Eucommia Ulmoides. J Beijing For Univ 35:53–58
Myburg AA, Potts BM, Marques CM, Kirst M, Gion GM, Grattapaglia D, Grima-Pettenatti J (2007) Eucalyptus. In: Kole C (ed) Genome mapping and molecular breeding in plants, volume 7, forest trees. Springer, Berlin, pp 115–160
Okazaki K, Kurimoto K, Miyajima I, Enami A, Mizuochi H, Matsumoto Y, Ohya H (2005) Induction of 2n pollen in tulips by arresting the meiotic process with nitrous oxide gas. Euphytica 143:101–114
Pereira RC, Ferreira MTM, Davide LC, Pasqual M, Mittelmann A, Techio VH (2014) Chromosome duplication in Lolium multiflorum Lam. Crop Breed Appl Biotechnol 14:251–255
Potts BM, Dungey HS (2004) Interspecific hybridization of Eucalyptus: key issues for breeders and geneticists. New Forest 27:115–138
Potts BM, Jackson WD (1986) Evolutionary processes in the Tasmanian high altitude eucalypts. In: Barlow BA (ed) Flora and fauna of alpine Australasia. Ages and origins. CSIRO, Melbourne, pp 511–527
Potts BM, Reid JB (1988) Hybridisation as dispersal mechanism. Evolution 42:1245–1255
Takamura T, Miyajima I (1996) Colchicine induced tetraploids in yellow-flowered cyclamens and their characteristics. Sci Hortic 65:305–312
van Tuyl JM, Meijer B, van Diën MP (1992) The use of oryzalin as an alternative for colchicine in in vitro chromosome doubling of Lilium and Nerin e. Acta Hortic 352:625–630
Wang J, Kang XY, Li DL, Chen HW, Zhang PD (2010) Induction of diploid eggs with colchicine during embryo sac development in populus. Silvae Genet 59:40–41
Wang J, Kang XY, Li DL (2012a) High temperature-induced triploid production during embryo sac development in Populus. Silvae Genet 61:85–93
Wang J, Li D, Kang X (2012b) Induction of unreduced megaspores with high temperature during megasporogenesis in Populus. Ann For Sci 69:59–67
Weisgerber H, Rau HM, Gartner EJ, Baumeister G, Kohnert H, Karner L (1980) 25 years of forest tree breeding in Hessen. Allg For 26:665–712
Xi XJ, Jiang XB, Li D, Guo LQ, Zhang JF, Wei ZZ, Li BL (2011) Induction of 2n pollen by colchicine in Populus × popularis and its triploids breeding. Silvae Genet 60:155–160
Xi X, Guo L, Xu W, Zhang J, Li B (2014) Megasporogenesis, megagametogenesis, and induction of 2n eggs with colchicine in poplar section. Scand J For Res 29:527–536
Yang J, Kang X (2015) Microsporogenesis and flower development in Eucalyptus urophylla × E. tereticornis. Breed Sci 65:138–144
Yang J, Lan J, Yao P, Huang Z, Kang X (2016) Comparative microsporogenesis and flower development in Eucalyptus urophylla × E. grandis. J For Res 27:257–263
Zhu ZT, Kang XY, Zhang ZY (1998) Studies on selection of natural triploids of Populus tomentosa. Sci Silave Sin 34:22–31
Acknowledgments
This research was financially supported by the Special Fund for Forest Scientific Research in the Public Welfare (201404113), the 111 Project (B13007) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT13047). The authors would like to thank Jun Lan and Donglin Chen from Guangxi Dongmen Forest Farm for additional help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Yao, P., Li, Y. et al. Induction of 2n pollen with colchicine during microsporogenesis in Eucalyptus . Euphytica 210, 69–78 (2016). https://doi.org/10.1007/s10681-016-1699-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-016-1699-x