Abstract
Key message
Apocynin is a natural organic compound structurally related to vanillin. We demonstrated that hydrogen peroxide and heme oxygenase participated in apocynin-induced lateral root formation in rice.
Abstract
Apocynin, also known as acetovanillone, is a natural organic compound structurally related to vanillin. Information concerning the effect of apocynin on plants is limited. In this study, we examined the effect of apocynin on lateral root (LR) formation in rice. Treatment with apocynin induced LR formation and increased H2O2 production, but had no effect on nitric oxide production. Diphenyleneiodonium chloride, an inhibitor of H2O2 generating NADPH oxidase, was effective in reducing apocynin-induced H2O2 production and LR formation. Apocynin treatment also increased superoxide dismutase activity and decreased catalase activity. H2O2 application was able to increase the number of LRs. Moreover, H2O2 production caused by H2O2 and apocynin was localized in the root area corresponding to the LR emergence. Treatment with H2O2 and apocynin also increased heme oxygenase (HO) activity and induced OsHO1 mRNA expression. Lateral root formation and HO activity induced by H2O2 and apocynin were reduced by Zn protoporphyrin IX (the specific inhibitor of HO). Our data suggest that both H2O2 and HO are required for apocynin-induced LR formation in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen peroxide is a constituent of oxidative metabolism and is itself a reactive oxygen species (ROS). The accumulation of H2O2 increases the probability of hydroxyl radical formation via a Fenton-type reaction. This leads to the phenomenon known as oxidative stress (Foyer and Noctor 2000). Initially, H2O2 was only considered damaging to cells (Gechev et al. 2006). More recently, H2O2 emerged as ubiquitous signaling molecule (Gechev et al. 2006). H2O2 is involved in many developmental and physiological processes (Gapper and Dolan 2006; Kwak et al. 2006). It acts as a signal molecule in the formation of adventitious roots, lateral roots (LRs), and root hairs (Su et al. 2006; Dunand et al. 2007; Li et al. 2007, 2009a, b; Huang et al. 2011). Nitric oxide (NO) is now emerging as an important signaling molecule in many important physiological processes in plants (Lamattina et al. 2003; Besson-Bard et al. 2008). There is increasing evidence indicating that NO also plays a critical role in root development such as LR formation (Correa-Aragunde et al. 2004; Guo et al. 2008; Chen and Kao 2012).
Heme oxygenase (HO) is a ubiquitous and highly active enzyme, which catalyzes the degradation of heme to produce carbon monoxide (CO), free iron, and biliverdin IXα (BV) (Kicuchi et al. 2005). HO is a small family with several members. It has been found that HO1 is clearly the one most highly expressed, followed by HO2, with both HO3 and HO4 expressed at low levels (Matsumoto et al. 2004). In plants, HO1 has been shown to be associated with LR formation (Cao et al. 2007; Guo et al. 2008; Chen et al. 2012; Han et al. 2012). The expression of HO1 has been shown to be induced by H2O2 (Yannarelli et al. 2006; Chen et al. 2009) and NO (Noriega et al. 2007).
Apocynin (4-hydroxy-3-methoxyacetophenone, acetovanillone) is a compound originally isolated from the medicinal plant Picrorhiza kurroa, a small perennial herb that grows in the Himalayas. It not only acts as an inhibitor of phagocyte NADPH oxidase but also as a ROS production stimulator in non-phagocytic cells (Vejrazka et al. 2005). Riganti et al. (2008) demonstrated that apocynin induces NO synthesis in N11 mouse cells. In maize leaves, apocynin increased NO production (Tossi et al. 2009). This is the only work showing that apocynin increases NO production in plants. To date, it is not known whether apocynin increases H2O2 in plants. Neither do we know whether apocynin promotes LR formation.
In this work, we first examined the effect of apocynin on the formation of LRs and production of NO and H2O2 in rice. In our recent study, we demonstrated that HO is involved in LR formation in the rice root system (Chen et al. 2012). Thus, the possible role of H2O2, NO, and HO in regulating apocynin-induced LR formation in rice is also examined.
Materials and methods
Plant material and growth conditions
Seeds of rice (Oryza sativa L., cv. Taichung Native 1, an Indica type) were sterilized with 3 % sodium hypochlorite for 15 min and washed extensively with distilled water. To obtain more uniformly germinated seeds, rice seeds in a Petri dish (20 cm) containing distilled water were pretreated at 37 °C for 1 day under dark conditions. Uniformly germinated seeds were then selected and transferred to a Petri dish (20 cm) containing two sheets of filter paper moistened with distilled water for 2 days. Two-day-old seedlings were then transferred to Petri dishes (9 cm) containing distilled water, apocynin, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), diphenyleneiodonium chloride (DPI), zinc protoporphyrin IX (ZnPPIX), at the desired concentration. Root growth of rice seedlings grown in distilled water is similar to that grown in medium containing inorganic salts; thus, seedlings grown in distilled water were used as the control. Each Petri dish contained five seedlings and each treatment was replicated four times. The seedlings were allowed to grow at 27 °C in darkness. The seminal roots of rice seedlings at the times specified were used for the analysis of LR formation, HO activity, and OsHO1 transcripts.
LR formation
To show LR formation in seminal roots for each treatment, the number of LRs longer than 1 mm per seedling was counted. For some experiments, photographs of seedlings were taken. Representative photographs of seedlings were shown in figures.
Detection of endogenous NO and H2O2
The NO and H2O2 were detected by fluorescence microscopy. Nitric oxide and H2O2 imaging were conducted according to Xiong et al. (2009) and Shin and Schachtman (2004), respectively. For NO detection, rice roots were incubated in 20 μM of the cell-permeable fluorescence probe 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA) in 20 mM HEPES (pH 7.5) for 15 min, whereas for H2O2 detection, rice roots were incubated in 50 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, dissolved in 0.0025 % dimethyl sulfoxide) in the dark for 30 min. A Nikon SMA 1500 stereoscopic fluorescence microscope was used for fluorescence image. Fluorescence was visualized by excitation and extinction at 495 and 515 nm, respectively.
For the in situ histochemical detection of H2O2, root segments stained with CM-H2DCFDA were cut into approximately 1-cm segments. The segments were embedded in 5 % agar and then longitudinally cut into section of 30–50 μm using vibrating microslicer (DTK-1000; Dosaka EM Co. Ltd., Kyoto, Japan). The longitudinal sections were observed with an Axio Scope A1 fluorescence microscope, following the manufacturer’s instructions (Carl Zeiss, Jena, Germany). Axio Scope A1 images were acquired with an AxioCam camera and processed with Axiovision software.
Enzyme extraction and assays
For the extraction of superoxide dismutase (SOD) and catalase (CAT), ten roots were homogenized with 100 mM sodium phosphate buffer (pH 6.8) in a chilled pestle and mortar. The homogenate was centrifuged at 12,000×g. SOD activity was determined according to Paoletti et al. (1986). The reaction mixture (2.73 mL) contained 100 mM triethanolamine–diethanolamine buffer (pH 7.4), 7.5 mM NADH, EDTA/MnCl2 (100/50 mM, pH 7.4), 10 mM of 2-mercaptoethanol, and enzyme extract (0.2 mL). The reaction was started by adding NADH. The reaction was allowed to proceed for 10 min. The absorbance was measured at 340 nm. One unit of SOD was defined as the amount of enzyme that inhibits by 50 % the rate of NADH oxidation observed in blank sample. CAT activity was assayed according to Kato and Shimizu (1987). The decrease in H2O2 was defined as the decline in the absorbance at 240 nm, and the activity was calculated using extinction coefficient (40 mM−1 cm−1 at 240 nm) for H2O2. One unit of CAT was defined as the amount of enzyme which degraded 1 μmol H2O2 per min.
HO activity was analyzed basically as described (Xuan et al. 2008). For extraction of HO, 25 roots were homogenized with 3 mL of 25 mM HEPES–Tris (pH 7.4) containing 250 mM mannitol, 1 mM EDTA, 1 % (w/v) polyvinylpyrrolidone, 10 % (v/v) glycerol, and 1 mM dithiothreitol. The whole isolation procedure was carried out at 4 °C. The homogenate was centrifuged at 15,000×g for 30 min, and the resulting supernatant was used for determining the activity of HO as described (Xuan et al. 2008). The reaction mixture (1 mL) contained 2 mM deferrioxamine in 100 mM HEPES–NaOH (pH 7.2), 100 μM NADPH, 10 μM Hm, 0.15 mg mL−1 bovine serum albumin, 50 μg mL−1 (4.2 μM) spinach ferredoxin, 0.025 units mL−1 spinach ferredoxin-NADP+ reductase, 5 mM ascorbate and enzyme extract (250 μL). The reaction was started by adding NADPH and allowed to proceed at 37 °C for 30 min. The absorbance of BV was measured at 650 nm. The increase in BV concentration was determined by the extinction coefficient 6.25 mM−1 cm−1 at 650 nm. One unit of activity for HO was defined as the amount of enzyme that produced 1 μmol of BV per 30 min. Rice roots contained very low protein. Thus, SOD, CAT, and HO activities were expressed on a dry weight (DW) basis.
Semi-quantitative RT-PCR
Total RNA was isolated from the roots of seedlings by the TRIzol reagent method (Invitrogen, CA, USA). To prevent DNA contamination, RNA was treated with Turbo DNase I (Ambion, TX, USA) for 30 min at 37 °C before performing RT-PCR. Control PCR amplifications involved RNA as a template after DNase I treatment to verify the elimination of contaminated DNA. Reverse-transcription reactions involved 200 ng of total RNA and the SuperScript III first-strand synthesis RT-PCR system (Invitrogen, CA, USA).
We searched the rice genome annotation project (http://rice.plantbiology.msu.edu/) for the sequence of rice heme oxygenase 1 (OsHO1, LOC_Os06g40080) with Arabidopsis HY1 (Davis et al. 1999) (AtHO1) used as a reference. Gene-specific primers were designed from the 5′ UTR of the rice OsHO1 gene. The sequences used and the predicted amplicon are shown in Table 1. The RT-PCR conditions were 94 °C denaturation for 5 min, then 27–30 cycles of 94 °C for 45 s, 60 °C for 45 s, 72 °C for 45 s, 72 °C extension for 5 min, and finally 16 °C. PCR was optimized for a number of cycles to insure product intensity within the linear phase of amplification. For all treatments, RT-PCR was performed three times with three batches of total RNA isolated independently. PCR products were resolved by electrophoresis in 3 % agarose gel and stained with ethidium bromide. The gel images were captured with the use of a SynGene gel documentation system and analyzed by Genetools (Syngene, MD, USA). The rice OsUbiquitin gene was used for normalization.
Statistical analysis
Data were analyzed by Duncan’s multiple range test. P < 0.05 was considered statistically significant.
Results
Effect of apocynin on the formation of LRs
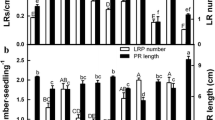
To examine the effect of apocynin on LR formation, 2-day-old seedlings were treated with various concentrations of apocynin for 3 days. In comparison with water treatment, apocynin (1–30 μM) was effective in inducing LR formation (Fig. 1). Apocynin at 10 μM was proved to be an optimal concentration for the promotion of LR formation.
Hydrogen peroxide but no NO is involved in apocynin-induced LR formation
To examine whether apocynin-induced LR formation was the result of the production of H2O2 or NO, 1 μM diphenyleneiodonium chloride (DPI, a NADPH oxidase inhibitor) or 100 μM cPTIO (a NO-specific scavenger), was applied along with 10 μM apocynin. The effect of apocynin on LR formation was significantly inhibited by DPI but not by cPTIO (Fig. 2). Clearly, the effect of apocynin may be attributed to H2O2 produced.
Effect of cPTIO and DPI on LR number of rice seedlings treated with apocynin. Two-day-old rice seedlings were pretreated with 100 μM cPTIO or 1 μM DPI for 3 h and then treated with 10 μM apocynin, respectively for 3 days. Bars indicates standard errors (n = 20). Values with the same letter are not significantly different at P < 0.05
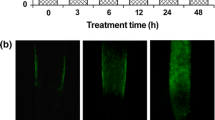
In vivo detection of H2O2 or NO in rice seminal roots was carried out using fluorescence probes. CM-H2DCFDA and DAF-FM DA were used to follow H2O2 and NO production, respectively. As shown in Fig. 3a, apocynin did not induce green fluorescence of NO. However, apocynin induced green fluorescence of H2O2 (Fig. 3b). Interestingly, when DPI at 1 μM was added along with 10 μM apocynin, the apocynin-induced H2O2 fluorescence was completely suppressed (Fig. 3b).
Effect of cPTIO and DPI on NO production (a) and H2O2 production (b) in rice seedlings treated with apocynin. Two-day-old rice seedlings were treated H2O, 100 μM cPTIO, 1 μM DPI, 10 μM apocynin, 10 μM apocynin + 100 μM cPTIO, 10 μM apocynin + 1 μM DPI for 24 h. Experiments were repeated four times with similar results. Representative photograph of rice seedlings were shown. Bars 1 mm
Effect of apocynin on SOD and CAT activities
To examine the effect of apocynin on SOD and CAT activities, 2-day-old rice seedlings were treated with 10 μM apocynin for 24 h. As compared with water treatment, apocynin was effective in increasing SOD activity (Fig. 4a) and decreasing CAT activity (Fig. 4b).
Effect of exogenous H2O2 on LR formation
To examine the effect of H2O2 on LR formation, 2-day-old seedlings were treated with various concentrations of H2O2 for 3 days. In comparison with water treatment, H2O2 (1–2.5 μM) was effective in inducing LR formation (Fig. 4). Hydrogen peroxide at 1 μM was proved to be an optimal concentration for inducing LR formation.
Localization of H2O2
In the longitudinal section examination of seminal roots, H2O2 production in H2O2- and apocynin-treated roots was observed to be localized in the root area corresponding to the LR emergence (Fig. 6).
Effect of apocynin and H2O2 on LR formation and HO activity
To examine whether zinc protoporphyrin IX (ZnPPIX) affects apocynin- and H2O2-promoted LR formation and HO activity, 2-day-old rice seedlings were treated with apocynin and H2O2 for 1 or 3 days. As compared with water treatment, apocynin and H2O2 were effective in inducing LR formation (Fig. 7a) and increasing HO activity (Fig. 7b). It has been shown that ZnPPIX (a potent HO1 inhibitor) inhibits HO activity in plants (Liu et al. 2007; Xuan et al. 2008). Application of ZnPPIX alone had no effect on LR formation and HO activity (Fig. 7a, b). However, pretreatment with 200 μM of ZnPPIX inhibited apocynin- and H2O2-increased LR formation and HO activity (Fig. 7a, b).
Apocynin and H2O2 increase OsHO1 mRNA expression
To examine whether apocynin and H2O2 affect the expression of OsHO1, 2-day-old seedlings were treated with apocynin and H2O2 for 24 h, which increased the mRNA level of OsHO1 (Fig. 8).
Discussion
Lateral roots play important roles in increasing the absorptive capacity of roots to absorb water and mineral nutrients as well as to anchor the plants in the soil (Hao and Ichii 1999; López-Bucio et al. 2003; Wang et al. 2006). Recently, Den Herder et al. (2010) suggest that root architecture, including LR, could be considered as one of the promising features of crops in a new green revolution. Information regarding the effect of apocynin on plants is scarce. It has been shown that apocynin confers antioxidant protection in maize leaves (Tossi et al. 2009). However, the effect of apocynin on LR formation remains largely unknown. In the present study, we observed that apocynin promoted LR formation in rice (Fig. 1). Moreover, we found that HO plays a role in H2O2 signaling leading to LR formation in apocynin-treated rice.
In animal system, apocynin induces NO synthesis (Riganti et al. 2008). Tossi et al. (2009) also demonstrated that apocynin increases NO production in maize leaves. In contrast, we have shown that, in rice roots, apocynin is unable to induce NO production (Fig. 3a).
Apocynin is often used as a specific inhibitor of NADPH oxidase (Riganti et al. 2006). However, Vejrazka et al. (2005) found that apocynin not only acts as an inhibitor of phagocyte NADPH oxidase but also as H2O2 production stimulator in non-phagocytic cells. In the present study, the in vivo detection of H2O2 in rice roots was carried out using a fluorescence probe, CM-H2DCFDA (Fig. 3b). The probe specificity has been checked using ascorbic acid, an H2O2 scavenger (data not shown). Interestingly, we observed that apocynin treatment resulted in an accumulation of H2O2-dependent CM-H2DCFDA fluorescence in rice roots (Fig. 3b).
In several model systems investigated in plants, the accumulation of H2O2 appears to be mediated by the activation of a plasma membrane-bound NADPH oxidase complex (Pei et al. 2000; Orozco-Cardenas et al. 2001; Zhang et al. 2001). DPI is known to inhibit plasma membrane NADPH oxidase (Orozco-Cardenas et al. 2001). DPI (1 μM) can prevent the increased production of H2O2 (Fig. 2b) in seminal roots of rice induced by apocynin (Fig. 2b). These data strongly suggest apocynin-induced accumulation of H2O2 was mediated, at least in part, through the activation of NADPH oxidase. However, apocynin has been shown to be an inhibitor of phagocyte NADPH oxidase (Vejrazka et al. 2005). It is not known whether apocynin induces H2O2 production in other plant species. In this regard, further work is thus required.
The increase in SOD activity and the decrease in CAT activity could result in H2O2 accumulation. We demonstrated that apocynin enhanced SOD activity and deactivated CAT activity in rice roots (Fig. 4a, b). Thus, apocynin-induced H2O2 accumulation is also mediated through the increase in SOD activity and the decrease in CAT activity in rice roots. These results suggest that apocynin may exert other effects besides its ability to induce NADPH oxidase, for instance, it increases SOD activity and decreases CAT activity.
The involvement of H2O2 in the formation of adventitious roots, LRs, and root hairs has been reported (Su et al. 2006; Dunand et al. 2007; Li et al. 2007, 2009a, b; Huang et al. 2011). Our data demonstrate that (a) H2O2 per se is able to induce LR formation (Fig. 5) and increase endogenous H2O2 level (Lin and Kao 2001) in rice seminal roots, (b) apocynin-promoted LR formation (Fig. 1) and H2O2 production (Fig. 3b) could be blocked by DPI (Figs. 2, 3), (c) H2O2 production occurred 24 h after apocynin treatment, whereas LR primordia were observed 42 h after apocynin treatment, indicating apocynin-caused H2O2 production is prior to apocynin-promoted LR formation, and (d) apocynin- and H2O2-induced H2O2 production were localized in root area corresponding to the emergence of LRs (Fig. 6). Thus, this evidence supports the suggestion that H2O2 mediates apocynin-induced LR formation.
H2O2 localization in H2O-, H2O2-, and apocynin-treated rice roots. Two-day-old seedlings wee treated with H2O, H2O2, or apocynin for 42 h. The concentration of H2O2 and apocynin were 1 μM and 10 μM, respectively. Arrowheads indicate LR primordia. Experiments were repeated four times with similar results. Representative photographs of rice seminal roots were shown. Bar 0.2 mm
Our recent results revealed that HO is involved in NO and auxin-induced LR formation in rice (Chen et al. 2012). We found that apocynin and H2O2, which increased HO activity (Fig. 7b) and OsHO1 mRNA level (Fig. 8), could induce LR formation in rice roots (Figs. 1, 5, 6). In addition, apocynin and H2O2-promoted LR formation and HO activity could be blocked by ZnPPIX, a potent HO inhibitor (Fig. 7a, b). Collectively, these data indicated that HO might be involved in apocynin- and H2O2-promoted LR formation in rice roots. More recently, similar function of HO1 from maize in the induction of LR formation was also reported (Han et al. 2012). It is known that CO and BV are generated by HO. It has been shown that CO could mimic or mediate the effect of auxin on the promotion of LR in rapeseed and tomato seedlings (Cao et al. 2007; Guo et al. 2008). Our previous results revealed that HO is involved in NO- and auxin-induced LR formation in rice (Chen et al. 2012).We also provided indirect evidence to show that CO increases HO activity in rice (Chen et al. 2012). More recently, we reported that HO is required for BV-induced LR formation in rice (Hsu et al. 2012a). It is interesting and necessary in the future to investigate the specific role of HO1/CO and HO1/BV in the apocynin-induced LR formation process.
Effects of ZnPPIX on H2O2- and apocynin-increased LR number (a) and HO activity (b) in rice. Two-day-old seedlings were pretreated with or without ZnPPIX for 3 h and then treated with H2O, H2O2, or apocynin, respectively, for 1 day (b) or 3 days (a). The concentrations of H2O2, apocynin, and ZnPPIX were 1, 10, and 200 μM, respectively. Bar indicates standard errors (n = 4). Values with the same letter are not significantly different at P < 0.05
Effect of H2O2 and apocynin on OsHO1 mRNA level in rice roots. Two-day-old seedlings were treated with H2O, 1 μM H2O2, or 10 μM apocynin for 1 day. Semi-quantitative RT-PCR analysis of mRNA levels relative to that of OsUbiquitin. Bar indicates standard errors (n = 3). Values with the same letter are not significantly different at P < 0.05
Jasmonic acid and methyl jasmonate are a class of plant hormones, which mediate various aspects of developmental and stress response (Wasternack 2007). Recently, we have shown that HO is required for methyl jasmonate-induced LR formation in rice (Hsu et al. 2012b). Data in Figs. 7 and 8 suggest that H2O2 is not as effective as apocynin in promoting the LR formation, enhancing the HO activity and inducing the HO1 transcription. Therefore, the effect of apocynin on the LR formation could also be mediated by some other pathway unrelated to H2O2, such as methyl jasmonate.
In summary, this is the first study investigating the effect of apocynin on LR formation in the rice root systems. Our data strongly support that H2O2 is responsible for apocynin-promoted LR formation and HO possesses a central role in determining apocynin- and H2O2-induced LR formation in rice. Apocynin has also been shown to confer antioxidant protection in maize leaves (Tossi et al. 2009). It is not known whether apocynin could protect against oxidative stress in rice seedlings, so further work in this direction seems warranted.
References
Besson-Bard A, Pugin A, Wendehenne D (2008) New insight into nitric oxide signaling in plants. Annu Rev Plant Biol 59:21–39
Cao ZY, Xuan W, Liu ZY, Li XN, Zhao N, Xu P, Wang Z, Guan RZ, Shen WB (2007) Carbon monoxide promotes lateral roots formation in rapeseed. J Integr Plant Biol 49:1070–1079
Chen Y-H, Kao CH (2012) Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 249:187–195
Chen XY, Ding X, Xu S, Wang R, Xuan W, Cao ZY, Chen J, Wu HH, Ye MB, Shen WB (2009) Endogenous hydrogen peroxide plays a positive role in the upregulation of heme oxygenase and acclimation to oxidative stress in wheat seedling leaves. J Integr Plant Biol 51:951–960
Chen Y-H, Chao Y-Y, Hsu YY, Hong C-Y, Kao CH (2012) Heme oxygenase is involved in nitric oxide- and auxin-induced later root formation in rice. Plant Cell Rep 31:1085–1091
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Davis SJ, Kurepa J, Vierstra RD (1999) The Arabidopsis thaliana HY1 locus, required for phytochrome–chromophore biosynthesis encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA 96:6541–6546
Den Herder G, Van Isterdael G, Beckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 5:600–607
Dunand C, Crevecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Foyer CH, Noctor G (2000) Oxygen processing in photosynthesis regulation and signaling. New Phytol 146:359–389
Gapper C, Dolan I (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 28:1091–1101
Guo K, Xia K, Yang Z-M (2008) Regulation of tomato lateral root development by carbon monooxide and involvement in auxin and nitric oxide. J Exp Bot 59:3443–3452
Han B, Xu S, Xie Y-J, Huang J-J, Wang L-J, Yang Z, Zhang C-H, Sun Y, Shen W-B, Xie G-S (2012) ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci 184:63–74
Hao ZB, Ichii M (1999) A mutant RM109 of rice (Oryza sativa L.) exhibiting altered lateral root initiation and gravitropism. Jpn J Crop Sci 68:245–252
Hsu YY, Chao Y-Y, Kao CH (2012a) Biliverdin-promoted lateral root formation is mediated through heme oxygenase in rice. Plant Signal Behav 7:885–887
Hsu YY, Chao Y-Y, Kao CH (2012b) Methyl jasmonate-induced lateral root formation in rice: the role of heme oxygenase and calcium. J Plant Physiol. http://dx.doi.org/10.1016/j.jplph.2012.08.015
Huang A-X, She XP, Cao B-H, Ren Y (2011) Distribution of hydrogen peroxide during adventitious roots initiation and development in mung bean hypocotyls cutting. Plant Growth Regul 64:109–118
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation. Can J Bot 65:729–735
Kicuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567
Kwak JM, Nguyen V, Schroeder JI (2006) The role of reactive oxygen species in hormonal responses. Plant Physiol 141:323–329
Lamattina L, Garcia MC, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Li S-W, Xue L, Xu S, Feng H, An L (2007) Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul 52:173–180
Li S-W, Xue L, Xu S, Feng H, An L (2009a) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65:63–71
Li S-W, Xue L, Xu S, Feng H, An L (2009b) IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: the role of H2O2. Environ Exp Bot 66:442–450
Lin CC, Kao CH (2001) Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant Soil 230:135–143
Liu K, Xu S, Xuan W, Ling T, Cao Z, Huang B, Sun Y, Fan L, Liu Z, Zhao N, Shen WB (2007) Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa. Plant Sci 172:544–555
López-Bucio J, Cruz-Ramiroz A, Herresra-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 5:280–287
Matsumoto F, Obayashi T, Sasaki-Dekimoto Y, Ohta H, Takamiya K-I, Masuda T (2004) Gene expression profiling of the tetrapyrrole metabolic pathway in Arabidopsis with a mini-array system. Plant Physiol 135:2379–2391
Noriega GO, Yannarelli GG, Balestrasse KB, Batle A, Tomaro ML (2007) The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 228:1155–1163
Orozco-Cardenas ML, Arvaez-Váaqjez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Paoletti F, Aldinucci D, Mocali A, Capparrini A (1986) A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem 154:536–541
Pei X, Murata Y, Benning G, Thomin S, Klusener B, Allen G, Grill E, Schroeder J (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406:731–734
Riganti C, Costamagna C, Bosia A, Ghigo D (2006) The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicol Appl Pharmacol 212:179–187
Riganti C, Costamagna C, Doublier S, Miraglia E, Polimeni M, Bosia A, Ghigo D (2008) The NADPH oxidase inhibitor apocynin induces nitric oxide synthesis via oxidative stress. Toxicol Appl Pharmacol 228:277–285
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101:8827–8832
Su G-X, Zhang W-H, Liu Y-L (2006) Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in soybean. J Integr Plant Biol 48:426–432
Tossi V, Cassia R, Lamattina L (2009) Apocynin-induced nitric oxide production confers antioxidant protection in maize leaves. J Plant Physiol 166:1336–1341
Vejrazka M, Micek R, Stipek S (2005) Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722:143–147
Wang H, Taketa S, Miyao A, Hirochika H, Ichii M (2006) Isolation of a novel lateral-rootless mutant in rice (Oryza sativa L.) with reduced sensitivity to auxin. Plant Sci 170:70–77
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Xiong J, Lu H, Lu K, Duan Y, An L, Zhu C (2009) Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordial initiation in rice seedlings. Planta 230:599–610
Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen W-B (2008) The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious shoot process. Plant Physiol 148:881–893
Yannarelli G, Noriega GO, Battle A, Tomaro ML (2006) Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 224:1154–1162
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Acknowledgments
This work was supported by a research Grant from the National Science Council of the Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Q. Zhao.
Rights and permissions
About this article
Cite this article
Chen, YH., Chao, YY., Hsu, Y.Y. et al. Heme oxygenase is involved in H2O2-induced lateral root formation in apocynin-treated rice. Plant Cell Rep 32, 219–226 (2013). https://doi.org/10.1007/s00299-012-1356-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1356-3