Abstract
Key message
β-Cyclodextrin–hemin complex-induced tomato lateral root formation was associated with nitric oxide and heme oxygenase 1 by modulating cell cycle regulatory genes.
Abstract
β-Cyclodextrin–hemin complex (β-CDH), a complex by combining β-cyclodextrin (β-CD) with hemin, a heme oxygenase 1 (HO1) inducer, was a trigger of cucumber adventitious root formation by enhancing HO1 gene expression. In this report, our results identified the previously unknown function of β-CDH in plants: the inducer of tomato lateral root (LR) formation. β-CDH-triggered LR formation is hemin-specific, since β-CD failed to induce LR development. Because nitric oxide (NO) is involved in LR formation, the correlation of β-CDH with NO and HO1 was investigated. Our analysis suggested that β-CDH induced an increase in endogenous NO production, followed by up-regulation of tomato HO1 gene and LR formation, all of which were mimicked by hemin and two NO-releasing compounds (SNP and GSNO). The induction of HO1 gene expression and LR formation triggered by β-CDH or hemin were significantly blocked by an inhibitor of HO1. Further results revealed that both β-CDH- and SNP-stimulated HO1 gene expression and thereafter LR formation were sensitive to the removal of NO with a potent NO scavenger, and the responses of SNP were significantly blocked by an inhibitor of HO1. Molecular evidence illustrated that representative cell cycle regulatory genes, including SlCDKA1, SlCYCA3;1, SlCYCA2;1, and SlCYCD3;1, were significantly up-regulated by β-CDH and SNP, but obviously blocked when seedlings were co-treated with the scavenger of NO or the inhibitor of HO1. In summary, our physiological and molecular evidence demonstrated that both NO and HO1 were involved in the β-CDH-induced LR formation with, at least partially, HO1 acting downstream of NO signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lateral root (LR) formation is one of the auxin-regulated developmental processes (Blakely et al. 1988; Casimiro et al. 2001, 2003). It was well known that auxin activates xylem pole pericycle cells for asymmetric cell division. Besides auxin, other phytohormones and environmental stimuli, including heavy metal exposure and salinity stress, could induce LR development (Potters et al. 2007; Cao et al. 2011). Further evidence revealed that some signaling molecules/systems, including reactive oxygen species (ROS; Montiel et al. 2013; Cao et al. 2014), nitric oxide (NO; Correa-Aragunde et al. 2004; Chen et al. 2012; Chen and Kao 2012), heme oxygenase/carbon monoxide (HO/CO; Xu et al. 2011b; Han et al. 2012), hydrogen sulfide (H2S; Fang et al. 2014a, b), and cyclic GMP (cGMP; Li and Jia 2013) are individually or simultaneously involved in the induction of LR. It was well known that NO in plants is produced by nitrate reductase (NR), and nitric oxide synthase (NOS)-like protein. Meanwhile, polyamine-mediated NO production and non-enzymatic synthesis of NO were also suggested (Gupta et al. 2011; Simontacchi et al. 2013). Further characterization of molecular events, especially the activation of cell cycle regulatory genes, including cyclin-dependent kinases (CDKs) as well as their interacting regulatory components triggered during the early phase of LR development, is an initial step toward understanding this developmental process (Casimiro et al. 2001; Himanen et al. 2002; Vanneste et al. 2005). Related works revealed that there are four stages that precede the cell division in the pericycle, characterized by G1 cell cycle block, auxin perception and signal transduction, followed by progression over G1/S transition and G2/M transition (Himanen et al. 2004).
β-Cyclodextrin (β-CD), a cyclic oligosaccharide of seven α-(1,4) linked glucose units, can improve the solubility of lipophilic medicinal and biological molecules (Fetzner et al. 2004). For instances, a complex by combining β-CD with hemin (ferroprotoporphyrin IX, a potent inducer of HO1, a major and inducible isoform of HO), called as β-cyclodextrin–hemin (β-CDH), could improve the solubility of hemin in neutral aqueous solution. The induction of adventitious root development in cucumber explants (Lin et al. 2012a) and the alleviation of cadmium (Cd) toxicity in alfalfa seedlings (Fu et al. 2011) were previously observed when β-CDH was exogenously applied. Stirringly, above mentioned physiological roles induced by β-CDH were more efficiently than its prosthetic group model hemin. Similar results were reported previously in the comparison with the effectiveness of peroxidase activity on the o-phenylenediamine (OPDA)-hydrogen peroxide (H2O2) system catalyzed by β-CDH and hemin (Zhang et al. 2000). However, whether β-CDH can induce LR formation is still unclear.
In this paper, first, we examined and compared the effects of β-CDH and its two constituent components, β-CD and hemin, on LR formation to elucidate another novel physiological role of β-CDH in plants. Previous results showed that HO1 mediates β-CDH response during the adventitious rooting process in cucumber (Lin et al. 2012a). However, the crosstalk between HO1 and NO in root organogenesis triggered by β-CDH still remains to be elucidated. The subsequent findings reported here are the results of research on this hypothetical link in tomato LR formation. From this study, we found that both NO and HO1 were involved in β-CDH-induced LR formation with, at least partially, HO1 acting downstream of NO signaling.
Materials and methods
Chemicals
All chemicals were purchased from Sigma (St Louis, MO, USA) unless stated otherwise. The preparation of β-CD-hemin (β-CDH) was according to Liu et al. (1999) and Dentuto et al. (2007). Hemin (H, an inducer of HO1; Xu et al. 2011a; Lin et al. 2012b; Jin et al. 2013) and β-CD with a suitable molar ratio were mixed by grinding for 60 min after the addition of de-ionized water. The mushy mixture was then freeze-dried, and all products were sieved and used. The brown powder was named as β-CD-hemin (β-CDH). Afterwards, a stock standard solution (10−4 M, calculated based on the concentration of hemin) was prepared by simply dissolving 1.63 g of β-CDH (0.1 % hemin) in 25 mL of de-ionized water. The stock solution was diluted and used immediately. The corresponding hemin (1 nM) or β-CD (500 nM) was, respectively, regarded as the control of 1 nM β-CDH (a complex of 1 nM hemin and 500 nM β-CD), which displayed the maximal inducible effect in the induction of LR formation. Meanwhile, the indicated concentrations of hemin or β-CD were also applied in our pilot test. Three NO-releasing compounds sodium nitroprusside (SNP), S-nitrosoglutathione (GSNO), and diethylamine NONOate (NONOate) were used at a concentration of 200 μM (Ederli et al. 2006; Xie et al. 2008, 2013; Chen et al. 2010), or the indicated concentrations. Both bilirubin (BR) and Fe-EDTA (Fe2+) were used as the catalytic by-products of HO1 at a concentration of 10 μM. Zinc protoporphyrin IX (ZnPP), a specific inhibitor of HO1 (Xuan et al. 2008; Cao et al. 2011; Han et al. 2014), was used at 50 μM. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) chosen as a NO scavenger (Tipsmark and Madsen 2003; Kováčik et al. 2014), was used at 200 μM.
Additionally, the Old SNP/NONOate solutions were, respectively, used as the negative controls by maintaining the separated solution of SNP/NONOate for at least 10 days in the light in a specific open tube to eliminate the entire NO (Di Fulvio et al. 2003; Tossi et al. 2009; Xie et al. 2013; Han et al. 2014).
CO aqueous solution preparation
The preparation of CO aqueous solution was carried out according to the method described previously (Xuan et al. 2008). The saturated stock solution (100 % saturation) was diluted immediately with distilled water to the specific concentration with a maximal inducible response (30 % of saturation [v/v]).
Plant material and growth conditions
Tomato (Solanum lycopersicum L.) seeds “baiguoqiangfeng” were purchased from Jiangsu Academy of Agricultural Sciences. Selected seeds with nearly identical size, shape and color were surface-sterilized with 2 % NaClO for 10 min, rinsed extensively and germinated in distilled water at 25 ± 1 °C in the dark for 2 days. Afterwards, seedlings were transferred to an illuminating incubator and maintained at 25 ± 1 °C with a 14-h photoperiod at 200 μmol m−2 s−1 for 1 day. Then, the selected identical seedlings with radicles of 2–3 mm were incubated with 4 ml of the various solutions as indicated in the legends for the indicated time points. At least three independent experiments were performed for each treatment, and 15 seedlings were included in each experiment. All solutions were renewed each day to maintain identical concentration of active constituent. Afterwards, photographs were taken. The number of emerged lateral roots (LRs; >1 mm) per seedling and the length of primary root (PR), as well as the emerged LR density (the number of LR per cm primary root; LRs/cm) were quantified with Image J software. LR primordia (LRP) per seedling were also observed after 3 days of treatments by root squash preparations and quantified by a light microscope (model Stemi 2000-C; Carl Zeiss, Germany). According to the previous methods (Himanen et al. 2002; Correa-Aragunde et al. 2006; Fang et al. 2014a), the root apical meristems of tomato seedlings at the indicated time points were cut off and the shoots were removed by cutting below the root-shoot junction to obtain samples of only lateral root-inducible segments for the following biochemical and molecular determinations.
Western blot analysis for tomato HO1
Rabbit polyclonal antibody was made against the mature tomato HO1 (mSlHO1) with a molecular mass of 26 kDa. Homogenates obtained for corresponding HO activity assays (Xu et al. 2011b) were also analyzed by western blotting. Forty micrograms of protein from homogenates were subjected to SDS-PAGE using a 12.5 % acrylamide resolving gel (Mini Protean II System, Bio-Rad, Hertz, UK), according to the method described in our previous report (Xuan et al. 2008). Separated proteins were then transferred to polyvinylidene difluoride (PVDF) membranes and non-specific binding of antibodies was blocked with 5 % non-fat dried milk in phosphate buffered saline (PBS, pH 7.4) for 2 h at room temperature. Membranes were then incubated overnight at 4 °C with primary antibodies diluted 1:3,000 in PBS plus 1 % non-fat milk. Immune complexes were detected using horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. The color was developed with a solution containing 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a HRP substrate.
NO content determination by using Greiss reagent assay
Nitric oxide content was determined using the previous methods (Xu et al. 2005; Zhou et al. 2005; Xuan et al. 2012) with some modifications. Samples were ground in a mortar and pestle in 1 ml of 50 mM cool acetic acid buffer (pH 3.6, containing 4 % zinc diacetate). The homogenates were centrifuged at 10,000×g for 15 min at 4 °C. Then, the supernatant was collected. The pellet was washed by 1 ml of extraction buffer and centrifuged as before. The two supernatants were combined and 0.1 g of charcoal was added. After vortex and filtration, the filtrate was leached and collected. The mixture of 1 ml of filtrate and 1 ml of the Greiss reagent was incubated at room temperature for 30 min. Meanwhile, after various treatments, the identical seedlings which were preincubated in 200 μM PTIO (the scavenger of NO), for 30 min, were regarded as the blank samples. Absorbance was assayed at 540 nm. NO content was calculated by comparison to a standard curve of NaNO2.
Confocal determination of NO production
Endogenous NO level was assayed by confocal microscopy using a fairly specific NO fluorescent probe 4-amino-5-methyl-amino-2′,7′-di-fluorofluorescein diacetate (DAF-FM DA) (Graziano and Lamattina 2007; De Michele et al. 2009; Han et al. 2014). Tomato seedlings were collected at the indicated times and loaded with 10 μM DAF-FM DA for 30 min before washing in 20 mM HEPES buffer (pH 7.8) three times for 5 min each time, and then immediately analyzed using Zeiss LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany, excitation at 488 nm, emission at 500–530 nm for NO analysis). All manipulations were performed at 25 ± 1 °C. Photographs are representative of identical results obtained after the processing and analysis of ten seedlings for each condition in three independent experiments. Data are presented as relative units of pixel intensities calculated by the ZEN software.
Real-time RT-PCR analysis
Total RNA was isolated using the Trizol reagent (Invitrogen, Gaithersburg, MD, USA), according to the manufacturer’s instructions. The RNA samples were treated with RNAase-free DNase (TaKaRa Bio Inc., Dalian, China) to eliminate traces of DNA, followed by the quantification using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). Afterwards, total RNA (2 μg) was reverse-transcribed using an oligo d(T) primer and M-MLV reverse transcriptase (BioTeke, Beijing, China). Real-time quantitative RT-PCR reactions were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Premix Ex Taq™ (TaKaRa Bio Inc, China), according to the manufacturer’s instructions. The primer sequence information was listed in Supplementary Table S1. Relative expression levels of SlHO1 (accession no. AF320028), SlCDKA1 (accession no. Y17225), SlCYCA2;1 (accession no. AJ243452), SlCYCA3;1 (accession no. AJ243452), SlCYCD3;1 (accession no. AJ245415), SlRSI-1 (accession no. NM_001247737.1), and SlARF7 (accession no. EF121545.1), were presented as values relative to the control samples at the indicated time points, after normalization with SlActin (accession no. BT012695) transcript levels.
Data analysis
Where indicated, results were expressed as the mean values ± SE of at least three independent experiments with similar results. Statistical analysis was performed using SPSS 17.0 software. For statistical analysis, t test (P < 0.05 or P < 0.01) or Duncan’s multiple test (P < 0.05) was chosen as appropriate.
Results
Effects of β-CDH, hemin, SNP, GSNO, and β-CD on tomato LR formation
Several parameters of root development, namely lateral root (LR) density and number, the number of lateral root primordia (LRP) and primary root (PR) length per seedlings, in tomato seedlings upon the indicated concentrations of β-CDH, hemin (an inducer of HO1), SNP, and GSNO (two NO-releasing compounds), were analyzed and compared. The results in Fig. 1 showed that exogenously applied β-CDH between the concentrations of 0.1 and 1,000 nM was able to promote LR formation in a dose-dependent fashion, with a maximal response at 1 nM β-CDH (a complex of 1 nM hemin and 500 nM β-CD), but to a lesser extent respect to the inducing responses of 200 μM SNP and 200 μM GSNO (especially compared in the LR density; Supplementary Fig. S1). A decrease in PR length was observed when 1 nM β-CDH was used. Under the similar experimental conditions, the addition of hemin with the concentrations ranging from 1 to 100 μM differentially influenced LR formation, and a maximal inducing response in LR formation appeared when 10 μM hemin was used individually, but also to a lesser extent respect to the responses of 1 nM β-CDH (Supplementary Fig. S1c). In comparison with the control samples, however, no difference in LR formation was individually observed in tomato seedlings treated with hemin at concentrations not more than 0.1 μM.
Effects of β-cyclodextrin–hemin (β-CDH) and its two constituent components, hemin (H), and β-cyclodextrin (β-CD), as well as sodium nitroprusside (SNP), old sodium nitroprusside (Old SNP) and S-nitrosoglutathione (GSNO) on lateral root (LR) formation. Three-day-old tomato seedlings were treated with 0, 0.1, 1, 10, 102 and 103 nΜ β-CDH, 104 nΜ hemin, 2 × 105 nΜ SNP, 2 × 105 nΜ Old SNP, 2 × 105 nΜ GSNO, 500 nM β-CD for 4 days. Afterwards, the number of emerged LRs (>1 mm) per seedling and the emerged LR density (a), the number of emerged lateral root primordia (LRP) and the length of primary root (PR) per seedling (b) were analyzed. Mean and SE values were calculated from at least three independent experiments (n = 45). Within each set of experiments, bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple range test
To assess the nature of the β-CDH-induced LR formation, the changes in LR formation upon different concentrations of β-CD were further compared. In fact, the inhibition of LR formation was differentially observed in β-CD-treated samples (Supplementary Fig. S1b). Compared to the control samples, a significant inhibition of LR density was observed when 500 nM β-CD was applied (Fig. 1). Meanwhile, the PR was increased. In the subsequent work, 1 nM β-CDH was used throughout our experiments.
Time-course analyses of NO production, SlHO1 expression and thereafter LR formation in response to β-CDH, hemin, β-CD, SNP, and GSNO
The results of the experiment given in Fig. 2b showed that, 1 nM β-CDH, 200 μM SNP and GSNO treatments for 96 h could time-dependently increase LR number per seedling, respect to the similar weaker responses in the control and 500 nM β-CD-treated alone samples. With 10 μM hemin, the induction of emerged LR formation was moderate, but also sustained for 96 h. We also observed that in comparison with the control and β-CD-treated samples, the treatments with β-CDH, hemin, SNP, and GSNO could increase LRP formation, presenting an accelerated anatomic structure (Fig. 2a).
NO production, SlHO1 transcript, and thereafter LR formation were progressively induced by β-CDH, hemin, SNP, and GSNO, but not β-CD. Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 10 μΜ hemin, 200 μΜ SNP, 500 nM β-CD, and 200 μΜ GSNO. After various treatments for 3 days, photographs showing the representative morphology of lateral root primordia (LRP; about 80 % of LRP at the shown stages) under the indicated treatments were taken (a). Bar 1 mm. Meanwhile, the time-course of the emerged LR number per seedling (n = 45; b), endogenous NO content (spectrometer method; c), and SlHO1 transcript (real-time RT-PCR; d) was investigated. Mean and SE values were calculated from at least three independent experiments. Within the same treated time points, bars with asterisks were significantly different between H2O and β-CDH treatment at P < 0.05 or P < 0.01 according to t test
Both NO and HO1 were suggested to be required for the LR formation in tomato seedlings (Correa-Aragunde et al. 2004; Xu et al. 2011b). To further investigate the contribution of NO and HO1 during LR formation, the time-course changes of intracellular NO production and SlHO1 transcripts during the similar time periods were analyzed. As expected, the NO production determined spectrophotometrically peaked at 6 h after the treatments of SNP and GSNO (in particular), and β-CDH, then gradually decreased till 48 h but still higher than the control and β-CD alone (Fig. 2c). Comparatively, hemin alone triggered NO levels as early as 3 h after treatments followed by the rapid decrease and returned to the basal level at 12 h.
Expression of tomato HO1 gene was determined by real-time RT-PCR (Fig. 2d). In comparison with a weaker increase in the levels of SlHO1 transcript during the early period of control treatment, the up-regulation of SlHO1 mRNA was strengthened within 48 h of exposure to β-CDH, SNP and GSNO (in particular), hemin, but not β-CD, peaking at 12 h (β-CDH, SNP, and GSNO) or 24 h (hemin) after treatments. We also noticed that the enhancement of NO synthesis and SlHO1 transcript induced by β-CDH apparently preceded LR formation, indicating a possible interrelationship between NO and HO1 in β-CDH responses.
Both β-CDH- and hemin-induced HO1 gene expression and thereafter LR formation were sensitive to zinc protoporphyrin IX, an inhibitor of HO1
The role of HO1 in β-CDH-induced responses was further examined. Co-treatment of tomato seedlings with the potent inhibitor of HO1, zinc protoporphyrin IX (ZnPP) at 50 μM, a concentration confirmed to be effective in our pilot test, showed pronounced and statistically significant effects on β-CDH- and hemin-elicited responses. For example, both β-CDH- and hemin-caused obvious increases in the amount of SlHO1 protein levels and corresponding transcripts, and thereafter LR formation, were, respectively, blocked by the presence of ZnPP (Fig. 3). In addition, a treatment of tomato seedlings with ZnPP alone significantly resulted in the down-regulation of HO1 gene expression and the inhibition of LR development. The ZnPP-mediated response in LR formation was sensitive to CO aqueous solution, which was previously confirmed to be the inducer of LR development in rapeseed (Cao et al. 2007) and Brassica napus (Cao et al. 2011). The moderate induction of tomato HO1 gene expression and LR formation triggered by CO alone was also observed in our experimental conditions. However, similar to previous report in Arabidopsis (Ma et al. 2014), no such induction in LR formation appeared in tomato seedlings supplemented with other two catalytic products of HO1, including BR and Fe2+ (Supplementary Fig. S2a). Above pharmacological evidence also confirmed that HO1 and its catalytic product CO could induce LR development.
LR formation, tomato HO1 protein levels, and SlHO1 transcripts in response to β-CDH, zinc protoporphyrin IX (ZnPP, a specific inhibitor of HO1), carbon monoxide (CO) aqueous solution, and hemin. Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 30 % saturated CO aqueous solution, 10 μΜ hemin, 50 μΜ ZnPP alone or their combinations. After 4 days of treatments, corresponding photographs were taken (a). Bar 1 cm. The number of emerged LRs (>1 mm) per seedling and the emerged LR density (b) were calculated (n = 45). Meanwhile, tomato HO1 protein level (c) and SlHO1 transcripts (e) were determined after 12 h of different treatments. The number below the band (c) indicates the relative abundance of the corresponding tomato HO1 protein level compared with that of the control sample. Coomassie Brilliant Blue-stained gels are present to show that equal amounts of proteins were loaded (d). Relative SlHO1 transcripts were also presented relative to the control sample (e). Mean and SE values were calculated from at least three independent experiments. Within each set of experiments, bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple test
Both β-CDH- and two NO-releasing compounds-induced intracellular NO production and thereafter LR formation were sensitive to PTIO, a scavenger of NO
To further elucidate the possibility that NO is required for the β-CDH-elicited LR formation as described above, tomato seedlings were treated with PTIO, which is considered as a scavenger of NO (Tipsmark and Madsen 2003; Kováčik et al. 2014). As two positive controls, the exogenous application of SNP and GSNO (two NO-releasing compounds; Dautov et al. 2013; Xie et al. 2013; Han et al. 2014) obviously increased NO content detected by LSCM and Greiss reagent in tomato seedlings, followed by the obvious induction of LR formation (Fig. 4). Similar responses were observed in β-CDH-treated plants. By contrast, Old SNP and Old NONOate, which were, respectively, used as the corresponding negative controls by maintaining the separated solution of SNP/NONOate for at least 10 days in the light in a specific open tube to eliminate the entire NO, had no such inducible effects in the induction of LR formation (Fig. 1; Supplementary Fig. S2b). These results clearly indicated that NO, but not any other by-products of SNP/NONOate degradation, is responsible for the corresponding responses. Therefore, besides GSNO, SNP could be used as an NO donor thereafter in our experimental conditions.
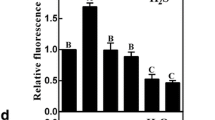
LR formation and endogenous NO production in response to β-CDH, two NO-releasing compounds (SNP and GSNO), and a NO scavenger (PTIO). Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 200 μΜ SNP, 200 μΜ GSNO, 200 μΜ PTIO alone or their combinations. After 4 days of treatments, corresponding photographs were taken (a). Bar 1 cm. The number of emerged LRs (>1 mm) per seedling and the emerged LR density (n = 45; b) were also calculated. After various treatments for 6 h, the DAF-FM fluorescence in seedling roots was detected by LSCM, and corresponding confocal images were provided (c). Bar 100 μm. The relative DAF-FM fluorescence presented as values relative to control samples (left), and NO contents (right) detected by using Greiss reagent, were also given (d). Mean and SE values were calculated from three independent experiments. Within each set of experiments, bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple test
Further comparison showed that both intracellular NO accumulation and LR formation stimulated by β-CDH, SNP or GSNO (both in particular) were greatly impaired by PTIO (Fig. 4). Meanwhile, PTIO alone also inhibited NO production and LR development. Above results clearly indicated that NO production is, at least partly, involved in β-CDH-induced LR formation.
HO1 might be related to β-CDH-induced NO-mediated LR formation
A subsequent work was to investigate the possibility of an interaction between HO1 and NO in β-CDH-induced LR formation. The results showed that, similar to the inhibitory responses of ZnPP, the application of PTIO dramatically blocked the up-regulation of SlHO1 transcript in tomato seedlings triggered by β-CDH (Fig. 5a). Afterwards, β-CDH-induced LR formation evaluated by LR density and number was, respectively, impaired (Fig. 5b). Similar inhibitory responses were observed when the combination of SNP together with ZnPP or PTIO. It is noteworthy that, when applied alone, ZnPP and PTIO could differentially inhibit SlHO1 transcript, both of which were consistent with the decreased LR development, with respect to the control samples. These results suggested that HO1 might be related to β-CDH-induced NO-mediated LR formation.
Effects of β-CDH, SNP, ZnPP and PTIO on relative amount of SlHO1 transcripts and LR formation. Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 200 μΜ SNP, 50 μΜ ZnPP, 200 μΜ PTIO alone or their combinations. After 12 h of treatments, SlHO1 transcripts were analyzed by real-time RT-PCR, and presented relative to the control sample (a). After 4 days of treatments, the number of emerged LRs (>1 mm) per seedling and the emerged LR density were also calculated (n = 45; b). Mean and SE values were calculated from at least three independent experiments. Within each set of experiments, bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple test
To confirm above possibility, Arabidopsis wild-type (WT), nia1/2 and hy1-100 mutants which exhibit defects in NR and HY1 (Xie et al. 2013; Han et al. 2014) were used. As shown in Supplementary Fig. S3, the increased LR formation was observed in WT when subjected to GSNO (in particular) and β-CDH. In comparison with the wild-type, a slight inhibition of LR formation was observed in nia1/2 and hy1-100 mutants as evaluated by LR number, rather than LR density since shorter primary roots were observed in two mutants. When plants were fed with exogenous GSNO and β-CDH, similar or strong inducing effects in LR number and density were observed in nia1/2 mutant with respect to the wild-type, indicating that besides nitrate reductase (NR), there may be other NO synthetic pathway(s) involved in β-CDH-induced NO-mediated LR formation. Compared with the inducing responses in nia1/2 mutant, impaired LR formation evaluated by LR density was observed in hy1-100 mutant when treated with GSNO and β-CDH.
Expression profiles of cell cycle regulatory genes and auxin signaling genes
Previous results suggested that cell cycle regulatory genes are targets during NO- and HO1-mediated LR formation (Correa-Aragunde et al. 2006; Han et al. 2012). Our next work was to investigate the time-course changes of cell regulatory genes assessed by real-time RT-PCR during the 48 h after various treatments.
The results of the experiment given in Fig. 6 revealed that, both β-CDH and hemin up-regulated SlCDKA1, SlCYCA3;1; SlCYCA2;1 and SlCYCD3;1 transcripts during the first 24 h of treatment, separately peaked at 12 h (except 6 h in SlCYCA2;1), in comparison with the moderate increases or the basal levels of above mentioned transcripts observed in the control samples and β-CD alone. Most importantly, above up-regulation in corresponding transcripts elicited by β-CDH were differentially impaired when SlHO1 mRNA or intracellular NO production was decreased by ZnPP or PTIO (Figs. 4, 5, 7). Similar responses were observed when SNP was added together with ZnPP or PTIO. These patterns of induction or repression exhibited good agreement with the phenotypic observations of LR development (Fig. 5b). Differential inhibitions in representative cell cycle regulatory genes (Fig. 7) and LR formation (Fig. 5b) were consistently observed in ZnPP or PTIO applied alone samples.
Changes of representative cell cycle regulatory genes. Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 10 μΜ hemin, or 500 nM β-CD for 2 days. The amount of SlCDKA1, SlCYCA3;1, SlCYCA2;1, and SlCYCD3;1 transcripts was analyzed by real-time RT-PCR, and presented relative to the control sample at time zero. Mean and SE values were calculated from three independent experiments. Within the same treated time points, bars with asterisks were significantly different between H2O and β-CDH treatment at P < 0.05 or P < 0.01 according to t test
Effects of β-CDH, SNP, ZnPP and PTIO on SlCYCD3;1, SlCDKA1, SlCYCA2;1, and SlCYCA3;1 transcripts. Three-day-old tomato seedlings were incubated with H2O, 1 nM β-CDH, 200 μΜ SNP, 50 μΜ ZnPP, 200 μΜ PTIO alone or their combinations for 12 h. Afterwards, the amount of corresponding transcripts was analyzed by real-time RT-PCR, and presented relative to the control sample. Mean and SE values were calculated from three independent experiments. Within each set of experiments, bars denoted by the same letter did not differ significantly at P < 0.05 according to Duncan’s multiple test
Comparatively, another two auxin signaling genes related to root organogenesis, SlRS1-1 and SlARF-7, exhibited the similar tendencies (Supplementary Fig. S4).
Discussion
Induction of lateral root formation: a new function of β-CDH in plants
Previous research discovered that β-CDH triggered cucumber adventitious rooting (Lin et al. 2012a). In this report, by using a pharmacological approach, we provide evidence that β-CDH was able to dose-dependently induce LR formation in tomato seedlings (Fig. 1), one of the phenotypes of root organogenesis. The maximal inducing response was observed in 1 nM β-CDH-treated tomato plants. The induction of LR formation by β-CDH was in the same way that three NO-releasing compounds SNP, GSNO, and NONOate treatments did (Supplementary Figs. S1, S2; Correa-Aragunde et al. 2004).
The comparison results of the experiments given in Fig. 1 and Supplementary Fig. S1 further illustrated that β-CDH-triggered LR formation is hemin-specific, since β-CD, another component of β-CDH, failed to induce LR development. Besides, in view of the transcription changes of two auxin signaling genes, SlRS1-1 and SlARF-7, which might be related to LR formation in plants (Supplementary Fig. S4; Taylor and Scheuring 1994; Lee et al. 2009; Wu et al. 2011b), the possible roles of auxin signaling in β-CDH response should be further elucidated. Together, our above results, combined with those reported previously (Lin et al. 2012a), led us to deduce that β-CDH might be a novel inducer responsible for root organogenesis, as does NO and HO1, which were previously confirmed in plants (Correa-Aragunde et al. 2004, 2006; Bai et al. 2012; Chen et al. 2012; Chen and Kao 2012; Cao et al. 2014). Since the beneficial role of β-CDH in the alleviation of Cd toxicity was also reported in alfalfa seedlings (Fu et al. 2011), we subsequently deduced that exogenously applied β-CDH might play key roles not only in the induction of root architecture, but also in plant responses against abiotic stress. In fact, the formation of LR belongs to stress-induced morphogenic response (SIMR; Potters et al. 2007).
Stirringly, compared with the maximal effect of 10 μM hemin, 1 nM β-CDH exhibited a 10,000-fold more potent in the induction of LR formation. Although no mechanism regarding to this phenomenon was investigated in our present research, this observation might be explained by the higher efficient increase in the solubility and stability of hemin, compared to that of hemin applied alone in neutral aqueous solution, which has been confirmed in pharmaceutical research (Nitalikar et al. 2012).
The roles of NO and HO1 in β-CDH-induced LR formation
The synergistic effects of NO and HO1/CO, two important signaling molecules in plants (Delledonne 2005; Shekhawat and Verma 2010; Wu et al. 2011a), have been well defined in the regulation of some physiological processes such as plant adaption against salinity in wheat seedlings (Xie et al. 2008, 2013), cucumber adventitious root development (Xuan et al. 2008, 2012), and stomatal closure in Vicia faba (Cao et al. 2007). Ample evidence also confirmed that NO is involved in LR formation (Simontacchi et al. 2013). However, the crosstalk between NO and HO1 in LR formation is not fully elucidated. In a subsequent study, we focused on the contribution of HO1 and NO during β-CDH-induced LR development, and our data suggested that besides their individual roles in β-CDH-elicited LR formation, a possible lineal signal transduction cascade involving NO production and subsequently HO1 up-regulation was downstream of β-CDH action.
The following results support above conclusion. First, β-CDH-induced NO production and thereafter HO1 gene expression exhibited a time-dependent fashion in tomato seedlings (Fig. 2). Afterwards, corresponding LR formation was observed. These phenomenons were mimicked by the application of hemin, SNP, and GSNO. A plausible explanation for these results is that NO might operate downstream of β-CDH promoting tomato LR formation through the up-regulation of HO1.
To prove this supposition, we firstly examined the requirement of NO in β-CDH-induced LR formation. Similar to previous reports (Correa-Aragunde et al. 2004, 2006; Chen and Kao 2012; Wang et al. 2013), two NO-releasing compounds SNP and GSNO treatments not only increased NO production, but also notably triggered LR formation (Fig. 4). These inducible effects in LR formation were not observed in seedlings treated with Old SNP/NONOate (Supplementary Fig. S2b), both of which were, respectively, used as a negative control of SNP or NONOate (Di Fulvio et al. 2003; Tossi et al. 2009; Xie et al. 2013; Han et al. 2014). Subsequent work revealed that the NO scavenger PTIO significantly counteracted β-CDH-mediated NO production and subsequent LR formation, with similar inhibiting effects seen following the application of SNP or GSNO together with PTIO (Fig. 4). Above results clearly indicated that NO might be a downstream messenger in the β-CDH-promoted LR formation.
In mammalian systems and recently in plants, one of the most investigated downstream targets of NO signaling is HO1 (Noriega et al. 2007; Pae et al. 2010; Santa-Cruz et al. 2010; Chen et al. 2012; Wu et al. 2013). Previous results suggested the contribution of HO1 in β-CDH-induced adventitious rooting in cucumber explants (Lin et al. 2012a). To further assess the possible involvement of HO1 in β-CDH-induced NO-mediated LR formation, we examined the changes of SlHO1 transcription and corresponding protein level using pharmacological agents to manipulate endogenous NO levels. Our results indicated that, similar to the positive control, hemin, the SlHO1 mRNA and/or its protein level were up-regulated by β-CDH and SNP, respectively (Figs. 3, 5). Besides these trends were strongly impaired by the presence of the HO1 inhibitor, ZnPP, the increased SlHO1 transcripts were also separately blocked by the inhibition of NO production when PTIO was co-treated (Fig. 5). Importantly, the above changes of tomato HO1 protein or its transcriptional levels triggered by β-CDH and SNP were matched with the changes of LR formation, showing that the reduction of HO1 gene expression inhibits LR formation, while the increase of this leads to the induction of LR development (Figs. 3, 5). These results confirmed the involvement of HO1 in β-CDH-induced LR development, and suggested that tomato HO1 gene acts downstream of NO signaling. Further genetic evidence in Arabidopsis (Supplementary Fig. S3) partly supported this conclusion. Additionally, since nia1/2 could not fully decrease all NO levels in Arabidopsis (Han et al. 2014), we admitted that the crosstalk between NO and HO1, or NO/HO1 acting in parallel, in β-CDH-induced Arabidopsis LR formation could not be fully ruled out. These possibilities should be investigated in the near future.
Molecular evidence and microarray data suggested that the modulation of multiple cell cycle regulatory genes is the important molecular event during LR formation triggered by auxin (Himanen et al. 2002, 2004). Supporting this conclusion, another important observation in this study is the activation of representative cell cycle regulatory genes, including SlCDKA1, SlCYCA3;1; SlCYCA2;1, and SlCYCD3;1, during the beginning of LR formation driven by β-CDH, hemin or SNP (Figs. 6, 7). During LR formation, an increase in tomato CYCA2;1, CYCA3;1, CYCD3;1, CDKA1 transcripts, as well as coordinated down-regulation of the Kip-Related Protein KRP2 mRNA, was previously reported in tomato seedlings supplemented with SNP (Correa-Aragunde et al. 2006) or cobalt, another inducer of LR formation (Xu et al. 2011b). Remarkably, further molecular evidence suggested that the above mentioned up-regulation of representative cell cycle regulatory genes was significantly blocked when seedlings were co-treated by PTIO or ZnPP together with β-CDH (Fig. 7). A similar reduction in the above transcripts was also observed in the combination with SNP and PTIO or ZnPP. These counteracting responses corroborated a reduction in LR density and number (Fig. 5b), suggesting that NO-mediated HO1 favors LR formation by the modulation of cell cycle regulatory genes. The observation that tomato seedlings treated with PTIO or ZnPP alone displayed reduced transcript levels of these genes combined with the inhibition of LR formation further supports the above notion. However, it is careful to note that although the possible linearity in β-CDH-governing NO and HO1 signaling has been primarily observed in LR formation, we cannot exclude the possibility that a NO-independent pathway might contribute to the β-CDH-triggered HO1-mediated LR formation.
Together, our study discovers a main branch of β-CDH-regulated LR formation, in which the increased intracellular NO production, subsequent SlHO1 activation and modulation of multiple cell cycle regulatory genes, were causally involved, at least in our experimental conditions (Fig. 8). Thus, our findings not only broadened the physiological roles of β-CDH exogenously applied in plants, but also obtained the detailed molecular mechanism: the interaction between HO1 and NO, which was not yet reported. The ongoing investigation of the roles of the other β-CDH target genes responsible for LR formation should open new windows in the understanding of the biological roles of β-CDH in plants. Additionally, our work not only identifies the previously unknown function of β-CDH in LR formation, but also raises the possibility of the potential field utilization of this compound for improving root development.
Author contribution statement
LH, JL and DZ conceived and designed the experiments, JL, DZ, RW, WS, YG, YR and WS performed the experiments, JL, WS, YR and LH analyzed the data and wrote the paper.
Abbreviations
- β-CD:
-
β-Cyclodextrin
- β-CDH:
-
β-Cyclodextrin–hemin
- CO:
-
Carbon monoxide
- GSNO:
-
S-Nitrosoglutathione
- HO1:
-
Heme oxygenase 1
- LR:
-
Lateral root
- LRP:
-
Lateral root primordia
- NO:
-
Nitric oxide
- NONOate:
-
Diethylamine NONOate
- PTIO:
-
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- SNP:
-
Sodium nitroprusside
- ZnPP:
-
Zinc protoporphyrin IX
References
Bai X, Todd CD, Desikan R, Yang Y, Hu X (2012) N-3-oxo-decanoyl-l-homoserine-lactone activates auxin-induced adventitious root formation via hydrogen peroxide- and nitric oxide-dependent cyclic GMP signaling in mung bean. Plant Physiol 158:725–736
Blakely LM, Blakely RM, Colowit PM, Elliott DS (1988) Experimental studies on lateral root formation in radish seedling roots: II. Analysis of the dose-response to exogenous auxin. Plant Physiol 87:414–419
Cao ZY, Huang BK, Wang QY, Xuan W, Ling TF, Zhang B, Chen X, Nie L, Shen WB (2007) Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull 52:2365–2373
Cao Z, Geng B, Xu S, Xuan W, Nie L, Shen W, Liang Y, Guan R (2011) BnHO1, a haem oxygenase-1 gene from Brassica napus, is required for salinity and osmotic stress-induced lateral root formation. J Exp Bot 62:4675–4689
Cao Z, Fang T, Chen M, Li J, Shen W, Huang L (2014) Involvement of haem oxygenase-1 in hydrogen peroxide-induced lateral root formation in tomato. Acta Physiol Plant 36:931–943
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes arabidopsis lateral root initiation. Plant Cell 13:843–852
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8:165–171
Chen YH, Kao CH (2012) Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 249:187–195
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819
Chen YH, Chao YY, Hsu YY, Hong CY, Kao CH (2012) Heme oxygenase is involved in nitric oxide- and auxin-induced lateral root formation in rice. Plant Cell Rep 31:1085–1091
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L (2006) Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J Exp Bot 57:581–588
Dautov RF, Ngo DT, Licari G, Liu S, Sverdlov AL, Ritchie RH, Kemp-Harper BK, Horowitz JD, Chirkov YY (2013) The nitric oxide redox sibling nitroxyl partially circumvents impairment of platelet nitric oxide responsiveness. Nitric Oxide 35:72–78
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, Sanità di Toppi L, Lo Schiacvo F (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396
Dentuto PL, Catucci L, Cosma P, Fini P, Agostiano A, Hackbarth S, Rancan F, Roeder B (2007) Cyclodextrin/chlorophyll a complexes as supramolecular photosensitizers. Bioelectrochemistry 70:39–43
Di Fulvio M, Lauf PK, Shah S, Adragna NC (2003) NONOates regulate KCl cotransporter-1 and -3 mRNA expression in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 284:1686–1692
Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S (2006) Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol 142:595–608
Fang T, Cao Z, Li J, Shen W, Huang L (2014a) Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol Biochem 76:44–51
Fang T, Li J, Cao Z, Chen M, Shen W, Huang L (2014b) Heme oxygenase-1 is involved in sodium hydrosulfide-induced lateral root formation in tomato seedlings. Plant Cell Rep 33:969–978
Fetzner A, Böhm S, Schreder S, Schubert R (2004) Degradation of raw or film-incorporated β-cyclodextrin by enzymes and colonic bacteria. Eur J Pharm Biopharm 58:91–97
Fu G, Zhang L, Cui W, Wang Y, Shen W, Ren Y, Zheng T (2011) Induction of heme oxygenase-1 with β-CD-hemin complex mitigates cadmium-induced oxidative damage in the roots of Medicago sativa. Plant Soil 345:271–285
Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52:949–960
Gupta KJ, Fernie AR, Kaiser WM, van Dongen JT (2011) On the origins of nitric oxide. Trends Plant Sci 16:160–168
Han B, Xu S, Xie YJ, Huang JJ, Wang LJ, Yang Z, Zhang CH, Sun Y, Shen WB, Xie GS (2012) ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci 184:63–74
Han B, Yang Z, Xie Y, Nie L, Cui J, Shen W (2014) Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol Plant 7:388–403
Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14:2339–2351
Himanen K, Vuylsteke M, Vanneste S, Vercruysse S, Boucheron E, Alard P, Chriqui D, Van Montagu M, Inzé D, Beeckman T (2004) Transcript profiling of early lateral root initiation. Proc Natl Acad Sci USA 101:5146–5151
Jin Q, Zhu K, Xie Y, Shen W (2013) Heme oxygenase-1 is involved in ascorbic acid-induced alleviation of cadmium toxicity in root tissues of Medicago sativa. Plant Soil 366:605–616
Kováčik J, Babula P, Klejdus B, Hedbavny J, Jarošová M (2014) Unexpected behavior of some nitric oxide modulators under cadmium excess in plant tissue. PLoS ONE 9:e91685
Lee HW, Kim NY, Lee DJ, Kim J (2009) LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol 151:1377–1389
Li J, Jia H (2013) cGMP modulates Arabidopsis lateral root formation through regulation of polar auxin transport. Plant Physiol Biochem 66:105–117
Lin Y, Li M, Huang L, Shen W, Ren Y (2012a) Involvement of heme oxygenase-1 in β-cyclodextrin–hemin complex-induced cucumber adventitious rooting process. Plant Cell Rep 31:1563–1572
Lin YT, Li MY, Cui WT, Lu W, Shen WB (2012b) Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul 31:519–528
Liu Z, Cai R, Mao L, Huang H, Ma W (1999) Highly sensitive spectrofluorimetric determination of hydrogen peroxide with β-cyclodextrin–hemin as catalyst. Analyst 124:173–176
Ma F, Wang L, Li J, Samma MK, Xie Y, Wang R, Wang J, Zhang J, Shen W (2014) Interaction between HY1 and H2O2 in auxin-induced lateral root formation in Arabidopsis. Plant Mol Biol 85:49–61
Montiel J, Arthikala MK, Quinto C (2013) Phaseolus vulgaris RbohB functions in lateral root development. Plant Signal Behav 8:144–146
Nitalikar MM, Sakarkar DM, Jain PV (2012) The cyclodextrins: a review. J Curr Pharm Res 10:1–6
Noriega GO, Yannarelli GG, Balestrasse KB, Batlle A, Tomaro ML (2007) The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 226:1155–1163
Pae HO, Son Y, Kim NH, Jeong HJ, Chang KC, Chung HT (2010) Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide 23:251–257
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105
Santa-Cruz DM, Pacienza NA, Polizio AH, Balestrasse KB, Tomaro ML, Yannarelli GG (2010) Nitric oxide synthase-like dependent NO production enhances heme oxygenase up-regulation in ultraviolet-B-irradiated soybean plants. Phytochemistry 71:1700–1707
Shekhawat GS, Verma K (2010) Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 61:2255–2270
Simontacchi M, García-Mata C, Bartoli CG, Santa-María GE, Lamattina L (2013) Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep 32:853–866
Taylor BH, Scheuring CF (1994) A molecular marker for lateral root initiation: the RSI-1 gene of tomato (Lycopersicon esculentum Mill) is activated in early lateral root primordia. Mol Gen Genet 243:148–157
Tipsmark CK, Madsen SS (2003) Regulation of Na+/K+-ATPase activity by nitric oxide in the kidney and gill of the brown trout (Salmo trutta). J Exp Biol 206:1503–1510
Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181:871–879
Vanneste S, De Rybel B, Beemster GT, Ljung K, De Smet I, Van Isterdael G, Naudts M, Iida R, Gruissem W, Tasaka M, Inzé D, Fukaki H, Beeckman T (2005) Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17:3035–3050
Wang H, Xiao W, Niu Y, Jin C, Chai R, Tang C, Zhang Y (2013) Nitric oxide enhances development of lateral roots in tomato (Solanum lycopersicum L.) under elevated carbon dioxide. Planta 237:137–144
Wu M, Huang J, Xu S, Ling T, Xie Y, Shen W (2011a) Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism. J Exp Bot 62:235–248
Wu J, Wang F, Cheng L, Kong F, Peng Z, Liu S, Yu X, Lu G (2011b) Identification, isolation and expression analysis of auxin response factor (ARF) genes in Solanum lycopersicum. Plant Cell Rep 30:2059–2073
Wu M, Wang F, Zhang C, Xie Y, Han B, Huang J, Shen W (2013) Heme oxygenase-1 is involved in nitric oxide- and cGMP-induced a-Amy2/54 gene expression in GA-treated wheat aleurone layers. Plant Mol Biol 81:27–40
Xie Y, Ling T, Han Y, Liu K, Zheng Q, Huang L, Yuan X, He Z, Hu B, Fang L, Shen Z, Yang Q, Shen W (2008) Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defence in wheat seedling roots. Plant Cell Environ 31:1864–1881
Xie Y, Mao Y, Lai D, Zhang W, Zheng T, Shen W (2013) Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J Exp Bot 64:3045–3060
Xu MJ, Dong JF, Zhu MY (2005) Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol 139:991–998
Xu S, Lou T, Zhao N, Gao Y, Dong L, Jiang D, Shen W, Huang L, Wang R (2011a) Presoaking with hemin improves salinity tolerance during wheat seed germination. Acta Physiol Plant 33:1173–1183
Xu S, Zhang B, Cao ZY, Ling TF, Shen WB (2011b) Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals 24:181–191
Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen WB (2008) The Heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 148:881–893
Xuan W, Xu S, Li M, Han B, Zhang B, Zhang J, Lin Y, Huang J, Shen W, Cui J (2012) Nitric oxide is involved in hemin-induced cucumber adventitious rooting process. J Plant Physiol 169:1032–1039
Zhang K, Mao L, Cai R (2000) Stopped-flow spectrophotometric determination of hydrogen peroxide with hemoglobin as catalyst. Talanta 51:179–186
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31201617, J1210056 and J1310015), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Xian Sheng Zhang.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Zhu, D., Wang, R. et al. β-Cyclodextrin–hemin complex-induced lateral root formation in tomato: involvement of nitric oxide and heme oxygenase 1. Plant Cell Rep 34, 381–393 (2015). https://doi.org/10.1007/s00299-014-1716-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1716-2











