Abstract
The advantages of gene ‘stacking’ or ‘pyramiding’ are obvious in genetically modified (GM) crops, and several different multi-transgene-stacking methods are available. Using linker peptides for multiple gene transformation is considered to be a good method to meet a variety of needs. In our experiment, the Bt cry1Ah gene, which encodes the insect-resistance protein, and the mG 2 -epsps gene, which encodes the glyphosate-tolerance protein, were connected by a 2A or LP4/2A linker. Linker 2A is a peptide from the foot-and-mouth disease virus (FMDV) that has self-cleavage activity. LP4 is a peptide from Raphanus sativus seeds that has a recognition site and is cleaved by a protease. LP4/2A is a hybrid peptide that contains the first 9 amino acids of LP4 and 20 amino acids from 2A. We used the linker peptide to construct four coordinated expression vectors: pHAG, pHLAG, pGAH and pGLAH. Two single gene expression vectors, pSAh and pSmG2, were used as controls. The six expression vectors and the pCAMBIA2301 vector were transferred into tobacco by Agrobacterium tumefaciens-mediated transformation, and 529 transformants were obtained. Molecular detection and bioassay detection data demonstrated that the transgenic tobaccos possessed good pest resistance and glyphosate tolerance. The two genes in the fusion vector were expressed simultaneously. The plants with the genes linked by the LP4/2A peptide showed better pest resistance and glyphosate tolerance than the plants with the genes linked by 2A. The expression level of the two genes linked by LP4/2A was not significantly different from the single gene vector.
Key message The expression level of the two genes linked by LP4/2A was higher than those linked by 2A and was not significantly different from the single gene vector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant genetic engineering developed rapidly and can be used to manipulate various traits, including biotic stress resistance, abiotic stress resistance, qualitative traits and quantitative traits, which shows the potential of genetic engineering to promote the evolution of agriculture, industry, nutrition and even medicine. According to ISAAA statistics, the global area of biotech crops has reached 160 million hectares, and 26 % of these crops contain stacked traits with two or three genes that result from gene stacking or pyramiding. These data indicate that hybrid gene products will become increasingly important for biotech crop development (James 2011). It has been shown that hybrid gene expression in transgenic plants has obvious advantages. The insertion of multiple genes into plants generates multi-function transgenic plants and makes the regulation of metabolic pathways possible. “Golden rice” is a famous example of a genetically engineered plant in which three different genes in the metabolic pathway of carotenoid synthesis were combined to obtain a new rice variety that was rich in vitamin A (Ye et al. 2000). The advantages of ‘stacking’ or ‘pyramiding’ traits in crops are obvious, and it is known that there are several different multi-transgene-stacking methods available for co-expressing multiple transgenes in plants (Cammue et al. 2002). Although many methods have already been used in the commercial production of GM crops, no single method is able to meet all of the requirements, including the coordinate, high-level and stable expression of multiple transgenes (Halpin 2005).
Currently, using linker peptides for the transformation of multiple genes is considered a better method for a variety of reasons. The peptides used included 2A, LP4/2A, the internal ribosome entry sites (IRESs) (Douin et al. 2004; Urwin et al. 2000), NIa protease (Marcos and Beachy 1994) and native protease (Zhang et al. 2011). The 2A sequence has been widely used because of its short sequence and high efficiency of cleavage (El Amrani et al. 2004; Halpin et al. 1999; Ralley et al. 2004). The 2A peptide is present in various types of viruses, and the sequence containing the D-x-E-x-N-P-G-P motif is called a 2A-like sequence (Donnelly et al. 2001; Palmenberg et al. 1992; Ryan et al. 1991). Other sequences include the picorna virus and ‘picorna virus-like’ 2A sequences, insect virus ‘2A-like’ sequences and type C rotavirus ‘2A-like’ sequences. A similar sequence exists in bacteria, but it does not possess a self-cleavage function. The 2A sequence used in plants is the peptide from the foot-and-mouth virus. The 2A sequence has been widely used in mammals (Varnavski et al. 2000), humans (Ryan and Drew 1994), fungal cells (Suzuki et al. 2000), insects (Blazar et al. 2005) and viruses (Funston et al. 2008). The GUS-2A-COMT-2A-F5H vector was transformed into tobacco to manipulate the biosynthesis of lignin and yield the GUS-2A and F5H or GUS-2A, COMT-2A and F5H products. This was the first time the function of 2A was demonstrated in a plant (Halpin et al. 2001). Using 2A for gene fusions has also been applied to tomato and potato plants (Kwon et al. 2004; Ralley et al. 2004; Randall et al. 2004). In recent years, people attempted to use 2A for multi-gene transformations in staple crops (Park et al. 2009). In 2010, researchers at the University of Tokyo used 2A to link the phytoene synthase gene from a pepper and the pan-genus carotene desaturase gene. The vector was transformed in rice to generate the high carotenoid content for “Golden Rice” (Ha et al. 2010). The application of 2A from the model plant to major crops indicates that the 2A linker has potential in other genetic engineering applications.
LP4 is a peptide from the seeds of Impatiens balsamina L. (Tailor et al. 1997). In the seed of I. balsamina L., the pre-antibacterial peptide is composed of signal peptides and six units of 20 amino acids. Every unit is separated by the LP peptides of different length (16–35 amino acids), which have conserved protease sites. After its translation, the pre-protein is cut by a protease to result in the mature peptide in the cytoplasm. LP4 is one of the LP peptides, which has already been used as the linker peptide to connect antimicrobial proteins in transgenic Arabidopsis thaliana (Francois et al. 2002, 2004). In 2009, Indian scholars used the LP4 linker peptide to connect the antibacterial proteins DmAMP1 and RsAFP2, and they transformed these proteins into rice by Agrobacterium-mediated transformation. The transgenic rice possessed an increased resistance to rice blast fungus and Rhizoctonia bacteria by 90 and 79 %, respectively, when compared with non-transgenic rice (Chattoo and Jha 2009).
In 2004, the laboratory of Francois connected the first nine amino acids of the LP4 and 2A peptides to generate a hybrid linker peptide, LP4/2A. They connected the gene DmAMP1 and RsAFP2 with LP4/2A and achieved higher antibacterial activity in Arabidopsis plants compared to the genes linked with the LP4 peptide (Francois et al. 2004). The cleavage site of the LP4/2A sequence included both the enzyme-digested positions of the LP4 peptides and the self-cleaving position of 2A. LP4/2A sequence possesses one more cleavage site than either the 2A sequence or the LP4 sequence alone, so the excess amino acid residues of the mature protein can be removed to avoid influencing the protein activity. There have been no reports comparing the 2A and LP4/2A linkers until now.
The crises of pests and weeds are important factors that reduce crop production. To improve crop production, we must learn how to control pests and weeds. The Bacillus thuringiensis (Bt) cry gene (Schnepf et al. 1998) and the epsps (5-enolpyruvylshikimate-3-phosphate synthase) gene are two important genes for culturing pest-resistant and glyphosate-tolerant crops. The cry1Ah gene is a novel insecticidal gene cloned from the BT8 isolate (Xue et al. 2008). This gene shows high toxicity towards the Lepidoptera insect. G2-epsps is a new epsps gene isolated from glyphosate-contaminated soil and was designed as mG 2 -epsps after being optimised with a plant codon preference. The G2-epsps gene is an isoenzyme of the plant endogenous EPSP synthase, but the conformational changes reduce its affinity for glyphosate. Therefore, even if endogenous epsps is inhibited, the transgenic plant expression of exogenous epsps can be maintained at normal levels to meet the plant’s metabolic needs. Thus, the stacking of these two genes in one plant will have an important impact on the genetic engineering of plants. The aims of our study are to produce a plant that is not only resistant to pests, but also tolerant of glyphosate and to provide a foundation for the future production of crops with these traits.
In this report, 2A and LP4/2A were used as linker peptides to construct polyprotein vectors to express the two functional proteins, Bt Cry1Ah and mG2-EPSPS. Four fusion genes vectors, pHAG, pHLAG, pGAH and pGLAH, were constructed and transformed into tobacco by Agrobacterium tumefaciens-mediated transformation to acquire plants with both pest resistance and glyphosate tolerance.
Materials and methods
Plant materials
The tobacco seeds of wild-type NC89 were sown on Murashige and Skoog (MS) medium (Gamborg et al. 1976) and stored at 28 °C for 5 days in the dark. The plants were then transferred to light conditions with 16 h light/8 h dark photoperiods at 28 °C.
Primers design
According to the published oligopeptide of 2A (RQLLNFDLLKLAGDVESNPGP) (Ryan and Drew 1994), a series of primers were designed. The primers used in this study are shown in Table 1.
Plasmid construction
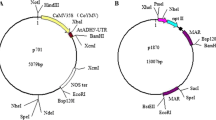
The series of plant transformation constructs are displayed in Fig. 1. The promoter driving the expression of the polyprotein construct was the cauliflower mosaic virus (CaMV) 35S promoter.
pSAh. The HindIII–EcoRI fragment of the pBI121 plasmid was cloned into the corresponding restriction sites of the pUC19 plasmid to yield pUC19SGN. The BamHI–EcoRI fragment of the pUCUbiAh plasmid, which was deposited in the laboratory, was cloned into the corresponding restriction sites of the pUC19SGN plasmid to yield pUCS1Ah. The HindIII–EcoRI fragment of the pUCS1Ah plasmid was cloned into the corresponding restriction sites of the pCAMBIA2301 plasmid to yield pSAh.
pSmG2. The HindIII–XbaI fragment of the pUC19SGN plasmid was cloned into the corresponding restriction sites of the pUCUbimG2 plasmid, which was deposited in the laboratory, to yield pUCSmG2. The HindIII–EcoRI fragment of the pUCSmG2 plasmid was cloned into the corresponding restriction sites of the pCAMBIA2301 plasmid to yield pSmG2.
pHAG (pAh-2A-mG2). The 2A3F and 2A3R primers were annealed to form dsDNA, which was digested with XbaI and SacI and cloned into the XbaI and SacI sites of pGEM-7Z (Promega) to form p7Z2AB. Using the AHF and AH2R1 primers, AG2R2 (a cry1Ah fragment carrying partial 2A gene at its 3′-end) was amplified from the pUCUbiAh plasmid by PCR. The cry1Ah fragment was digested with XbaI and HindIII and cloned into XbaI- and HindIII-digested p7Z2AB to form p7ZBH2A. The XbaI–ClaI fragment of the pUCS1Ah plasmid was cloned into the corresponding restriction sites of the p7ZBH2A plasmid to yield p7ZH2A. The NcoI–EcoRI fragment of the pUCSmG2 plasmid was cloned into the corresponding restriction sites of the p7ZH2A plasmid to yield p7ZHAG. The HindIII–EcoRI fragment of the p7ZHAG plasmid was cloned into the corresponding restriction sites of the pCAMBIA2301 plasmid to yield pHAG.
pGAH (pmG2-2A-Ah). The construction of pGAH was similar to that of pHAG, except that the pUCUbimG2 plasmid was used as the template and BmG2F, AG2R1 and AG2R2 were used as the primers for the PCR amplification.
pHLAG (pAh-LP2A-mG2). The construction of pHLAG was similar to that of pHAG, except that the pUCUbiAh plasmid was used as the template and AHF, LPAH2R1, LPAG2R2 and LPAG2R3 were used as the primers for the PCR amplification.
pGLAH (pmG2-LP2A-Ah). The construction of pGLAH was similar to that of pHAG, except that the pUCUbimG2 plasmid was used as the template and BmG2F, LPAG2R1, LPAG2R2 and LPAG2R3 were used as primers for the PCR amplification.
Plant transformation
The Agrobacterium tumefaciens strain LBA4404, transformed with pSAh, pSmG2, pHAG, pGAH, pHLAG, pGLAH and pCAMBIA2301, was used to transform the tobacco plants. The inoculated plantlets were placed on MS plates with filter paper for 3 days. The plantlets were then placed onto MS plates with 0.2 mg/L naphthalene acetic acid, 3 mg/L 6-benzyl amino purine, 500 mg/L carbenacillin and 100 mg/L kanamycin. The shoots growing from calluses were planted into pots of MS media with 500 mg/L carbenacillin and 100 mg/L kanamycin for root induction.
PCR analysis
The presence of the foreign gene cassette in the tobacco genome was verified using polymerase chain reaction (PCR) analysis. The genomic DNA was isolated from the leaves of the tobacco plants. PCR was performed by amplifying the coding regions of the transgenes using the following sets of oligonucleotide primers: forward primer AHF and reverse primer AH2R1 for the cry1Ah gene and forward primer BmG2F and reverse primer AG2R1 for the mG2-epsps gene. The expected size of the PCR products was 704 bp for both genes. The standard procedure for the PCR reactions was as follows: cycling began with initial denaturation at 94 °C for 5 min, then 30 cycles of denaturation at 94 °C for 40 s, annealing at 56 °C for 40 s, and extension at 72 °C for 50 s, followed by a final extension at 72 °C for 10 min. The PCR products were resolved by electrophoresis in 1 % (w/v) agarose gels.
Reverse transcription-PCR
The total RNA was isolated using Trizol solution from the leaves of the transgenic tobacco plants and a non-transformed control. The amount of mRNA extracted was determined spectrophotometrically, and 1 μg was used as a template in the reverse transcript reaction primed with oligo (dT)15 (Promega). Reverse transcription-PCR (RT-PCR) assays were performed according to the directions and materials supplied in the RT-PCR kit (Invitrogen Super Script™III). The PCR amplifications of the pHAG and pHLAG transformed plants were performed with the same primers, HGF and HGR. The PCR amplifications of the pGAH and pGLAH transformed plants were performed with the same primers, GHF and GHR. The ActincDNA fragment was amplified with the primers ActinF and ActinR as the control.
Southern blot analysis
The total DNA was extracted from the leaves of the transgenic plants and the wild-type plant using the CTAB method. Thirty micrograms of each genomic DNA sample were digested with the BamHI restriction enzyme and separated on 0.8 % (w/v) agarose gel. The 704-bp DNA fragment containing cry1Ah gene was amplified with the primers AHF and AH2R1 and used as probe. Southern blotting was performed using a DIG High Prime Labeling and Detection Starter Kit II, which is a kit for chemiluminescent detection with CSPD (Roche Co.), following the manufacturer’s protocol.
Detection of protein in the transgenic plants
Western blot analysis and enzyme-linked immunosorbent assay (ELISA) of the transgenic plant materials were performed. The leaves of 1-month-old plants grown in the greenhouse were used for the protein extraction. The plant material was ground in liquid nitrogen and dissolved in buffer containing 200 mM Tris–HCl pH 8.0, 100 mM NaCl, 400 mM sucrose and 1 mM PMSF, with 14 mM 2-mercaptoethanol and 0.05 % Tween 20. The dissolved plant material was vortexed and centrifuged for 15 min at 12,000 rpm. The amount of the total soluble proteins were determined by the Bradford assay (1976) with the BioRad protein dye concentrate using a standard curve derived from bovine serum albumin (BSA). Twenty micrograms of soluble protein were separated by SDS-PAGE (8–10 %) and transferred to a PVDF membrane. The primary antibodies (anti-Cry1Ah and anti-mG2-EPSPS) were used at 1:5,000 dilution, and the IgG-AP was used at a 1:20,000 dilution. Detection was performed using the NPT/BCIP kit (CWBIO company, China) as described by the manufacturer. The Bt cry1Ab/1Ac ELISA detect kit (Envirologix) was used to detect the Cry1Ah protein. The mG2-EPSPS protein was detected using the method described below. One hundred microliters of the tobacco extract was added to the plate, and the coating buffer was added. The plate was then incubated at 4 °C overnight. Each well was washed three times with 0.05 % phosphate-buffered saline–Tween-20 (PBST) and incubated at 37 °C for 2 h with the antibodies (anti-mG2-EPSPS) at 1/1,000 dilutions in PBST + 1 % BSA. The wells were then washed three times with PBST, a solution of IgG-HRP at a 1/10,000 dilution in PBST + 1 % BSA was added to each well, and they were incubated for 1 h at 37 °C. The enzyme activity was detected using a TMB kit (CWBIO company, China), as described by the manufacturer.
Insect bioassays
Insect bioassays were conducted on the leaves from transgenic tobacco plants and age-matched WT controls. Each of the leaves were placed on filter paper in a plate with a moist pledget on the bottom stem and were infested with Helicoverpa armigera larvae (<24 h old) on the upper surface of the leaf section. Three replicates were set-up for each transgenic tobacco line and the WT controls. The plates were then sealed with parafilm, and 500 μl ddH2O was added to the pledget each day. The number of surviving insects was recorded after 3 days at 25 °C.
Glyphosate tolerance spray tests
The plants were sprayed with Roundup™ (Monsanto, 41.0 %) herbicide at the 5- to 6-leaf stage approximately 2 weeks after transplanting. Vegetative injury and fertility data were obtained by visual observation 2 weeks after the spray.
Glyphosate germination tests
Tobacco seeds were placed in the labelled 1.5 ml tubes and soaked with 500 μl 75 % ethanol for 30 s, washed once with 500 μl ddH2O, soaked with 500 μl 2.5 % sodium hypochlorite for 15 min and washed with ddH2O three times. The glyphosate concentrations used were 0, 2, 4, 5 and 6 mM in MS medium. A marker was used to divide the dish into seven sections. Each species of the seeds were sown in 1/7 of the area of the plate (approximately 20 seeds) (Fig. 9f). After 2 weeks, the germination of each seed was observed.
Results
Identification of the transgenic plants
The regenerated tobacco plants were screened with PCR (Fig. 2) for the presence of Cry1Ah and mG2-epsps genes. The amplified fragments were of the expected size. The positive control, PCR with the pHAG plasmid, also gave amplicons of similar size. However, these fragments were not amplified in the non-transformed plants. The results of the PCR analysis showed that there were 73 pHAG-transformed tobacco plants, 42 pHLAG-transformed tobacco plants, 27 pGAH-transformed tobacco plants and 58 pGLAH-transformed tobacco plants. The transformation rate was 49.3 %. Southern analysis was carried out on the DNA obtained from the young leaves of the transgenic plants (Fig. 3). The Southern blot analysis indicated that the transferred cry1Ah gene in the pHAG-28, pHLAG-60, pGAH-11, pGAH-56, pGLAH-95 and pSAh-40 transgenic tobacco plants had integrated into a single site in the genome. In the pGAH-51 transgenic tobacco, the transferred cry1Ah gene was inserted into two sites in the genome. The patterns of the hybridization bands differed from plant to plant, indicating independent transformation events and random integration. The single copy plants were further analysed.
PCR analysis of the transgenic plants. a PCR detection of the mG2-epsps gene. b PCR detection of the cry1Ah gene. The primers used for detecting the mG2-epsps gene were AHF and AH2R1. The primers used for detecting the cry1Ah gene were BmG2F and AG2R1. The total genomic DNA was isolated from young leaves by the CTAB (cetyltrimethylammonium bromide) method. The plasmid DNA from the Agrobacterium carrying pHAG was used as a positive control. M DNA size marker 500 bp ladder, W wild-type tobacco, P positive control, numbers indicate the lines of transgenic tobacco
Southern blot analysis for the number of transgene copies. The number of gene copies was assessed by Dig-labelled cry1Ah DNA fragments. M 1 Kb DNA marker, P positive control, 1 pHAG-28 transgenic tobacco, 2 pHLAG-60 transgenic tobacco, 3 pGAH-51 transgenic tobacco, 4 pGLAH-95 transgenic tobacco, 5 pSAh-40 transgenic tobacco, 6 pSmG2 transgenic tobacco, 7 pCAMBIA2301 transgenic tobacco, 8 pGAH-11 transgenic tobacco, 9 pGAH-56 transgenic tobacco
Analysis of the splicing period of the linkers
To determine the splicing period of the linkers (2A and LP4/2A), the total RNA was isolated and subjected to RT-PCR analysis. The primers were designed according to the sequences of the Bt cry1Ah gene and the mG2-epsps-gene. The locations are shown in Fig. 4a. These results showed that the foreign fusion genes were transcriptionally expressed as intact mRNA in the transgenic tobacco plants. We obtained the 113-bp segments of the pHAG vector, the 140-bp segments of the pHLAG vector, the 162-bp segments of the pGAH vector and the 189-bp segments of the pGLAH vector (Fig. 4b). The amount of the actin RT-PCR product was approximately equal among the transgenic and non-transgenic plants (Fig. 4c). Comparison of the different lanes suggests that the quantity of transcript is nearly the same in the different vectors.
RT-PCR analysis for the mRNA expression of cry1Ah and mG 2 -epsps in the transgenic tobacco plants. The primers used for detecting the transgenic plants harbouring pHAG or HLAG were HGF and HGR. The primers used for detecting the transgenic plants harbouring pGAH or pGLAH were GHF and GHR. M DNA size marker, CK wild-type tobacco plant
Analysis of the splicing cleavage efficiency of the linkers
When probed with antiserum raised against the Cry1Ah protein, the extracts from the transgenic tobacco plants showed a major immunoreactive band of approximately 70 KDa (Fig. 5a). Similarly, when probed with antiserum raised against mG2-EPSPS, the extracts from the transgenic tobacco plants showed a major band of 56 KDa (Fig. 5b). As a control, no band could be detected in the empty vector transgenic plant. These data indicate that the expected dissociation of the 2A-polyprotein and the LP4/2A-polyprotein to yield their component polypeptides occurred during translation in the plants. There was a small amount of uncleaved polyprotein present in some of the plants, which is noted in the figures. The uncleaved polyprotein constituted 20–30 % of the target protein in the plant tissue, according to the band intensity as measured by ImageJ software.
WB analysis of the cleavage efficiency of LP4/2A and 2A in the transgenic plants. The cleavage efficiency was assessed using Cry1Ah and G2-EPSPS antibodies (a, b). An anti-β actin antibody was used as a control (c). M protein marker, P1 Cry1Ah in E. coli, P2 mG2 -EPSPS in E. coli, 1 pHAG transgenic tobacco, 2 pHLAG transgenic tobacco, 3 pGAH transgenic tobacco, 4 pGLAH transgenic tobacco, 5 pSAh transgenic tobacco, 6 pSmG2 transgenic tobacco, 7 pCAMBIA2301 transgenic tobacco
Analysis of the expression levels of the protein
The expression levels of the foreign proteins in the different transgenic lines were detected by ELISA, and the levels of total protein were detected by Bradford assay. Ten plants from each vector were randomly selected to analyse the protein expression. The mean level of the Cry1Ah expression of the vector linked by 2A was 1.31 μg per mg of total soluble protein. The mean level of the Cry1Ah expression of the vector linked by LP4/2A was 1.58 μg per mg of total soluble protein. The mean level of the mG2-EPSPS expression of the vector linked by 2A was 1.20 μg per mg of total soluble protein. The mean level of the mG2-EPSPS expression of the vector linked by LP4/2A was 1.44 μg per mg of total soluble protein (Fig. 6).
From the results of the statistical analysis, the Cry1Ah and mG2-EPSPS expression levels of pHAG were lower than those of a single gene vector (pSAh and pSmG2) (P (1Ah) = 0.010 < 0.05; P (mG2) = 0.020 < 0.05). However, the Cry1Ah and mG2-EPSPS expression levels of pGAH and the single gene vector were not significantly different (P (1Ah) = 0.525 > 0.05; P (mG2) = 0.566 > 0.05). The two genes in the pHAG and pGAH vector were both linked by 2A, the only difference is the gene location. Therefore, the gene location has an effect on the expression levels of the target gene when linked with 2A. However, this situation will not occur when the genes are linked with LP4/2A. The Cry1Ah and mG2-EPSPS expression levels of pHLAG and the single gene vector were not significantly different (P (1Ah) = 0.429 > 0.05; P (mG2) = 0.331 > 0.05). The Cry1Ah and mG2-EPSPS expression levels of pGLAH and the single gene vector were also not significantly different (P (1Ah) = 0.082 > 0.05; P (mG2) = 0.566 > 0.05). The ELISA results also showed that the gene expression level for the LP4/2A peptide vector was higher than that of the 2A peptide vector, regardless of whether the Cry1Ah gene (P (1Ah) = 0.001 < 0.05; P (mG2) = 0.004 < 0.05) or the mG2-EPSPS gene was positioned first (P (1Ah) = 0.038 < 0.05; P (mG2) = 0.036 < 0.05).
Analysis of the insect resistance of the transformed tobacco plants
To determine the role of cry1Ah in the resistance of the tobacco plants to Lepidoptera pests, wild-type and transgenic plants were challenged with Helicoverpa armigera Hubner larvae (<24 h old). In three independent trials, the transgenic plants displayed greater resistance than the wild-type plants. The results showed that all of the transgenic plants contained high toxin quantities and that the death levels of the pests were greater than 90 % in all of the transgenic plants (Table 2). The wild-type plant was clearly eaten and possessed many holes made by the insects, but the transgenic plant was intact with only a few small holes (Fig. 7).
Analysis of the glyphosate tolerance of the transformed tobacco plants
To measure glyphosate tolerance, the transgenic tobacco lines containing the mG 2 -epsps gene were tested by spraying Roundup, whose active ingredient is the isopropylamine salt of glyphosate (41.0 %), at the 5- to 6-leaf stage approximately 2 weeks after transplantation. The level of vegetative tolerance for each line was scored 1 week after spraying. From the results of the preliminary experiments, 0.2 % Roundup was selected to test the glyphosate tolerance of the plants (data are not shown). The results from the 0.2 % Roundup spray treatment are shown in Table 3. The wild-type plants showed severe vegetative damage and died within several weeks of being sprayed at the lowest dose tested (0.05 %). Figure 8 shows the transgenic plant with the mG2 gene that possessed good tolerance to 0.2 % glyphosate.
Analysis of the glyphosate tolerance of the T1 generation seeds
The seeds of the transgenic tobacco plant transformed with the pHAG vector were able to grow in medium containing 2 mM glyphosate. The seeds of the transgenic tobacco plants transformed with the pHLAG, pGAH, pGLAH and pSmG2 vectors showed a better tolerance to glyphosate and were able grow in medium containing 5 mM glyphosate. Figure 9 displays the growth of seeds in the MS containing various concentrations of glyphosate (0, 2, 4, 5 and 6 mM).
Discussion
Because of the obvious advantages of gene-stacking, multi-gene transformation has become an area of intense study in GM research. Multi-gene transformation is becoming more prominent in commercial GM crops. For the traits that are not controlled by a single gene, multi-gene transformation is advantageous. For example, there are several branches of some metabolic pathways, and changing a single gene rarely achieves the required trait change.
Strategies used to introduce multi-step pathways into higher plants have classically been preceded by single transformation experiments, followed by characterising the progeny and subsequently crossing suitable lines. Co-transformation or re-transformation approaches have also been used, but they are unlikely to achieve coordinated expression (Halpin 2005; Halpin et al. 1999). Currently, there is no single ideal method to meet the requirements for the coordinated, high-level and stable expression of multiple transgenes. The use of the self-cleavage features of the 2A peptide from the foot-and-mouth disease virus and LP4 from the seeds of impatiens is an alternative method for multiple gene fusion in genetic engineering. The construction of multiple genes in the same vector and transformation of the fusion gene expression vector into plants will save manpower and resources. The coordinated expression of multiple genes will resolve a number of gene separations and recombinations and will overcome the gene silencing caused by the linked-gene transformation.
In this study, we describe a versatile and simple strategy for introducing multiple proteins into plants that allows for coordinated expression from a single promoter. Four fusion gene plant transformation vectors (pHAG, pHLAG, pGAH and pGLAH) were constructed. All of the constructs were successfully transformed into tobacco plants and showed good insect resistance and glyphosate tolerance.
The results of the RT-PCR analysis showed that the mRNA were intact during transcription. This indicates that the cleavage happened at the translation level, which is consistent with the cleavage mechanism of the linker we used.
The results of the western blot analysis showed that the linker peptide system played a very significant role, but that the cleavage was not complete. This incomplete cleavage was caused by incomplete cleavage of the cleavage site (C-terminal between the G and P or N-terminal after amino acid N) of the linker peptide, which is a result that has been previous reported. Ryan and Drew constructed the pCAT2AGUS vector and transformed it into rabbit reticulocyte lysates and human HTK-143 cells. They found that 20 % of the product was present as the uncleaved protein ([CAT2AGUS]), 80 % of the CAT protein was in the form of CAT with a C-terminal extension of FMDV 2A ([CAT2A]) and 80 % of the GUS protein contained an additional N-terminal proline residue. The cleaved proteins were active (Ryan and Drew 1994). There are fewer relevant reports on LP4/2A. LP4/2A is the linker peptide that combines the first nine amino acids of the LP4 and 2A sequence. In theory, it will not only cleave after the second N-terminal amino acid (N) in the peptide, but also at the C-terminal end between the amino acids G and P (Francois et al. 2004), making the upstream and downstream proteins carry a small number of amino acids. This cleavage will not only minimise the impact on the protein activity or location, but it will also reduce the risk of bio-security for biological safety considerations. In our experiment, the LP4 site was mostly not cleaved, and there was a very small amount of the fusion protein, which suggests that the two sites are not cut simultaneously.
The ELISA results demonstrated that the two genes in the fusion vector were expressed co-ordinately, which is the advantage of using multiple gene transformation. The ELISA results showed that the gene expression of pHAG was lower than that of a single gene vector. There was no significant difference in the gene expression between pGAH and the single gene vector (pSAh and pSmG2). The two vectors contained the same linker 2A but had different gene locations, which means that the gene location has an effect on the expression level of the target gene when linked with 2A. The cleavage efficiency changed depending on the constructs, which has also been reported previously (de Felipe et al. 2003; Mitra and Ma 2002). This result may be caused by the different proteins upstream of 2A that affect the conformation of the protein, which determines the cleavage efficiency and the cellular location of the protein because the amino acid may be “slipstreaming”. The upstream protein may affect the cleavage efficiency and the location of the protein downstream of 2A. However, this situation does not occur when the genes are linked with LP4/2A. The location of the gene linked by LP4/2A has no effect on the gene expression level, which is in contrast to that of to 2A. The ELISA results also showed that the gene expression level with the LP4/2A peptide vector is higher than that with the 2A peptide vector, regardless of the order of the genes. We conclude that genes linked by the LP4/2A peptide are more efficiently expressed than those linked by 2A.
Recently, some researchers have made good suggestions for improving the cleavage efficiency of the linker in a 2A system. For example, increasing the 1D protein sequence of 2A at the 5′-end of 2A, adding some flexible amino acid sequence, such as glycine-serine-glycine, between the 2A sequence and the protein or adding other protease recognition sites have shown improvements (de Felipe et al. 2010). The proteins linked by the hybrid linker LP4/2A showed both cleavage mechanisms. If the efficiency is low for some reason, another cleavage system provides a good alternative approach, and the high efficiency of the fusion protein cleavage is ensured. In 2004, Francois used LP4/2A to fuse the antibacterial gene sequences RsAFP2 and DmAMP1 and integrated them into the Arabidopsis thaliana genome; this produced Arabidopsis plants with high disease resistance. The cleavage efficiency of LP4/2A was significantly improved over LP4 alone (Francois et al. 2004).
Currently, the method for gene fusion by linker peptides is beginning to be adopted for multiple gene transformations. As researchers gain a deeper understanding of the linker peptide, the use of linker peptides for multiple gene transformations will become more widely applied.
References
Blazar BR, Osborn MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell SK, Vignali DAA, Ryan MD, Wilber AC, McIvor RS, Tolar J (2005) A picornaviral 2A-like sequence-based tricistronic vector allowing for high-level therapeutic gene expression coupled to a dual-reporter system. Mol Therapy 12(3):569–574
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cammue BPA, Francois IE, Broekaert WF (2002) Different approaches for multi-transgene-stacking in plants. Plant Sci 163(2):281–295
Chattoo BB, Jha S (2009) Transgene stacking and coordinated expression of plant defensins confer fungal resistance in rice. Rice 2(4):143–154
de Felipe P, Hughes LE, Ryan MD, Brown JD (2003) Co-translational, intraribosomal cleavage of polypeptides by the foot-and-mouth disease virus 2A peptide. J Biol Chem 278:11441–11448
de Felipe P, Luke GA, Brown JD, Ryan MD (2010) Inhibition of 2A-mediated ‘cleavage’ of certain artificial polyproteins bearing N-terminal signal sequences. Biotechnol J 5(2):213–223
Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D, Ryan MD (2001) The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol 82(Pt 5):1027–1041
Douin V, Bornes S, Creancier L, Rochaix P, Favre G, Prats AC, Couderc B (2004) Use and comparison of different internal ribosomal entry sites (IRES) in tricistronic retroviral vectors. BMC Biotechnol 4:16
El Amrani A, Barakate A, Askari BM, Li X, Roberts AG, Ryan MD, Halpin C (2004) Coordinate expression and independent subcellular targeting of multiple proteins from a single transgene. Plant Physiol 135(1):16–24
Francois IE, De Bolle MF, Dwyer G, Goderis IJ, Motors PF, Verhaert PD, Proost P, Schaaper WM, Cammue BP, Broekaert WF (2002) Transgenic expression in Arabidopsis of a polyprotein construct leading to production of two different antimicrobial proteins. Plant Physiol 128:1346–1358
Francois IE, Hemelrijck WV, Aerts AM (2004) Processing in Arabidopsis thaliana of a heterologous polyprotein resulting in differential targeting of the individual plant defensins. Plant Sci 166(1):113–121
Funston GM, Kallioinen SE, de Felipe P, Ryan MD, Iggo RD (2008) Expression of heterologous genes in oncolytic adenoviruses using picornaviral 2A sequences that trigger ribosome skipping. J Gen Virol 89:389–396
Gamborg OL, Murashige T, Thorpe TA, Vasil IK (1976) Plant tissue culture media. In Vitro 12(7):473–478
Ha SH, Liang YS, Jung H, Ahn MJ, Suh SC, Kweon SJ, Kim DH, Kim YM, Kim JK (2010) Application of two bicistronic systems involving 2A and IRES sequences to the biosynthesis of carotenoids in rice endosperm. Plant Biotechnol J 8(8):928–938
Halpin C (2005) Gene stacking in transgenic plants—the challenge for 21st century plant biotechnology. Plant Biotechnol J 3(2):141–155
Halpin C, Barakate A, Askari BM, Abbott JC, Ryan MD (2001) Enabling technologies for manipulating multiple genes on complex pathways. Plant Mol Biol 47(1–2):295–310
Halpin C, Cooke SE, Barakate A, El Amrani A, Ryan MD (1999) Self-processing 2A-polyproteins—a system for co-ordinate expression of multiple proteins in transgenic plants. Plant J 17:453–459
James C (2011) Global status of commercialized biotech/GM crops: 2011. ISSSA Brief No.43. ISSSA, Ithaca
Kwon HB, Kwon SJ, Hwang EW (2004) Genetic engineering of drought resistant potato plants by co-introduction of genes encoding trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase of Zygosaccharomyces rouxii. Kor J Genet 26(2):199–206
Marcos JF, Beachy RN (1994) In vitro characterization of a cassette to accumulate multiple proteins through synthesis of a self-processing polypeptide. Plant Mol Biol 24(3):495–503
Mitra A, Ma CL (2002) Expressing multiple genes in a single open reading frame with the 2A region of foot-and-mouth disease virus as a linker. Mol Breed 9(3):191–199
Palmenberg AC, Parks GD, Hall DJ, Ingraham RH, Seng TW, Pallai PV (1992) Proteolytic processing of the cardioviral P2 region: primary 2A/2B cleavage in clone-derived precursors. Virology 190(2):754–762
Park S, Kang K, Kim YS, Back K (2009) Endosperm-specific expression of tyramine N-hydroxycinnamoyltransferase and tyrosine decarboxylase from a single self-processing polypeptide produces high levels of tyramine derivatives in rice seeds. Biotechnol Lett 31(6):911–915
Ralley L, Enfissi EM, Misawa N, Schuch W, Bramley PM, Fraser PD (2004) Metabolic engineering of ketocarotenoid formation in higher plants. Plant J 39(4):477–486
Randall J, Sutton D, Ghoshroy S, Bagga S, Kemp JD (2004) Co-ordinate expression of beta- and delta-zeins in transgenic tobacco. Plant Sci 167(2):367–372
Ryan MD, Drew J (1994) Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J 13(4):928–933
Ryan MD, King AM, Thomas GP (1991) Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol 72(Pt 11):2727–2732
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3):775–806
Suzuki N, Geletka LM, Nuss DL (2000) Essential and dispensable virus-encoded replication elements revealed by efforts to develop hypoviruses as gene expression vectors. J Virol 74(16):7568–7577
Tailor RH, Acland DP, Attenborough S, Cammue BP, Evans IJ, Osborn RW, Ray JA, Rees SB, Broekaert WF (1997) A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens balsamina is derived from a single precursor protein. J Biol Chem 272(39):24480–24487
Urwin P, Yi L, Martin H, Atkinson H, Gilmartin PM (2000) Functional characterization of the EMCV IRES in plants. Plant J 24(5):583–589
Varnavski AN, Young PR, Khromykh AA (2000) Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J Virol 74(9):4394–4403
Xue J, Liang G, Crickmore N, Li H, He K, Song F, Feng X, Huang D, Zhang J (2008) Cloning and characterization of a novel Cry1A toxin from Bacillus thuringiensis with high toxicity to the Asian corn borer and other lepidopteran insects. FEMS Microbiol Lett 280(1):95–101
Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I (2000) Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 287:303–305
Zhang B, Rapolu M, Huang L, Su WW (2011) Coordinate expression of multiple proteins in plant cells by exploiting endogenous kex2p-like protease activity. Plant Biotechnol J 9(9):970–981
Acknowledgments
The authors are very grateful to Dr. Liang Gemei at the Institute of Plant Protection, CAAS for test larvae supply and bioassay help. This work was supported by the National Natural Science Foundation of China (Grant No. 30771383)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by K. Chong.
Rights and permissions
About this article
Cite this article
Sun, H., Lang, Z., Zhu, L. et al. Acquiring transgenic tobacco plants with insect resistance and glyphosate tolerance by fusion gene transformation. Plant Cell Rep 31, 1877–1887 (2012). https://doi.org/10.1007/s00299-012-1301-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1301-5