Abstract
Appropriate responses of seeds and fruits to environmental factors are key traits that control the establishment of a species in a particular ecosystem. Adaptation of germination to abiotic stresses and changing environmental conditions is decisive for fitness and survival of a species. Two opposing forces provide the basic physiological mechanism for the control of seed germination: the increasing growth potential of the embryo and the restraint weakening of the various covering layers (seed envelopes), including the endosperm which is present to a various extent in the mature seeds of most angiosperms. Gibberellins (GA), abscisic acid (ABA) and ethylene signaling and metabolism mediate environmental cues and in turn influence developmental processes like seed germination. Cross-species work has demonstrated that GA, ABA and ethylene interact during the regulation of endosperm weakening, which is at least partly based on evolutionarily conserved mechanisms. We summarize the recent progress made in unraveling how ethylene promotes germination and acts as an antagonist of ABA. Far less is known about jasmonates in seeds for which we summarize the current knowledge about their role in seeds. While it seems very clear that jasmonates inhibit germination, the results obtained so far are partly contradictory and depend on future research to reach final conclusions on the mode of jasmonate action during seed germination. Understanding the mechanisms underlying the control of seed germination and its hormonal regulation is not only of academic interest, but is also the ultimate basis for further improving crop establishment and yield, and is therefore of common importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant seeds and fruits have evolved as the typical propagation and dispersal units of angiosperms (Linkies et al. 2010). Seeds are very complex and diverse plant organs; they store nutrients and allow far-distance dispersal as well as persistence of a species in the local habitat. A typical mature angiosperm seed consists of the embryo covered by the maternal seed coat (testa) and in most cases by a more or less abundant layer of endosperm tissue between the embryo and testa (Fig. 1). The endosperm is a product of double fertilization and serves as a nutrient source for the embryo during seed development. It may also serve as a nutrient source for the emerging embryo during germination and seedling establishment, but another important role of the endosperm in mature seeds is its involvement in the regulation of germination timing (see below). Due to their structure, low water content and mechanisms such as dormancy and desiccation tolerance, orthodox seeds are able to survive for long periods and harsh environmental conditions. These features enable the timing of seed germination to weather conditions that are favorable for germination and further seedling development. It has therefore been concluded that the evolution of seeds was one of the major reasons for the rapid distribution, diversification and dominance of seed plants, in particular angiosperms that evolved more than 130 million years ago (Crane et al. 1995; Friedman 1998; Linkies et al. 2010; Wang et al. 2009).
Diversity of eudicot seed structure and the presence of endosperm in mature seeds as widespread trait important for regulating germination. a Phylogenetic tree of the eudicots. b–h Seed structure of representative species for important eudicot clades. b–d Brassicaceae seeds as rosid representatives: b Lepidium sativum (garden cress), c Sisymbrium officinale (hedge mustard), d Arabidopsis thaliana (thale cress). e, f Solanaceae seeds as asterid representatives: e Solanum lycopersicum (tomato), f Nicotiana tabacum (tobacco). g Beta vulgaris (sugarbeet, Amaranthaceae) as representative for the caryophyllids. h Trollius spp. (globeflower, Ranunculaceae) as representative for the basal eudicots. Sources for seed images: b Müller et al. (2006), c Iglesias-Fernandez and Matilla (2010), e Hilhorst and Downie (1995), g Hermann et al. (2007), h Hepher and Roberts (1985b)
Seed dormancy and germination are complex traits that are regulated by the antagonistic action of the phytohormones abscisic acid (ABA) and gibberellins (GA)
The control of seed germination is a very sophisticated process which requires the concerted action of and interaction between diverse phytohormones (Kucera et al. 2005). In seed tissues, the best investigated are the antagonistic actions of abscisic acid (ABA) and gibberellins (GA) on dormancy and germination. Dormancy is an intrinsic seed state that can be described as the inability of an intact, viable seed to complete germination under favorable conditions. It is a mechanism of the plant to adapt the timing of germination to the surrounding environmental conditions to prevent germination during seasons when the conditions for the subsequent seedling establishment and plant growth would be unfavorable. Different seed tissues, such as the testa, endosperm and embryo can contribute to dormancy; it is a very complex trait determined by genetic factors and environmental cues, which has been reviewed in detail by Finch-Savage and Leubner-Metzger (2006) and Holdsworth et al. (2008a). Hormonally, the dormant seed state is induced and maintained by ABA and released by GA. This ABA–GA antagonism is also evident in a broader view on plant dormancy, in which it controls the sprouting of tuber and tree buds (Rentzsch et al. 2011; Rohde et al. 2007). ABA signaling components are already present in the non-vascular bryophytes including liverworts, which represent the most basal members of the extant land plant lineage. ABA is considered as a major stress hormone, and its accumulation is especially connected to dehydration-related processes in plants (Hauser et al. 2011). The conservation of ABA signaling across diverse plant taxa from basal land plants to core eudicots suggests that ABA and its roles related to stress tolerance, especially to drought stress and dehydration, are some of the key evolutionary steps and a fundamental process for the adaptation to air/land conditions (Takezawa et al. 2011). Desiccation also occurs during seed maturation of orthodox seeds, which occurs in concert with increased ABA contents and often also with the induction of dormancy (Kucera et al. 2005). In that context, ABA functions as a positive regulator of physiological dormancy, the most widespread seed dormancy class among gymnosperm and angiosperm species (Finch-Savage and Leubner-Metzger 2006; Nambara et al. 2010). ABA and its associated transcription factors such as ABI3/VP1 are described as ancient dormancy regulators because they are phylogenetically widespread and therefore evolutionarily old (Holdsworth et al. 2008b; Romanel et al. 2009; Graeber et al. 2010). ABA-insensitive mutants, such as A. thaliana abi3 (ABA-insensitive3), show impaired seed dormancy (Ooms et al. 1993), and the VP1 (Viviparous1) orthologs of cereals are involved in preventing precocious germination on the ears and thereby contribute to preharvest sprouting resistance (Gerjets et al. 2010; McKibbin et al. 2002). DOG1 (Delay-of-germination1), a key gene regulating seed dormancy, has been shown to be regulated by ABA in Lepidium sativum (Brassicaceae; garden cress) and A. thaliana (Graeber et al. 2010; Teng et al. 2008). A recent publication by Kendall et al. (2011) shows that DOG1 expression and dormancy induction during A. thaliana seed maturation is not only regulated by ABA, but also by low maturation temperature and GA.
In contrast to ABA, GA negatively regulates dormancy as it releases coat-mediated seed dormancy and promotes seed germination (Holdsworth et al. 2008b; Koornneef 2002; Kucera et al. 2005; Leubner-Metzger 2003). GA-deficient ga1-3 mutants of A. thaliana have enhanced dormancy and need GA treatment to complete germination (Koornneef and Veen 1980). Gibberellins are known as growth-promoting hormones, being involved in several processes during plant development, such as shoot growth, flower development, dormancy release and seed germination. While parts of the GA signaling pathway, such as the GID1 receptor and DELLA repressor homologs, were already evident in early land plants such as the moss Physcomitrella patens, the complete GA-dependent signaling pathway arose later during land plant evolution (Yasumura et al. 2007; Vandenbussche et al. 2007; Depuydt and Hardtke 2011). Since no interaction between GID1-like (GLP1) and DELLA proteins is evident in P. patens (Hirano et al. 2007; Yasumura et al. 2007), GA signaling quite likely arose after the bryophyte divergence around 430 million years ago. Besides this, GA application does not lead to the typical growth-promoting effects in P. patens. Yasumura et al. (2007) also showed that a DELLA-protein-deficient P. patens mutant line lacks the de-repressed growth characteristics of DELLA-protein-deficient angiosperms, which shows that DELLA proteins of early land plants do not repress growth in situ.
Seed after-ripening, which is a longer period of air-dry storage at ambient temperature, regulates dormancy potential and comprises other non-dormancy related changes in the seed state; after-ripening is often accompanied by dormancy release (Carrera et al. 2008; Holdsworth et al. 2008a). This goes in concert with a decrease in ABA sensitivity and with increased sensitivity to germination-promoting hormones such as GA. Not only hormone sensitivities, but also hormone contents between fresh and after-ripened seeds differ (Cadman et al. 2006). Once dormancy is released, germination can take place if conditions are favorable. This process starts with the imbibition of a dry non-dormant seed and is completed when the radicle has protruded all covering layers, such as testa and endosperm (Figs. 1, 2a), and in many species endosperm rupture is the visible event that accompanies the completion of germination (Bewley 1997b; Finch-Savage and Leubner-Metzger 2006; Weitbrecht et al. 2011). Plant hormones control seed germination as internal mediators of developmental and environmental factors, such as light, temperature, moisture, oxygen and nutrients. It has been shown that endosperm rupture is promoted by GA and inhibited by ABA, which seems to involve the exchange of ABA- and GA-related signals between endosperm and embryo. A lot is known about the antagonistic action of ABA as germination-inhibiting and GA as germination-promoting hormone in several species (reviewed by Finch-Savage and Leubner-Metzger (2006) and Kucera et al. (2005). The ABA-insensitive A. thaliana mutant abi3 (Bassel et al. 2006; Nambara et al. 2000) and the ABA-deficient tomato mutant sitiens (Groot and Karssen 1992; Hilhorst and Downie 1995) show premature germination, while some GA-insensitive A. thaliana GID1 receptor mutants show impaired germination (Voegele et al. 2011). Voegele et al. (2011) have shown in A. thaliana and L. sativum that GID1a and GID1c are the major GA receptors involved in seed germination, while GID1b seems to be less important. In angiosperm seeds, the GID1-type GA receptors interact with DELLA repressor proteins which negatively regulate GA signaling in a stage-specific pattern. Therefore, non-functional DELLA repressor mutants show increased germination (Cao et al. 2005; Lee et al. 2002). A complex interaction between DELLA repressors, GA and ABA contents, and ABA-related transcription regulators seems to control seed germination (Piskurewicz et al. 2008). GA and ABA thereby antagonistically regulate downstream mechanisms that mediate the two key processes important for the completion of germination of endospermic seeds: embryo elongation and endosperm weakening (Figs. 1, 2a).
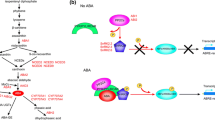
a Opposing forces during seed germination: germination is promoted by the growth potential of the embryonic axis (radicle–hypocotyl) and inhibited by the restraint of the seed covering layers (testa, endosperm). The completion of germination by radicle emergence and endosperm rupture occurs when the increasing embryo growth potential overcomes the restraint of the micropylar endosperm (cap). Lepidium sativum seeds are a rosid model to study endosperm weakening as an important developmental process that precedes endosperm rupture. Taken from Graeber et al. (2010). b Proposed model for the hormonal regulation of endosperm cap weakening and rupture, involving the coordinated interaction of radicle and endosperm tissues and the hormones ABA, GA and ethylene. Modified from Linkies et al. (2009). ACC 1-aminocyclopropane-1-carboxylic acid, ACO ACC oxidase, CTR1 constitutive triple response1, EIN3 ethylene insensitive3, ERFs ethylene response factors, GID1 gibberellin insensitive dwarf1
Endosperm tissue weakening during seed germination involves the action of cell wall remodeling proteins (CWRPs) and apoplastic reactive oxygen species (ROS)
In angiosperm species, double fertilization leads to the formation of the endosperm, which is a nutritional tissue for the embryo during seed development and is thereby partially (see examples below) or completely (for example pea or Brassica ssp.) obliterated upon seed maturation (Finch-Savage and Leubner-Metzger 2006; Forbis et al. 2002; Linkies et al. 2010). The abundance of the endosperm layer, which is located between embryo and testa (seed coat) in mature seeds, can therefore differ to a great extent (Fig. 1). Basal eudicots, e.g., Trollius spp. (Ranunculaceae; Fig. 1h), often have tiny embryos embedded in highly abundant endosperm tissue (Hepher and Roberts 1985a, b). Solanaceous species such as tomato (Fig. 1e) or tobacco (Fig. 1f) have mature seeds with an abundant endosperm layer (Petruzzelli et al. 2003a; Leubner-Metzger 2002; Toorop et al. 2000). In contrast to this, mature seeds with a thin endosperm of one to three cell layers include lettuce (Lactuca sativa, Asteraceae), and the Brassicaceae species L. sativum (Fig. 1b), Sisymbrium officinale (Fig. 1c) and A. thaliana (Fig. 1d) (Liu et al. 2005; Pritchard et al. 2002; Müller et al. 2006; Iglesias-Fernández and Matilla 2010). In many endospermic seeds, germination progresses in two visible steps, with testa rupture and endosperm rupture being two sequentially and differentially regulated events (Leubner-Metzger 2002; Liu et al. 2005; Müller et al. 2006; Petruzzelli et al. 2003a; Iglesias-Fernández and Matilla 2010). Figure 2a provides a general mechanistic model for seed germination and dormancy in which the net sum of the embryo growth potential and the weakening of the opposing restraint of the covering layers determine if a seed completes germination or not and also the germination timing and uniformity within a seed population (Finch-Savage and Leubner-Metzger 2006; Nonogaki 2006). Embryo elongation growth requires cell expansion in defined regions of the radicle and lower hypocotyl, which is promoted by GA and inhibited by ABA (da Silva et al. 2008; Sliwinska et al. 2009; Nonogaki 2006). Germination is therefore regulated by two opposing forces, the increasing growth potential of the radicle, which is counteracted by the resistance of the testa and endosperm tissues covering the radicle (Fig. 2a). After testa rupture, the growth potential of the radicle must overcome the resistance of the micropylar endosperm to protrude and thereby complete germination by endosperm rupture. At the same time, endosperm resistance decreases through tissue softening, a process called endosperm weakening, and this has been quantified by direct biomechanical measurements in several Asterid species, including tomato, coffee and lettuce (Bewley 1997a; Müller et al. 2006; Ni and Bradford 1993; Toorop et al. 2000; da Silva et al. 2004), and in the Rosid model system L. sativum (Graeber et al. 2010; Linkies et al. 2009; Müller et al. 2006). Indirect evidence supports the view that endosperm weakening also occurs in small seeds such as the Brassicaceae A. thaliana (Bethke et al. 2007; Debeaujon et al. 2000; Linkies et al. 2009) and S. officinale (Iglesias-Fernández and Matilla 2010), as well as in seeds of the Solanaceae Petunia spp. and tobacco (Leubner-Metzger 2003; Petruzzelli et al. 2003a). In L. sativum, it has been shown that ABA delays not only endosperm rupture, but also the onset of endosperm weakening and decreases the rate of weakening progression after its onset (Müller et al. 2006; Linkies et al. 2009). Endosperm weakening in tomato and coffee has been shown to be biphasic: the first phase is ABA insensitive, and this is followed by a second phase which is inhibited by ABA (Bewley 1997a; Müller et al. 2006; Ni and Bradford 1993; Toorop et al. 2000; da Silva et al. 2004). Promotion of endosperm weakening by GA appears to be a widespread phenomenon (Finch-Savage and Leubner-Metzger 2006; Bewley 1997a) and this suggests that GA regulates molecular mechanisms of endosperm weakening that are, at least in part, evolutionarily conserved across distinct taxa.
Endosperm tissue weakening is associated with the action of cell wall remodeling proteins (CWRPs) (Finch-Savage and Leubner-Metzger 2006; Nonogaki et al. 2007; Holdsworth et al. 2008b). This has been intensively investigated in Solanaceae species, such as tomato, where it has been shown that for example endo-β-1,4-mannanase, endo-β-1,3-glucanase and expansins are specifically expressed in the micropylar endosperm during germination (Chen and Bradford 2000; Nonogaki et al. 2000; Leubner-Metzger 2003). Morris et al. (2011) have shown for the Brassicaceae L. sativum that endo-β-1,4-mannanase enzyme activity accumulates in the radicle and the micropylar endosperm during germination, which is at least partly due to the LesaMAN7 isoform. That it has a role in germination is in agreement with the results of Iglesias-Fernández et al. (2011), who show impaired germination for AtMAN-5, -6 and -7 T-DNA insertion lines of A. thaliana. In S. officinale, another endospermic Brassicaceae species (Fig. 1c), endo-β-1,4-mannanase enzyme activity increase was associated with seed after-ripening and up-regulated by both GA and ethylene (Iglesias-Fernández and Matilla 2009). Further, the expression of endo-β-1,3-glucanases has been analyzed in the context of seed germination in tobacco and other Solanaceous species (Leubner-Metzger 2002, 2003; Petruzzelli et al. 2003a).
Xyloglucan endotransglycosylases/hydrolases (XTHs) are involved in cell wall remodeling, which has been shown to either lead to a stiffening (Maris et al. 2009) or loosening (van Sandt et al. 2007) of the cell wall for diverse developmental processes, such as fruit ripening, seedling growth and seed germination. In tomato, expression of SlXET4 was induced in the micropylar endosperm during germination, which was promoted by GA and not inhibited by ABA (Chen et al. 2002). In contrast to that, Voegele et al. (2011) found a down-regulation by GA for XTH18 and XTH19 transcript abundance in both the micropylar endosperm and the radicle of L. sativum seeds during germination; also, ABA did not influence their expression levels. They also used knockout mutants of A. thaliana to show that the GA receptors GID1a and GID1c were involved in regulating the expression of XTH18 and XTH19 during seed germination. Morris et al. (2011) used trancriptome data of L. sativum seed tissues during germination to carry out a sophisticated temporal and spatial transcript expression analysis for diverse CWRPs. This revealed that there are complex and tissue-specific expression patterns for endo-β-1,4-mannanase, endo-β-1,3-glucanase, expansins and other CWRPs during seed germination.
Cell wall remodeling can also be achieved by reactive oxygen species (ROS), including short-lived molecules such as superoxide (O ·−2 ), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2), which are known to play a role in the regulation of germination timing (Bailly 2004). They may act indirectly as signaling components in diverse developmental processes and pathogen defense, but are also known to act directly by scission of cell wall polymers leading to cell wall loosening important for cell extension of embryos during germination and seedling growth, as well as weakening of the surrounding tissues during seed germination and fruit ripening (Fry et al. 2001; Schopfer et al. 2001; Müller et al. 2009b). Müller et al. (2009b) have shown that apoplastic ·OH play a role in seed germination and seedling growth of L. sativum and that they have tissue-specific targets for polysaccharide scission in the cell walls. They conclude that in vivo ·OH production is hormonally and developmentally regulated in a tissue-specific manner. ·OH production in the apoplast increases in the radicle and the micropylar endosperm prior to the onset of endosperm weakening and rupture. This increase can be inhibited by ABA, leading to a delay in endosperm weakening, radicle growth and endosperm rupture. Both effects of ABA can be reverted in the radicle by treatment with GA or 1-aminocyclopropane-1-carboxylic acid (ACC), the direct precursor of ethylene (Graeber et al. 2010). O ·−2 production in the apoplast of germinating L. sativum seeds is regulated in the same hormonal manner (Müller et al. 2009a; Oracz et al. 2011), which supports the proposed reaction scheme for apoplastic O ·−-2 and ·OH production that depend on regulated NADPH oxidase activity in seed tissues (Graeber et al. 2010; Schopfer et al. 2001).
Taken together, developmentally and hormonally regulated expression of cell wall remodeling proteins (CWRPs) and apoplastic reactive oxygen species (ROS) is required for seed germination. The regulation is characterized by hormonal interactions where ABA inhibits, while GA and ethylene promote seed germination. While the molecular mechanisms underlying the ABA–GA antagonism have been the subject of several recent reviews (e.g., Nambara et al. (2010) and Weitbrecht et al. (2011), the molecular mechanisms by which ethylene promotes and jasmonates inhibit seed germination have not been reviewed after 2008 (Kucera et al. 2005; Matilla and Matilla-Vázquez 2008). The recent progress in the research about ethylene and jasmonates during seed germination is therefore the focus of the next sections of our review.
Differential regulation of ACC oxidases (ACOs) as the key step for ethylene biosynthesis during seed germination; ethylene biosynthesis and signaling are both required for endosperm weakening and rupture
Ethylene has diverse effects on plant growth and development. Besides its participation in stress responses such as on high temperature, it is involved in the ripening of climacteric fruits, flowering, aging, seedling growth, bud and seed dormancy release and seed germination (Klee and Clark 2004; Matilla 2000). In general, seedling growth is inhibited by ethylene and ABA, which is well investigated at the molecular level (Etheridge et al. 2006; Nemhauser et al. 2006). Contrary to that, ethylene promotes seed germination and an antagonism between ABA and ethylene has been shown in A. thaliana (Beaudoin et al. 2000; Ghassemian et al. 2000; Linkies et al. 2009) and several other species (Kucera et al. 2005; Matilla and Matilla-Vázquez 2008; Linkies et al. 2009). That ethylene promotes seed germination and alleviates thermoinhibition of seed germination is beyond doubt and reviewed below. Whether in addition ethylene is also involved in breaking seed dormancy is less obvious and is discussed in detail in the excellent review by Matilla and Matilla-Vázquez (2008).
In higher plants, ethylene is produced from methionine through the Yang cycle (Fig. 3) (Yang and Hoffman 1984; Lin et al. 2009). The last two steps are mediated by two enzyme families: the conversion from S-adenosyl-methionine (SAM) to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) and the following conversion of ACC to the bioactive ethylene by ACC oxidase (ACO). ACO, the enzyme that mediates the final rate-limiting step in ethylene biosynthesis, controls the ethylene evolution during seed germination (Kucera et al. 2005; Matilla and Matilla-Vázquez 2008; Linkies et al. 2009). Accumulation of ACOs and enhanced ethylene evolution are connected with seed germination of several species (Chiwocha et al. 2005; Hermann et al. 2007; Leubner-Metzger et al. 1998; Petruzzelli et al. 2000; Linkies et al. 2009). The levels of ACO transcripts have been shown to be regulated by ethylene itself and other phytohormones (Lin et al. 2009). During pea seed germination, ethylene promotes ethylene biosynthesis by positive feedback regulation of Ps-ACO1 transcripts, whereas Ps-ACS1 mRNA levels and overall ACC contents are not affected by ethylene treatment. A promoting effect of ethylene on pea seed germination was associated with β-1,3-glucanase being specifically induced in the radicle of the embryonic axis, but not in the cotyledons (Petruzzelli et al. 2000; Petruzzelli et al. 2003b). Here, identical expression patterns were evident for β-1,3-glucanase and PsACO1 transcripts just after the completion of germination. Ethylene biosynthesis and responsiveness are localized to the elongation and differentiation zones of the pea radicle. Treatment of S. officinale (Brassicaceae) seeds with ethylene-generating ethrel also did not appreciably affect the SoACS7 mRNA levels and overall ACC contents (Iglesias-Fernández and Matilla 2010). In agreement with this, inhibition of ACS by AVG did not appreciably affect germination, while inhibition of ACO by Co2+ reduced the maximum germination percentage of S. officinale (Iglesias-Fernández and Matilla 2010). These authors showed that in S. officinale seeds, SoACO2 expression behaves in a germination-related pattern. Its expression increases during germination; this increase is inhibited by both the GA biosynthesis inhibitor paclobutrazol and the ethylene signaling and biosynthesis inhibitor mix IESS, which delays germination in association with a delay in the increase of SoACO2 transcripts. These findings for S. officinale ACO are in full agreement with a recent work using other Brassicaceae, the conclusions for which are supported by analyzing specific seed tissues (L. sativum) and mutant seeds (A. thaliana) (Linkies et al. 2009; Narsai et al. 2011; Voegele et al. 2011). Three A. thaliana ACOs, AtACO1, AtACO2 and AtACO4/EFE (ethylene-forming enzyme), have been described by Alonso and Ecker (2001) as functional enzymes and 13 more sequences with similarity to known ACOs are known in A. thaliana (Linkies et al. 2009). Figure 3c shows that the transcript levels of AtACO1 and AtACO2 (plus two other ACO-related genes), but not AtACO4, were up-regulated upon A. thaliana seed imbibition. ABA treatment did not affect AtACO2 transcript levels, and either did not or slightly decrease the AtACO1 transcript levels on ABA treatment. Cold stratification reduced the transcript abundances of all ACOs, which is in agreement with the data of Narsai et al. (2011), who detected down-regulation of ACO transcripts during cold stratification. In contrast to that, GA had gene-specific effects including up-regulation of AtACO1 and down-regulation of AtACO2 (Fig. 3c). The important point is that during seed germination upon hormone treatment of A. thaliana, the regulation of ethylene biosynthesis is mediated by ACO, while the transcript abundances of ACS are not appreciably affected by imbibition or treatment with ABA or GA (Fig. 3b). Narsai et al. (2011) have shown that cold stratification leads to a transient increase in expression of some ACS genes, which might lead to ACC accumulation. Linkies et al. (2009) have analyzed the role of the individual ACOs during seed germination of the Brassicaceae species, A. thaliana and L. sativum, and found that ACO1 and ACO2 seem to be the major ACOs in seeds. Analysis of the A. thaliana knockout mutant for ACC oxidase2 (aco2) demonstrated that ethylene production by ACO2 was important for counteracting the ABA-mediated inhibition on A. thaliana endosperm rupture. From a tissue-specific analysis of ACO expression during L. sativum seed germination, they concluded that ethylene biosynthesis was mainly achieved by LesaACO1 and LesaACO2 and was differentially regulated in the radicle and the micropylar endosperm (Fig. 2b). ABA inhibits transcript expression of LesaACO1 in the radicle, but does not affect the expression of LesaACO2. ABA inhibits the transcript expression of LesaACO1 and LesaACO2 in the micropylar endosperm (Fig. 2b). Total ACO activity is higher in the radicle than in the micropylar endosperm and is inhibited by ABA in the radicle. Ethylene does not affect the ABA levels of seeds; therefore, the counteraction of the ABA-mediated inhibition by ethylene has to go through interference with ABA signaling (Linkies et al. 2009). L. sativum, A. thaliana and S. officinale are all endospermic Brassicaceae seeds. The collective evidence from these three species suggests an evolutionarily conserved role for ethylene and the ACO2 orthologs in micropylar endosperm weakening and rupture (Linkies et al. 2009). High GA and ethylene sensitivity of the micropylar endosperm is a prerequisite for the ethylene-enhanced expression of genes that are involved in endosperm weakening and inhibited by ABA (Fig. 2b). These gene products then cause endosperm weakening by cell wall loosening and cell separation processes that finally lead to endosperm rupture and thereby the completion of germination.
Ethylene biosynthesis pathway and gene expression regulation during Arabidopsis thaliana seed germination. a Ethylene biosynthesis as discovered by Yang and Hoffman (1984). Key step during seed germination is the conversion of ACC to ethylene via ACC oxidases (Linkies et al. 2009; Matilla and Matilla-Vázquez 2008). b and c Relative transcript expression results obtained by eNorthern analyses of data from the BAR expression browser at http://www.bar.utoronto.ca (Toufighi et al. 2005) based on experiments for non-dormant, non-stratified after-ripened wild-type seeds (Nakabayashi et al. 2005; Preston et al. 2009), cold-stratified wild-type seeds (Yamauchi et al. 2004) and ABA-treated wild-type and GA-treated ga1-3 seeds (RIKEN transcriptome sets). Shown are relative transcript abundance ratios based on seed imbibition time in the light (24 h/0 h), ABA treatment (ABA/control at 24 h), cold stratification (4°C/22°C after 96 h imbibition in darkness) and GA treatment (GA/control at 6 h). ACC 1-aminocyclopropane-1-carboxylic acid, MTA methylthioadenosine, MTR methylthioribose, SAM S-adenosylmethionine
Ethylene signaling takes place via its receptor; the first receptor mutant being isolated in A. thaliana was etr1 (ethylene triple response 1), a dimer histidine kinase located to the ER membrane and a negative regulator of ethylene signaling (Matilla and Matilla-Vázquez 2008). In A. thaliana, five ethylene receptors are known: ETR1, ETR2, ERS1, ERS2, EIN4. Upon ethylene binding, the receptors get inactivated, which in turn leaves CTR1 (constitutive triple response1) inactive. CTR1 is a serine–threonine protein kinase that acts as a negative regulator in ethylene signaling. Inactive CTR1 leads to progression of a MAP-kinase cascade and controls the positive regulator EIN2 and its transcription factors located in the nucleus, such as EIN3, EIL1 and EREBPs/ERFs, which activate the transcription of ethylene-responsive genes. Ethylene-insensitive etr1 mutant seeds show shape alterations and enhanced dormancy, germinate poorly and their germination is ABA hypersensitive (Chiwocha et al. 2005; Beaudoin et al. 2000). This is at least partly caused by higher ABA contents in the etr1 seeds. Also, ein2 mutant seeds with decreased ethylene sensitivity show enhanced ABA sensitivity (Chiwocha et al. 2005; Beaudoin et al. 2000). It has been shown that the ctr1 loss-of-function A. thaliana mutant has reduced ABA sensitivity during seed germination (Beaudoin et al. 2000), and is impaired in the ACC-mediated reversion of the ABA inhibition of endosperm rupture (Linkies et al. 2009). In experiments with the ethylene action inhibitor 2,5-norbornadiene (NBD), it has been shown that signaling via the known ethylene receptors is important for seed germination of many species, such as the Brassicaceae A. thaliana and L. sativum (Siriwitayawan et al. 2003; Linkies et al. 2009), the Fabaceae pea (Petruzzelli et al. 2000), the Solanaceae tobacco (Leubner-Metzger et al. 1998) and species from many other clades (Kucera et al. 2005). The involvement of ethylene signaling and biosynthesis in promoting seed germination and counteracting ABA effects is therefore a phylogenetically widespread phenomenon. Several hypotheses have been proposed to explain the mechanisms of ethylene action in germinating seeds: by biomechanical quantification of the weakening in the micropylar endosperm of L. sativum seeds, compelling evidence shows that ethylene signaling and biosynthesis are required for this process (Linkies et al. 2009). Ethylene promotes weakening of the micropylar endosperm of this species by inducing the expression of CWRP and/or ROS that cause cell wall loosening or cell separation of this tissue (Graeber et al. 2010; Morris et al. 2011). It was proposed that in the embryonic axis, ethylene could act by promoting radial cell expansion or by decreasing the seed base water potential (Kucera et al. 2005; Matilla and Matilla-Vázquez 2008; Dutta et al. 1994). It is therefore clear that in future research on ethylene action during seed germination, tissue-specific mechanisms and interactions with other hormones must be considered.
It has been proposed that a GA–ethylene synergism exists for dormancy release, after-ripening and germination promotion (reviewed by Matilla and Matilla-Vázquez 2008). In Fagus sylvatica, the expression of the gibberellin 20-oxidase FsGA20ox1 is linked to the cross-talk between both hormones during breaking of seed dormancy (Calvo et al. 2004). In S. officinale, the expression of a gibberellin 3-oxidase, SoGA3ox, is stimulated, while the expression of SoACO2 is inhibited by seed after-ripening (Iglesias-Fernández and Matilla 2009). In GA-deficient ga1-3 mutants of A. thaliana, GA treatment leads to increase in ACO expression, which suggests that GA activates ethylene biosynthesis and/or response (Ogawa et al. 2003). Further, ethylene treatment can replace GA lacking in ga1-3 mutants and induce germination in the light (Karssen et al. 1989). Contrary to that, ethylene cannot replace GA treatment to induce germination of GA-deficient gib-1 tomato mutant seeds (Groot et al. 1987). Evidence for genetic interactions among ethylene, GA and ABA signaling pathways has been obtained for A. thaliana seed germination (see Fig. 2 in Kucera et al. 2005 and Fig. 2 in Holdsworth et al. 2008a, b). The detailed action of ethylene and interaction with GA and ABA during seed germination have been postulated in a model by Linkies et al. (2009) based on their comparative work with L. sativum and A. thaliana (Fig. 2b).
Ethylene and downstream cell wall remodeling proteins are involved in temperature responses such as thermoinhibition
High temperature during seed imbibition can delay or severely inhibit seed germination, a mechanism called thermoinhibition (Argyris et al. 2011). It has been shown that ethylene plays a role in the reversion of thermoinhibition in chickpea and lettuce seeds. In lettuce, ethylene is specifically needed to counteract thermoinhibition of thermosensitive genotypes at high temperatures, although the exact function is unknown (Nascimento et al. 2000b). In chickpea, germination at optimal temperature depends on ethylene production in the embryonic axis. At higher temperatures, a reversion of thermoinhibition by the addition of ACC or ethylene is possible (Matilla 1996; Gallardo et al. 1994, 1996). Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin and ethylene biosynthesis, metabolism and response genes (Argyris et al. 2008; Argyris et al. 2011). They have shown that germination of lettuce (L. sativa ‘Salinas’) seeds is inhibited when imbibition temperature exceeds 25–30°C, while seeds of an accession of Lactuca serriola do not show any thermoinhibition up to 37°C. Thermoinhibited Salinas seeds contained higher ABA contents, while the expression of several genes involved in ABA, GA and ethylene biosynthesis, metabolism, and response were differentially influenced in the two genotypes by elevated temperatures and light. Generally, ABA-related genes showed higher expression upon inhibition of germination, while expression of GA- and ethylene-related genes was higher when germination was permitted. LsNCED4, a gene that is involved in ABA biosynthesis, showed higher expression upon temperature elevation only in Salinas. The upper temperature sensitivity of LsNCED4 expression may specify the temperature limit for the germination of lettuce seeds. There are several investigations pointing toward a connection between ethylene and endo-β-1,4-mannanase activity increase leading to a reversion of thermoinhibition (Nascimento et al. 2000a, 2001; Matilla 1996). Endo-β-1,4-mannanases as CWRPs could be involved in the weakening of the endosperm tissue leading to progression of germination and in that way revert thermoinhibition. In lettuce, an endo-β-1,4-mannanase was identified by Wang et al. (2004), which is specifically expressed in the micropylar region of the endosperm just after the completion of germination. Further, the expression of endo-β-1,4-mannanase has been shown to increase during after-ripening and by ethylene treatment in endospermic seeds of S. officinale (Iglesias-Fernández and Matilla 2009). High temperature-induced ABA biosynthesis and its role in the inhibition of GA action were also investigated in seeds of A. thaliana (Toh et al. 2008). Thermoinhibition-tolerant germination of loss-of-function mutants of GA negative regulators, SPINDLY (SPY) and RGL2, suggests that repression of GA signaling is required for thermoinhibition. Interestingly, ABA-deficient aba2-2 mutant seeds show significant expression of GA synthesis genes and repression of SPY expression even at high temperatures. Toh et al. (2008) conclude that high temperature stimulates ABA synthesis and represses GA synthesis and signaling through the action of ABA in A. thaliana seeds. Watt et al. (2011) developed a hydrothermal time model that accurately characterizes how thermoinhibition regulates seed germination. While much is known about GA, ABA and ethylene, far less is known about other hormones during seed temperature stress responses. Jasmonates are known to be involved in the stress response of plants; their role in seed germination is reviewed in the following sections.
Jasmonate biosynthesis and signaling by diverse JA derivatives with biological activity
Jasmonates (JAs) and their biosynthetic precursor 12-oxo-phytodienoic acid (OPDA) are signals in plant stress responses, physiological reactions and developmental processes. Generally, JAs inhibit plant growth, regulate defense, wounding and stress responses and are involved in pollen and embryo development, seed germination, senescence, fruit ripening and allelopathy (reviewed in Delker et al. 2006; Browse 2009). Several genes are induced by both JA and ABA under stress conditions (Porta et al. 1999; Chao et al. 1999), showing the synergism in several physiological reactions. In contrast to that, both synergism and antagonism can occur between JA and ethylene pathways during stress responses (Rojo et al. 1999; Xu et al. 1994).
The pathway for jasmonate biosynthesis has first been described in Vicia faba by Vick and Zimmerman (1983). It initiates in the chloroplast with the conversion of α-linolenic acid to 12-oxo-phytodienoic acid (OPDA), which is mediated by 13-lipoxygenase (LOX), allene oxide synthase (AOS) and allene oxide cyclase (AOC) (Fonseca et al. 2009a) (Fig. 4a). Four genes (AOC1–AOC4) code for AOCs in A. thaliana, and these are expressed locally and systemically (Delker et al. 2006). Under the involvement of the ATP binding cassette (ABC) transporter COMATOSE (CTS), OPDA is then transported to the peroxisome, the site for ß-oxidation in plants. OPDA is then converted by OPDA reductase (OPR), for which only the A. thaliana OPR3 gene product carries a peroxisomal target sequence. The OPC-8:0 produced by OPR3 is activated by carboxyl-CoA ligase encoded in A. thaliana by OPCL1. By three rounds of ß-oxidation catalyzed by acyl-CoA oxidases (ACX), the multifunctional protein (MFP) and L-3-ketoacyl-CoA thiolase (KAT), it is then converted to jasmonic acid (JA). Both isomers of JA, the predominant (3R,7R)-isomer (−)-JA and the (3R,7S)-isomer (+)-7-iso-JA, are detected in plants (Fig. 4a). Severe JA-biosynthesis mutants are characterized by male sterility (Browse 2009). JA can be converted into a variety of derivatives, such as methyl jasmonate (MeJA) and jasmonoyl-l-isoleucine (JA-Ile) (reviewed by Browse 2009). Many of these derivates, including MeJA and JA-Ile, have been shown to be biologically active (Wasternack 2007). In several cases, it is however a matter of controversial debate which derivate or isoform is the biologically active compound, as in planta an inactive precursor upon plant treatment may be converted into the active compound (Delker et al. 2006; Fonseca et al. 2009a). This is for example debated for JA-Ile: (−)-JA-L-Ile has been described as the molecularly active form of the hormone (Staswick and Tiryaki 2004; Thines et al. 2007), while (+)-7-iso-JA-L-Ile has been proposed by others (Fonseca et al. 2009a, b). In A. thaliana, JA-Ile biosynthesis is mediated by JAR1 (Staswick et al. 2002; Suza and Staswick 2008). jar1 mutants show five- to tenfold reduced JA-Ile levels and impaired JA responses (Kang et al. 2006; Staswick and Tiryaki 2004; Browse 2009). The biosynthesis of JA-Ile is positively regulated by JA signaling. Most genes involved in the JA biosynthesis and signaling pathway, such as LOX2, LOX3, AOS, AOC, OPR3, OPCL1 and JAR1, are induced by JA treatment (Chung et al. 2008; Suza and Staswick 2008; Wasternack 2004). Interestingly, Stumpe et al. (2010) found in P. patens only cis-(+)-OPDA, but not JA and no other precursors or metabolites of JA, showing the rather recent evolution of jasmonates.
Jasmonate biosynthesis pathway, which has first been described in Vicia faba by Vick and Zimmerman (1983)
JA signaling takes place via its receptor, the F-box protein COI1 (Coronatine insensitive1) (Yan et al. 2009; Browse 2009; Xie et al. 1998). COI1 is part of the E3 ubiquitin ligase SCF-complex SCFCOI1 (Fig. 5a) that targets proteins for degradation through the 26S proteasome by ubiquitination (Devoto et al. 2002; Xu et al. 2002; Zheng et al. 2002; Fonseca et al. 2009a). The null mutant coi1-1 is JA insensitive and produces infertile pollen (Feys et al. 1994). Figure 5 shows that upon binding of JA-Ile to COI1, the SCFCOI1 complex targets JAZ (Jasmonate ZIM-domain) proteins for degradation, which otherwise inhibit JA responses (Thines et al. 2007; Browse 2009; Yan et al. 2007; Chini et al. 2007). It has been shown that the JAZ protein JAI3 (Jasmonate insensitive 3) negatively regulates MYC2, which represents the key transcriptional activator of jasmonate responses (Chini et al. 2007). Besides that, the transcription factor ethylene response factor 1 (ERF1) is known to act downstream of JA in stress response (Lorenzo et al. 2004).
Jasmonate signaling is mediated via MYC transcription factors, which are in turn negatively regulated by JAZ proteins that get targeted for degradation by the SCFCOI1 complex as reviewed by Browse (2009). COI1 coronatine insensitive1, JA-RE jasmonate-responsive element, JAZ jasmonate zim-domain, MYC2 MYC transcription factor, allelic to Jasmonate-insensitive1, TS transcription start, UQ ubiquitin
Various JA metabolites seem to play roles in the JA responses related to dormancy and germination, and their contents differ between species and change during the progression of germination
JA, its metabolites such as methyl jasmonate (MeJA), jasmonoyl-L-isoleucine (JA-Ile) and its precursor 12-oxo-phytodienoic acid (OPDA) are all involved in biotic and abiotic stress responses (Wasternack and Kombrink 2009), as well as in the regulation of plant growth and development, such as senescence, reproduction, and pollen and embryo development (Staswick et al. 1992; Feys et al. 1994; Balbi and Devoto 2008; Wasternack and Kombrink 2009). Recent work with A. thaliana mutants (e.g., Dave et al. 2011; Preston et al. 2009) summarized below demonstrates that jasmonates have role(s) during seed germination. Further, it has been shown that JA/MeJA can inhibit seed germination of Solanum lycopersicum, Brassica napus, Linum usitatissimum, Lupinus luteus and Zea mays (Miersch et al. 2008; Oh et al. 2009; Wilen et al. 1991; Zalewski et al. 2010; Norastehnia et al. 2007). Thines et al. (2007) proposed that the JA-Ile conjugate, but not other jasmonate derivatives such as JA, OPDA or MeJA, play a central role in the jasmonate response. It promotes the interaction between COI1 and the JAZ1 proteins, which results in the ubiquitin-dependent degradation of JAZ1 that represses transcription of JA-responsive genes. Recently, Dave et al. (2011) proposed based on crossing several mutants impaired at several steps during β-oxidation that in fact OPDA, and not JA, has the stronger effect in inhibiting seed germination of A. thaliana. They found that OPDA was around ten times more efficient than JA. They further found a synergistic effect of OPDA and ABA on the germination inhibition of A. thaliana seeds.
The exogeneous application of both JA and ABA delays germination of A. thaliana, B. napus and L. usitatissimum (Brassicaceae) (Wilen et al. 1991; Ellis and Turner 2002; Nambara et al. 2010) and indicates a synergistic effect of both hormones. Contrary to that, two JA-insensitive mutants, coi1-16 and jar1, display ABA hypersensitivity during germination, indicating an antagonistic effect of JA toward ABA-mediated inhibition of germination (Ellis and Turner 2002; Staswick et al. 1992). Staswick et al. (1992) investigated the effects of jar1, an ethyl methanesulfonate mutant with decreased sensitivity to MeJA regarding root growth. They found that jar1 showed an ABA-hypersensitive phenotype with a much stronger germination delay by ABA compared to wild-type seeds, showing that this mutant with lower MeJA sensitivity was not ABA insensitive. The JA-perception mutants jin4 and coi1 also show a stronger delay in seed germination by ABA in A. thaliana (Ellis and Turner 2002; Berger et al. 1996). Further, a connection between JA and ABA had been shown by Kanai et al. (2010), who investigated the ped3 mutant, an allele of cts, which was impaired in germination potential. They found a very high transcript abundance of the bZIP-type transcription factor ABI5, which normally binds to ABA-responsive (ABRE) elements of gene promoters and thereby inhibits germination in the ped3 mutant. They further found that a mutation in ABA-insensitive 5 (abi5) could rescue the germination defects of ped3 and that promotion of seed germination of PED3 worked through the induction of pectin degradation.
Preston et al. (2009) determined the amounts of JA and its conjugate JA-Ile in dry seeds and during an imbibition period of 36 h for two different A. thaliana accessions: non-dormant Columbia (Col) seeds, which completed germination between 30 and 36 h, and dormant Cap Verde Islands (Cvi) seeds, which did not germinate during this imbibition period. In the dry state, non-dormant Col seeds contained ca. tenfold higher contents of JA and JA-Ile compared to dormant Cvi seeds. These JA and JA-Ile contents remained low in the imbibed Cvi seeds during the entire incubation period of 36 h (as these seeds are dormant, they did not germinate). The high JA contents detected in dry Col seeds rapidly declined during the early phase of imbibition to ca. tenfold lower levels at 5 h. Also, the high JA-Ile detected in dry Col seeds declined during imbibition, but the rate of this decline was much slower and evenly spread over the early and late phase of germination until ca. tenfold lower levels were reached at the time of endosperm rupture (30–36 h). Preston et al. (2009) propose that the reduction of JA-Ile in non-dormant Col seeds is an accession-specific mechanism to regulate germination. Dave et al. (2011) investigated ODPA, JA and JA-Ile contents during late seed development of Col and their temporal pattern showed that there was no massive increase in their contents during seed maturation, suggesting that their accumulation occurred during early seed development. Dave et al. (2011) and Preston et al. (2009) obtained similar values for the JA and JA-Ile contents in mature dry Col seeds. As the ODPA, JA and JA-Ile contents of dry Col and Wassilewskija (Ws) wild-type seeds are very similar (Dave et al. 2011), one may conclude that the rather non-dormant accessions Col and Ws are characterized by high jasmonate contents, whereas the deeply dormant accession Cvi is characterized by low jasmonate contents in dry seeds. The ODPA and JA contents of Ws seeds decreased during germination, as shown for JA in Col. However, while JA-Ile contents decreased during Col germination (Preston et al. 2009), the JA-Ile contents remained the same at dry-seed level during Ws germination (Dave et al. 2011). Dose–response curves for the percentage of endosperm rupture demonstrate that treatment with JA or OPDA inhibits germination of both ecotypes. While JA had only a comparably weak effect that was equal for both ecotypes, OPDA was a stronger inhibitor and the inhibition of endosperm rupture was more sensitive for the Ws ecotype compared to Col. These findings suggest that a decrease in JA-Ile contents is not a requirement for non-dormant seeds to complete germination (Col versus Ws), while a decline in JA is evident during germination of both ecotypes. JA-Ile and JA contents of dry seeds are high in the non-dormant Col/Ws ecotypes, whereas they are low and remain low in dry and imbibed seeds of the deeply dormant ecotype Cvi. Furthermore, OPDA is a stronger germination inhibitor compared to JA, and the sensitivity for this OPDA inhibition seems to be ecotype specific (Col versus Ws). Therefore, although it is without doubt that treatment of seeds with jasmonates inhibits germination, there is yet no clear relationship between endogenous jasmonate contents and germination or dormancy of A. thaliana. Taken together, more research is required to unravel the molecular mechanisms by which jasmonates regulate germination.
Several A. thaliana mutants of the β-oxidation pathway, such as cts, acx1 acx2 and kat2, which are impaired in their ability to catabolize fatty acids derived from storage oil and to synthesize jasmonate are not only defective in seedling establishment, requiring the addition of exogenous sucrose, but are also impaired in their germination potential. Interestingly, this phenotype cannot be reverted by the addition of exogeneous sucrose as an alternative carbon source (Footitt et al. 2002; Pinfield-Wells et al. 2005; Russell et al. 2000; Adham et al. 2005), indicating that the carbon deficiency is not the reason for the impaired germination potential. Other mutants impaired in storage oil breakdown do not show any delayed germination (Quettier et al. 2008; Fulda et al. 2004; Russell et al. 2000). The impaired germination of cts-1 can also not be reverted by the addition of GA. This mutant has been identified in a screen for mutants with increased seed dormancy (Russell et al. 2000). In agreement with this, it has been shown that CTS is epistatic to RGL2, a DELLA factor which inhibits germination and is targeted for degradation by GA (Carrera et al. 2007). Dave et al. (2011) showed that cts-1 (pxa1-1) and cts-2 mutants have increased OPDA, JA and JA-Ile contents, as well as altered transcript expression levels of JA and GA metabolism genes. They conclude that OPDA accumulation in cts/pxa1 seeds leads to the inhibition of seed germination. This may involve gene regulation via the SCFCOI1 complex and the JAZ repressors (Fig. 5).
MeJA also inhibits seed germination and root growth of cereal grains (Norastehnia et al. 2007). In cereal grains, the embryonic root is covered with the coleorhiza tissue; this covering layer is involved in regulating dormancy and germination (Barrero et al. 2009). These authors compared transcriptome data from root and coleorhiza tissues of dormant and after-ripened barley grains. They propose that the coleorhiza may not only play a role in the protection of the seminal roots during development and germination (Sargent and Osborne 1980) or serve as a water and food store (Walne et al. 1975; Debaene-Gill et al. 1994), but may also serve as a germination-regulating tissue by preventing root emergence in dormant seeds and in relation to ABA contents and sensitivity, just as the endosperm tissue in eudicot seeds (Linkies et al. 2009, 2010). Barrero et al. (2009) found for jasmonates an induction of several jasmonate-related genes in the coleorhiza tissue during imbibition of after-ripened seeds in comparison to dormant seeds, such as the biosynthesis genes jasmonate 12-oxophytodienoic acid reductase (HvOPR) and allene oxide synthase (HvAOS) and the putative jasmonate receptor coronatine insensitive1 (HvCOI1). In contrast to that, a lipoxygenase (HvLOX) was highly expressed in dormant coleorhiza tissue. Barrero et al. (2009) propose the involvement of jasmonate in dormancy and after-ripening and present a model linking ABA and jasmonate in the after-ripening in barley coleorhizae.
MeJA is not only a signaling molecule within an individual plant, but also an ethylene gaseous compound that can mediate communication between plants, as it is the case in the defense against chewing herbivores (Karban et al. 2000; Onkokesung et al. 2010) and in allelopathic interactions (Krock et al. 2002; Preston et al. 2002). Allelopathy is defined as a direct or indirect interaction, whereby allelochemicals released by one organism influence the physiological processes of another neighboring organism. Nicotiana attenuata, wild tobacco, is a postfire annual plant native to the Great Basin of western North America that often grows in close proximity to sagebrush, Artemisia tridentata, the defining and dominant plant of that region. Annually established populations of this native tobacco germinate from seed banks that respond to smoke cues from wildfires (Krock et al. 2002; Preston et al. 2002). Sagebush has well-documented allelopathic tendencies that have generally been ascribed to its most abundantly released secondary metabolites. However, as a minor component, sagebrush releases MeJA. Preston et al. (2002) propose that MeJA is an allelochemical of sagebrush that inhibits germination of neighboring tobacco, N. attenuata. With a combination of field and laboratory studies, they examined the role of MeJA, released from sagebrush and transported by both air and water, in inhibiting N. attenuata seed germination. Exposure to volatile and aqueous MeJA also inhibited germination of N. attenuata seeds at quantities that are released naturally by sagebrush. A. tridentata seeds were significantly more resistant to MeJA inhibition compared to N. attenuata seeds. Several other tobacco species that are not known to be associated with sagebrush are less sensitive to MeJA, suggesting an evolved sensitivity to MeJA. They conclude that MeJA release from sagebrush plays an allelopathic role for N. attenuata seed banks, but other unidentified compounds are also involved. Krock et al. (2002) demonstrated that the germination inhibitor MeJA did not act by altering ABA contents of imbibed seeds. Treatment with the ABA biosynthesis inhibitor, fluridone, inhibited the dormancy-inducing effects of MeJA, but surprisingly did not affect endogenous ABA levels in treated seeds. However, ABA leached from the litter of the species, which dominate the plant community before fires, plays an important role in germination control. They conclude that N. attenuata seeds, which can lie dormant in the soil for 150 years between fires, time their germination with the postfire environment by responding to smoke, ABA and four terpenes leaching from the litter of the dominant vegetation. Smoke breaks N. attenuata dormancy and induces germination mainly by increasing GA sensitivity and decreasing ABA contents of imbibed seeds (Schwachtje and Baldwin 2004). Wu and Bradford (2003) found that MeJA and wounding induce class I chitinase (Chi9), but not class I ß-1,3-glucanase (GluB) gene expression in the micropylar endosperm of tomato seeds during germination. In the jasmonate-deficient defenseless1 mutant of tomato expression of Chi9 was reduced, which could be restored by MeJA treatment. Wounding also did not induce the expression of the class I ß-1,3-glucanase (GluB) gene in tobacco seeds, where it has been shown to be up-regulated by GA and ethylene, and down-regulated by ABA (Leubner-Metzger 2003; Leubner-Metzger et al. 1995; Leubner-Metzger and Meins 2000; Leubner-Metzger et al. 1998). ß-1,3-Glucanase expression in the micropylar endosperm promotes endosperm rupture and has been proposed to act via weakening of the micropylar endosperm in tobacco (Leubner-Metzger and Meins 2000; Leubner-Metzger 2002) and other Solanaceous species (Leubner-Metzger 2003; Petruzzelli et al. 2003a).
During responses to stress and developmental processes, JA and ethylene can work in a synergistic or antagonistic way (reviewed by Lorenzo and Solano (2005) and references therein). During pathogen defense, both hormones act synergistically. Mutants that are affected in JA or ethylene biosynthesis or perception are impaired in their defense reactions (Lorenzo and Solano 2005; Vijayan et al. 1998; Knoester et al. 1998), while an antagonistic action has been described in the context of wounding response in A. thaliana (Rojo et al. 1999, 2003). Few studies have been carried out on the interaction between JA and ethylene biosynthesis and signaling during seed germination. Staswick and Tiryaki (2004) determined a connection between JA and ethylene signaling. They found conjugates of JA and ACC (aminocyclopropane-1-carboxylic acid; the direct ethylene precursor) in plant tissues, but this conjugate did not have the same inhibitory effect as JA-Ile on root growth. They propose the synthesis of JA-ACC as a mechanism to regulate the availability of both precursors for the synthesis of the bioactive hormones ethylene and JA-Ile and present a model showing the putative co-regulation of both enzymes′ biosynthesis, including JAR1 as negative regulator for the formation of JA-ACC. Direct interaction between JA and ethylene has been shown, as the ethylene response factor 1 (ERF1) is directly regulated by JA (Solano et al. 1998; Lorenzo et al. 2003). Further, Adams and Turner (2010) found that the jasmonate receptor COI1 plays a role in the ethylene-induced inhibition of root growth in the light, but not in the dark. They propose that a second ACC/ethylene-induced root growth inhibiting pathway exists, which is mediated by COI1, in parallel with the usual ethylene signaling pathway.
To sum up our review, the roles and molecular mechanisms of GA and ABA in seed dormancy and germination have been rather intensely studied. In recent research, the germination-promoting hormone ethylene has been quite well characterized and ethylene–ABA interactions in seeds and seedlings have been shown to differ. In contrast to that, only little research has been carried out on the roles and molecular mechanisms of jasmonates, which have germination-inhibiting effects, but for which the current knowledge is very limited and partly contradictory. To further unravel the multiple role(s) of hormones in seeds, more research and a deeper insight into JA biosynthesis and signaling during seed germination are required; this is a challenging research subject. A deeper understanding of the complex hormonal network and its interactions during seed dormancy and germination is a prerequisite to apply this knowledge to crop species in a way that leads to further improvement of seed and seedling performance important for crop productivity and yield even upon abiotic stress.
References
Adams E, Turner J (2010) COI1, a jasmonate receptor, is involved in ethylene-induced inhibition of Arabidopsis root growth in the light. J Exp Bot 61(15):4373–4386
Adham AR, Zolman BK, Millius A, Bartel B (2005) Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J 41(6):859–874
Alonso JM, Ecker JR (2001) The ethylene pathway: a paradigm for plant hormone signaling and interaction. Science’s STKE 2001 (70):RE1
Argyris J, Dahal P, Hayashi E, Still DW, Bradford KJ (2008) Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol 148(2):926–947
Argyris J, Truco M, Ochoa O, McHale L, Dahal P, Van Deynze A, Michelmore R, Bradford K (2011) A gene encoding an abscisic acid biosynthetic enzyme LsNCED4 collocates with the high temperature germination locus Htg6.1 in lettuce (Lactuca sp.). TAG Theor Appl Genet 122(1):95–108
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14(02):93–107
Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177(2):301–318
Barrero JM, Talbot MJ, White RG, Jacobsen JV, Gubler F (2009) Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiol 150(2):1006–1021
Bassel GW, Mullen RT, Bewley JD (2006) ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J Exp Bot 57:1291–1297
Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12(7):1103–1116
Berger S, Bell E, Mullet JE (1996) Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol 111(2):525–531
Bethke PC, Libourel IGL, Aoyama N, Chung Y–Y, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143(3):1173–1188
Bewley JD (1997a) Breaking down the walls—a role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci 2(12):464–469
Bewley JD (1997b) Seed germination and dormancy. Plant Cell 9(7):1055–1066
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60(1):183–205
Cadman CSC, Toorop PE, Hilhorst HWM, Finch-Savage WE (2006) Gene expression profiles of Arabidopsis Cvi seed during cycling through dormant and non-dormant states indicate a common underlying dormancy control mechanism. Plant J 46:805–822
Calvo AP, Nicolás C, Nicolás G, Rodríguez D (2004) Evidence of a cross-talk regulation of a GA 20-oxidase (FsGA20ox1) by gibberellins and ethylene during the breaking of dormancy in Fagus sylvatica seeds. Physiol Plant 120(4):623–630
Cao D, Hussain A, Cheng H, Peng J (2005) Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223(1):105–113
Carrera E, Holman T, Medhurst A, Peer W, Schmuths H, Footitt S, Theodoulou FL, Holdsworth MJ (2007) Gene expression profiling reveals defined functions of the ATP-binding cassette transporter COMATOSE late in phase II of germination. Plant Physiol 143(4):1669–1679
Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, Holdsworth MJ (2008) Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J 53:214–224
Chao WS, Gu Y-Q, Pautot V, Bray EA, Walling LL (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol 120(4):979–992
Chen F, Bradford KJ (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124:1265–1274
Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448(7154):666–671
Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42(1):35–48
Chung HS, Koo AJK, Gao X, Jayanty S, Thines B, Jones AD, Howe GA (2008) Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol 146(3):952–964
Crane PR, Friis EM, Pedersen KR (1995) The origin and early diversification of angiosperms. Nature 374(6517):27–33
da Silva EAA, Toorop PE, van Aelst AC, Hilhorst HWM (2004) Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta 220(2):251–261
da Silva EAA, Toorop PE, Van Lammeren AAM, Hilhorst HWM (2008) ABA inhibits embryo cell expansion and early cell division events during coffee (Coffea arabica ‘Rubi’) seed germination. Ann Bot 102(3):425–433
Dave A, Hernandez ML, He Z, Andriotis VME, Vaistij FE, Larson TR, Graham IA (2011) 12-oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. Plant Cell 23(2):583–599
Debaene-Gill SB, Allen PS, Gardner JS (1994) Morphology of the perennial ryegrass (Lolium perenne; Poaceae) coleorhiza and emerging radicle with continuous or discontinuous hydration. Am J Bot 81(6):739–744
Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122(2):403–414
Delker C, Stenzel I, Hause B, Miersch O, Feussner I, Wasternack C (2006) Jasmonate biosynthesis in Arabidopsis thaliana—enzymes, products, regulation. Plant Biol 8(3):297–306
Depuydt S, Hardtke S (2011) Hormone signalling crosstalk in plant growth regulation. Curr Biol 21(9):R365–R373
Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signalling and fertility to the SCF ubiquitin–ligase complex in Arabidopsis. Plant J 32(4):457–466
Dutta S, Bradford KJ, Nevins DJ (1994) Cell-wall autohydrolysis in isolated endosperms of lettuce (Lactuca sativa L.). Plant Physiol 104(2):623–628
Ellis C, Turner J (2002) A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta 215(4):549–556
Etheridge N, Hall B, Schaller G (2006) Progress report: ethylene signaling and responses. Planta 223(3):387–391
Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6(5):751–759
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171(3):501–523
Fonseca S, Chico JM, Solano R (2009a) The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 12(5):539–547
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009b) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5(5):344–350
Footitt S, Slocombe SP, Larner V, Kurup S, Wu Y, Larson T, Graham I, Baker A, Holdsworth M (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J 21(12):2912–2922
Forbis TA, Floyd SK, Queiroz Ad (2002) The evolution of embryo size in angiosperms and other seed plants: implications for the evolution of seed dormancy. Evolution 56(11):2112–2125
Friedman WE (1998) The evolution of double fertilization and endosperm: an ‘historical’ perspective. Sex Plant Reprod 11(1):6–16
Fry SC, Dumville JC, Miller JG (2001) Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem J 357(Pt 3):729–737
Fulda M, Schnurr J, Abbadi A, Heinz E, Browse J (2004) Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell Online 16(2):394–405
Gallardo M, Gallardo ME, Matilla A, Munoz de Ruedo P, Sanchez-Calle IM (1994) Inhibition of polyamine synthesis by cyclohexylamine stimulates the ethylene pathway and accelerates the germination of Cicer arietinum seeds. Physiol Plant 91:9–16
Gallardo M, Sanchez-Calle IM, Munoz de Ruedo P, Matilla A (1996) Alleviation of thermoinhibition in chick-pea seeds by putrescine involves the ethylene pathway. Aust J Plant Physiol 23:479–487
Gerjets T, Scholefield D, Foulkes MJ, Lenton JR, Holdsworth MJ (2010) An analysis of dormancy, ABA responsiveness, after-ripening and pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.) caryopses. J Exp Bot 61(2):597–607
Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12(7):1117–1126
Graeber K, Linkies A, Müller K, Wunchova A, Rott A, Leubner-Metzger G (2010) Cross-species approaches to seed dormancy and germination: conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol Biol 73(1):67–87
Groot SPC, Karssen CM (1992) Dormancy and germination of abscisic acid-deficient tomato seeds. Plant Physiol 99:952–958
Groot SPC, Bruinsma J, Karssen CM (1987) The role of endogenous gibberellin in seed and fruit development of tomato: studies with a gibberellin-deficient mutant. Physiol Plant 71(2):184–190
Hauser F, Waadt R, Schroeder Julian I (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21(9):R346–R355
Hepher A, Roberts JA (1985a) The control of seed germination in Trollius ledebouri: a model of seed dormancy. Planta 166(3):321–328
Hepher A, Roberts JA (1985b) The control of seed germination in Trollius ledebouri: the breaking of dormancy. Planta 166(3):314–320
Hermann K, Meinhard J, Dobrev P, Linkies A, Pesek B, Hess B, Machackova I, Fischer U, Leubner-Metzger G (2007) 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.): a comparative study of fruits and seeds. J Exp Bot 58:3047–3060
Hilhorst HWM, Downie B (1995) Primary dormancy in tomato (Lycopersicon esculentum cv. Moneymaker): studies with the sitiens mutant. J Exp Bot 47(294):89–97
Hirano K, Nakajima M, Asano K, Nishiyama T, Sakakibara H, Kojima M, Katoh E, Xiang H, Tanahashi T, Hasebe M, Banks JA, Ashikari M, Kitano H, Ueguchi-Tanaka M, Matsuoka M (2007) The GID1-mediated gibberellin perception mechanism is conserved in the Lycophyte Selaginella moellendorffii but not in the Bryophyte Physcomitrella patens. Plant Cell 19(10):3058–3079. doi:10.1105/tpc.107.051524
Holdsworth MJ, Bentsink L, Soppe WJJ (2008a) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179(1):33–54
Holdsworth MJ, Finch-Savage WE, Grappin P, Job D (2008b) Post-genomics dissection of seed dormancy and germination. Trends Plant Sci 13(1):7–13
Iglesias-Fernández R, Matilla A (2009) After-ripening alters the gene expression pattern of oxidases involved in the ethylene and gibberellin pathways during early imbibition of Sisymbrium officinale L. seeds. J Exp Bot 60(6):1645–1661
Iglesias-Fernández R, Matilla A (2010) Genes involved in ethylene and gibberellins metabolism are required for endosperm-limited germination of Sisymbrium officinale L. seeds. Planta 231(3):653–664
Iglesias-Fernández R, Rodríguez-Gacio M, Barrero-Sicilia C, Carbonero P, Matilla A (2011) Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta 233(1):25–36
Kanai M, Nishimura M, Hayashi M (2010) A peroxisomal ABC transporter promotes seed germination by inducing pectin degradation under the control of ABI5. Plant J 62(6):936–947
Kang J-H, Wang L, Giri A, Baldwin IT (2006) Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell 18(11):3303–3320
Karban R, Baldwin IT, Baxter KJ, Laue G, Felton GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125(1):66–71
Karssen CM, Zagorski S, Kepczynski J, Groot SPC (1989) Key role for endogenous gibberellins in the control of seed germination. Ann Bot 63(1):71–80
Kendall SL, Hellwege A, Marriot P, Whalley C, Graham IA, Penfield S (2011) Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell Online 23(7):2568–2580
Klee HJ, Clark DG (2004) Ethylene signal transduction in fruits and flowers. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action. Cluver Academic, London, pp 377–398
Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95(4):1933–1937
Koornneef M (2002) Seed dormancy and germination. Curr Opin Plant Biol 5(1):33–36
Koornneef M, Veen JH (1980) Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. TAG Theor Appl Genet 58(6):257–263
Krock B, Schmidt S, Hertweck C, Baldwin IT (2002) Vegetation-derived abscisic acid and four terpenes enforce dormancy in seeds of the post-fire annual, Nicotiana attenuata. Seed Sci Res 12(04):239–252
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Science Research 15(04):281–307
Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002) Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev 16(5):646–658
Leubner-Metzger G (2002) Seed after-ripening and over-expression of class I ß-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta 215(6):959–968
Leubner-Metzger G (2003) Functions and regulation of β-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Science Research 13(01):17–34
Leubner-Metzger G, Meins F (2000) Sense transformation reveals a novel role for class I β-1,3-glucanase in tobacco seed germination. Plant J 23(2):215–221
Leubner-Metzger G, Frundt C, Vogeli-Lange R, Meins F Jr (1995) Class I β-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiol 109(3):751–759
Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F (1998) Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I β-1,3-glucanase during tobacco seed germination. Plant Mol Biol 38(5):785–795
Lin Z, Zhong S, Grierson D (2009) Recent advances in ethylene research. J Exp Bot 60(12):3311–3336
Linkies A, Müller K, Morris K, Tureckova V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Linkies A, Gräber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186(4):817–831
Liu P–P, Koizuka N, Homrichhausen TM, Hewitt JR, Martin RC, Nonogaki H (2005) Large-scale screening of Arabidopsis enhancer-trap lines for seed germination-associated genes. Plant J 41(6):936–944
Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8(5):532–540
Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15(1):165–178
Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16(7):1938–1950
Matilla AJ (1996) Polyamines and seed germination. Seed Sci Res 6(03):81–93
Matilla AJ (2000) Ethylene in seed formation and germination. Seed Sci Res 10(02):111–126
Matilla AJ, Matilla-Vázquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175(1–2):87–97
McKibbin RS, Wilkinson MD, Bailey PC, Flintham JE, Andrew LM, Lazzeri PA, Gale MD, Lenton JR, Holdsworth MJ (2002) Transcripts of Vp-1 homeologues are misspliced in modern wheat and ancestral species. Proc Natl Acad Sci 99(15):10203–10208
Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 177(1):114–127
Morris K, Linkies A, Müller K, Oracz K, Wang X, Lynn JR, Leubner-Metzger G, Finch-Savage WE (2011) Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: a global tissue-specific transcript analysis. Plant Physiol 155(4):1851–1870
Müller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47(7):864–877
Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009a) The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184(4):885–897
Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G (2009b) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150(4):1855–1865
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41(5):697–709
Nambara E, Hayama R, Tsuchiya Y, Nishimura M, Kawaide H, Kamiya Y, Naito S (2000) The role of ABI3 and FUS3 loci in Arabidopsis thaliana on phase transition from late embryo development to germination. Dev Biol 220(2):412–423
Nambara E, Okamoto M, Tatematsu K, Yano R, Seo M, Kamiya Y (2010) Abscisic acid and the control of seed dormancy and germination. Seed Sci Res 20(02):55–67
Narsai R, Law SR, Carrie C, Xu L, Whelan J (2011) In depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis thaliana. Plant Physiol. doi:10.1104/pp.111.183129
Nascimento WM, Cantliffe DJ, Huber DJ (2000a) Endo-β-mannanase activity during lettuce seed germination at high temperature conditions. Acta Hortic 517:107–112
Nascimento WM, Cantliffe DJ, Huber DJ (2000b) Thermotolerance in lettuce seeds: association with ethylene and endo-β-mannanase. J Am Soc Hortic Sci 125:518–524
Nascimento WM, Cantliffe DJ, Huber DJ (2001) Endo-β-mannanase activity and seed germination of thermosensitive and thermotolerant lettuce genotypes in response to seed priming. Seed Sci Res 11(03):255–264
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126(3):467–475
Ni BR, Bradford KJ (1993) Germination and dormancy of abscisic acid- and gibberellin-deficient mutant tomato (Lycopersicon esculentum) seeds (sensitivity of germination to abscisic acid, gibberellin, and water potential). Plant Physiol 101(2):607–617
Nonogaki H (2006) Seed germination—the biochemical and molecular mechanisms. Breed Sci 56:93–105
Nonogaki H, Gee OH, Bradford KJ (2000) A germination-specific endo ß-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123:1235–1246
Nonogaki H, Chen F, Bradford KJ (2007) Mechanisms and genes involved in germination sensu stricto. In: Annual plant reviews: seed development, dormancy and germination, vol 27. Blackwell Publishing Ltd
Norastehnia A, Sajedi RH, Nojavan-Asghari M (2007) Inhibitory effects of methyl jasmonate on seed germination in maize (Zea mays): effect on α-amylase activity and ethylene production. Gen Appl Plant Physiol 33(1–2):13–23
Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G (2009) Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 21(2):403–419
Onkokesung N, Baldwin IT, Gális I (2010) The role of jasmonic acid and ethylene crosstalk in direct defense of Nicotiana attenuata plants against chewing herbivores. Plant Signal Behav 5(10):1305–1307
Ooms J, Leon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM (1993) Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana (a comparative study using abscisic acid-insensitive abi3 mutants). Plant Physiol 102(4):1185–1191
Oracz K, Voegele A, Tarkowská D, Jacquemoud D, Turecková V, Urbanová D, Strnad M, Sliwinska E, Leubner-Metzger G (2011) Myrigalone A inhibits Lepidium sativum seed germination by interference with gibberellin metabolism and apoplastic superoxide production required for embryo extension growth and endosperm rupture. Plant Cell Physiol, in press
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211(1):144–149
Petruzzelli L, Müller K, Hermann K, Leubner-Metzger G (2003a) Distinct expression patterns of ß-1,3-glucanases and chitinases during the germination of Solanaceous seeds. Seed Sci Res 13:139–153
Petruzzelli L, Sturaro M, Mainieri D, Leubner-Metzger G (2003b) Calcium requirement for ethylene-dependent responses involving 1-aminocyclopropane-1-carboxylic acid oxidase in radicle tissues of germinated pea seeds. Plant Cell Environ 26(5):661–671
Pinfield-Wells H, Rylott EL, Gilday AD, Graham S, Job K, Larson TR, Graham IA (2005) Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J 43(6):861–872
Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L (2008) The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20(10):2729–2745
Porta H, Rueda-Benítez P, Campos F, Colmenero-Flores JM, Colorado JM, Carmona MJ, Covarrubias AA, Rocha-Sosa M (1999) Analysis of lipoxygenase mRNA accumulation in the common bean (Phaseolus vulgaris L.) during development and under stress conditions. Plant Cell Physiol 40(8):850–858
Preston CA, Betts H, Baldwin IT (2002) Methyl jasmonate as an allelopathic agent: sagebrush inhibits germination of a neighboring tobacco Nicotiana Attenuata. J Chem Ecol 28(11):2343–2369
Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E (2009) Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant Cell Physiol 50(10):1786–1800
Pritchard SL, Charlton WL, Baker A, Graham IA (2002) Germination and storage reserve mobilization are regulated independently in Arabidopsis. Plant J 31(5):639–647
Quettier A-L, Shaw E, Eastmond PJ (2008) SUGAR-DEPENDENT6 encodes a mitochondrial flavin adenine dinucleotide-dependent glycerol-3-P dehydrogenase, which is required for glycerol catabolism and postgerminative seedling growth in Arabidopsis. Plant Physiol 148(1):519–528
Rentzsch S, Podzimska D, Voegele A, Imbeck M, Müller K, Linkies A, Leubner-Metzger G (2011) Dose- and tissue-specific interaction of monoterpenes with the gibberellin-mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta:1–15. doi:10.1007/s00425-011-1501-1
Rohde A, Ruttink T, Hostyn V, Sterck L, Van Driessche K, Boerjan W (2007) Gene expression during the induction, maintenance, and release of dormancy in apical buds of poplar. J Exp Bot 58(15–16):4047–4060
Rojo E, León J, Sánchez-Serrano JJ (1999) Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J 20(2):135–142
Rojo E, Solano R, Sánchez-Serrano J (2003) Interactions between signaling compounds involved in plant defense. J Plant Growth Regul 22(1):82–98
Romanel EA, Schrago CG, Counago RM, Russo CA, Alves-Ferreira M (2009) Evolution of the B3 DNA binding superfamily: new insights into REM family gene diversification. PloS one 4 (6): doi:10.1371/journal.pone.0005791
Russell L, Larner V, Kurup S, Bougourd S, Holdsworth M (2000) The Arabidopsis COMATOSE locus regulates germination potential. Development 127(17):3759–3767
Sargent JA, Osborne DJ (1980) A comparative study of the fine structure of coleorhiza and root cells during the early hours of germination of rye embryos. Protoplasma 104(1):91–103
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125(4):1591–1602
Schwachtje J, Baldwin IT (2004) Smoke exposure alters endogenous gibberellin and abscisic acid pools and gibberellin sensitivity while eliciting germination in the post-fire annual, Nicotiana attenuata. Seed Sci Res 14(01):51–60
Siriwitayawan G, Geneve RL, Bruce Downie A (2003) Seed germination of ethylene perception mutants of tomato and Arabidopsis. Seed Sci Res 13(04):303–314
Sliwinska E, Bassel GW, Bewley JD (2009) Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J Exp Bot 60(12):3587–3594
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12(23):3703–3714
Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16(8):2117–2127
Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89(15):6837–6840
Staswick P, Tiryaki I, Rowe ML (2002) Jasmonate Response Locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14(6):1405–1415
Stumpe M, Göbel C, Faltin B, Beike AK, Hause B, Himmelsbach K, Bode J, Kramell R, Wasternack C, Frank W, Reski R, Feussner I (2010) The moss Physcomitrella patens contains cyclopentenones but no jasmonates: mutations in allene oxide cyclase lead to reduced fertility and altered sporophyte morphology. New Phytol 188(3):740–749
Suza W, Staswick P (2008) The role of JAR1 in Jasmonoyl-l-isoleucine production during Arabidopsis wound response. Planta 227(6):1221–1232
Takezawa D, Komatsu K, Sakata Y (2011) ABA in bryophytes: how a universal growth regulator in life became a plant hormone? J Plant Res 124(4):437–453
Teng S, Rognoni S, Bentsink L, Smeekens S (2008) The Arabidopsis GSQ5/DOG1 Cvi allele is induced by the ABA-mediated sugar signalling pathway, and enhances sugar sensitivity by stimulating ABI4 expression. Plant J 55(3):372–381
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448(7154):661–665
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, Iuchi S, Kobayashi M, Yamaguchi S, Kamiya Y, Nambara E, Kawakami N (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146(3):1368–1385
Toorop PE, van Aelst AC, Hilhorst HWM (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51(349):1371–1379
Toufighi K, Brady SM, Austin R, Ly E, Provart NJ (2005) The Botany Array Resource: e-Northerns, Expression Angling, and promoter analyses. Plant J 43(1):153–163
Vandenbussche F, Fierro A, Wiedemann G, Reski R, Van Der Straeten D (2007) Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol 7(1):65–81
Vick BA, Zimmerman DC (1983) The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun 111(2):470–477
Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95(12):7209–7214
Voegele A, Linkies A, Müller K, Leubner-Metzger G (2011) Members of the gibberellin receptor gene family GID1 (GIBBERELLIN INSENSITIVE DWARF1) play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J Exp Bot. doi:10.1093/jxb/err214
Walne PL, Haber AH, Triplett LL (1975) Ultrastructure of auxin-induced tumors of the coleorhiza-epiblast of wheat. Am J Bot 62(1):58–66
Wang A, Li J, Bewley JD (2004) Molecular cloning and characterization of an endo-β-mannanase gene expressed in the lettuce endosperm following radicle emergence. Seed Sci Res 14(03):267–276
Wang H, Moore MJ, Soltis PS, Bell CD, Brockington SF, Alexandre R, Davis CC, Latvis M, Manchester SR, Soltis DE (2009) Rosid radiation and the rapid rise of angiosperm-dominated forests. Proceedings of the National Academy of Sciences 106(10):3853–3858
Wasternack C (2004) Jasmonates—biosynthesis and role in stress responses and developmental processes. Plant cell death processes. Elsevier, New York
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100(4):681–697
Wasternack C, Kombrink E (2009) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5(1):63–77
Watt MS, Bloomberg M, Finch-Savage WE (2011) Development of a hydrothermal time model that accurately characterises how thermoinhibition regulates seed germination. Plant Cell Environ 34(5):870–876
Weitbrecht K, Müller K, Leubner-Metzger G (2011) First off the mark: early seed germination. J Exp Bot 62(10):3289–3309
Wilen RW, van Rooijen GJH, Pearce DW, Pharis RP, Holbrook LA, Moloney MM (1991) Effects of jasmonic acid on embryo-specific processes in Brassica and Linum oilseeds. Plant Physiol 95(2):399–405
Wu C-T, Bradford KJ (2003) Class I chitinase and β-1,3-glucanase are differentially regulated by wounding, methyl jasmonate, ethylene, and gibberellin in tomato seeds and leaves. Plant Physiol 133(1):263–273
Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280(5366):1091–1094
Xu Y, Chang PFL, Liu D, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA (1994) Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6(8):1077–1085
Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCFCOI1 ubiquitin–ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14(8):1919–1935
Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16(2):367–378
Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19(8):2470–2483
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 Protein is a jasmonate receptor. Plant Cell 21(8):2220–2236
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35(1):155–189
Yasumura Y, Crumpton-Taylor M, Fuentes S, Harberd NP (2007) Step-by-step acquisition of the gibberellin-DELLA growth-regulatory mechanism during land-plant evolution. Curr Biol 17(14):1225–1230
Zalewski K, Nitkiewicz B, Lahuta LB, Glowacka K, Socha A, Amarowicz R (2010) Effect of jasmonic acid-methyl ester on the composition of carbohydrates and germination of yellow lupine (Lupinus luteus L.) seeds. J Plant Physiol 167(12):967–973
Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, Conaway RC, Conaway JW, Harper JW, Pavletich NP (2002) Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416(6882):703–709
Acknowledgments
Our work and the position of A.L. are funded by the Deutsche Forschungsgemeinschaft (Grant No. DFG Le720/6), which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Reski.
Rights and permissions
About this article
Cite this article
Linkies, A., Leubner-Metzger, G. Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31, 253–270 (2012). https://doi.org/10.1007/s00299-011-1180-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1180-1