Abstract
Life cycle of a plant (spermatophyte) sets off by germination of seeds that virtually transfer successfully genetic information from parents to off-springs across adverse environmental conditions. Considering the pivotal roles played by both hormones and ROS (especially H2O2 and ˙OH radical) during seed germination, it appears most likely that they function in a coordinated manner having one or more signaling cross-talks. Overwhelming evidences embossed the process of germination diligently controlled by GA-ABA balance; ROS probably being proactive in this event by modulating their metabolism. Ethylene can also be accommodated in this network of regulation, again, through ROS intervention. On the other hand, involvement of PM H+-ATPase in germination is also documented over time. Interestingly, both ROS and phytohormones (e.g. IAA, ethylene) have been reported to modulate PM H+-ATPase activity. Based on its activity of energy-driven transport of H+ across the PM, the H+-ATPase activates cell wall loosening enzymes and proteins like expansins in the context of a redox milieu maintained primarily by NADPH oxidase activity. Nitric Oxide (˙NO), another potential candidate to play a role in signaling, has been documented to regulate seed germination through modulation of hormonal metabolism in a ROS-mediated way. In this chapter, the probable signaling cross-talks among ROS and hormones during seed germination have been discussed with a special emphasis on the role PM H+-ATPase and ˙NO.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- NADPH oxidase
- Phytohormones
- PM H+-ATPase
- Reactive nitrogen species (RNS)
- Reactive oxygen species (ROS)
- Seed germination
3.1 Introduction
From the earliest simple life forms long course of evolution, in compliance with the changing environment, led to the emergence of complex multicellular organisms having structurally and functionally distinct parts or organs to survive the challenges of habitat. Gradual development of such architectural complexity in terms of cellular and tissue patterning calls for subtle coordination and communication between cells and tissues, a concept that was envisaged long back by German botanist Julius von Sachs (1832–1897) breeding to the idea of chemical messengers (Kucera et al. 2005; Taiz et al. 2015). Thus in higher plants well-regulated growth and development, based on harmonized functioning of cells and tissues of different organs, is possible with the involvement of such chemical messengers, aptly called hormones (Weyers and Paterson 2001).
Plant hormones, analogous to animal hormones, act at extremely low concentration and often transported away from their site of synthesis to target tissues; but, unlike animal hormones, these are unexpectedly simple molecules, e.g. ethylene, an olefin of molecular weight 28 only (Gray 2004; Davies 2010). Moreover, although functional similarities exist between animal and plant steroidal hormones (Brassinosteroids), their respective perception and signal transduction mechanisms are completely different (Thummel and Chory 2002; Lozano-Elena et al. 2018). Parallel to the discovery of auxins as the first class of plant hormones rigorous research by the plant physiologists of several countries established other classes of hormones that too have important role in plant growth and development and this list is still growing (Taiz et al. 2015). All these hormones act as signaling agents throughout the plant body, from root tip to the leaves, and throughout the life cycle, right from seed germination to senescence (Weyers and Paterson 2001; Shu et al. 2016). Apart from intrinsic regulation, some of these hormones also mediate responses to external oscillating environment. These messenger molecules are synthesized by most of the plant cells with differential capacity and, interestingly, function through both local [paracrine e.g. Brassinosteroid (Lozano-Elena et al. 2018) or autocrine e.g. GA (Arteca 1996)] and long distance [endocrine e.g. cytokinin (Kudo et al. 2010)] signaling pathways. Both ABA and cytokinin demonstrate combination of local and long distance signaling systems (Wasilewska et al. 2008; Wang and Irving 2011). Thus, plant hormones do not strictly adhere to the characteristics of hormones in mammalian sense i.e. transported chemical messengers (Davies 2010).
3.2 Seed Germination: First Sign of Perceptible Growth and Hormonal Interplay
Seed is a very unique structure having independent existence, but a part of it (embryo) is finally transformed into a plant. Germination of a seed marks the earliest event of growth that initiates in the metabolically hyperactive embryo (or embryonic axis) following a resting stage (Bewley 1997; Weitbrecht et al. 2011). Right from this stage hormones take over the control of well programmed developmental processes. However, truly speaking, hormones, particularly auxin and GA, set to work in cellular orientation of the axial embryo that already started growing, even before germination, following fertilization inside the developing seed, which is practically bridging two generations successfully transferring genetic information. During this seed maturation phase, when the seed is still attached with the mother plant, embryonic development is regulated by hormones from dual source – maternal and own. Although rapid growth of the embryonic axis occurs during the early stage of seed maturation under the guidance of set of hormones, when auxin is playing the pivotal role towards polarity, late stage of development is dominated by ABA and characterized by arrested growth and metabolism followed by dehydration (except viviparous seeds) entering into a resting break awaiting germination marked by a fresh spurt of growth.
New generation for a plant starts with the seed germination that passes through a set of complex developmental changes under the strict guidance of hormones. Quiescent seeds germinate upon receiving favorable conditions encompassing light, ambient temperature, oxygen and water. In case of dormant seeds, however, process of germination is suspended until receiving some extrinsic cue or after-ripening (endogenous). As orthodox seeds undergo maturation desiccation and generally contains less than 5–15% water by weight, rapid uptake of water (imbibition) ensues at the very commencement of germination (Bewley 1997; Weitbrecht et al. 2011). During this rehydration phase cellular structures are rebuilt with integrated membrane system with gradual water saturation. Along with, activities like respiration, protein and nucleic acid synthesis and other metabolic processes resume, either parallel or one by one to flag off embryonic growth. Strictly speaking, germination starts with imbibition and culminates in the protrusion of radicle through testa, radicle growth being relied mostly upon cell extension, not cell division (Barroco et al. 2005; Kucera et al. 2005).
Plant hormones that play a definite role in this earliest event of growth are most likely to influence the expression of several genes associated with seed germination process. Among the hormones, GA-ABA conflict is a well-known issue in this connection that has been dealt with seed biologists through ages (Taiz and Zeiger 2010). Most convincing evidence came up with elegant experiments done with mutants leading to the firm concept that GA and ABA act antagonistically- GA releases dormancy and promotes germination whereas ABA inhibits germination and maintains dormancy (Koornneef et al. 1982; Bentsink and Kooenneef 2002). In fact, ABA-GA balance behind dormancy/germination is a result of positive feedback loop involving transcription factors and DELLA proteins (Piskurewicz et al. 2008). Thus environmental factors influencing germination, like light (photoblastism) and cold temperature (stratification), act through modulation of GA metabolism and GA response (Sawada et al. 2008; Seo et al. 2009; Lee et al. 2018). Further studies also revealed the roles of other hormones, like ethylene, jasmonates, brassinosteroids and auxin (Taiz and Zeiger 2010; Linkies and Leubner-Metzger 2012; Shu et al. 2016) indicating for a complex signaling network with the possibility of crosstalk at several points that finally control the ‘commitment to growth of the next generation’ (Taiz and Zeiger 2010).
Storage mobilization, often considered as a part of germination process, is practically an event subsequent to germination proper, whereby reserve polymers in the storage organs (endosperm or cotyledons) are hydrolysed to provide soluble substrates for embryonic growth. Most popularly studied material is barley endosperm, a classical model system, with its outer aleurone layer that secretes hydrolytic enzymes (dominated by α-amylase) to the non-living starch grain loaded thin walled cells of endosperm. Storage mobilization thus results from synthesis of hydrolytic enzymes, α-amylase as an example, and their subsequent secretion, both of which are conducted by GA that reaches the aleurone layer through diffusion after being synthesized by the growing embryo. Further molecular biological approaches elucidated GA action towards α-amylase synthesis and secretion in great details. Binding of GA with its receptor (possibly GID1 protein) results in ubiquitin-26S proteasome mediated degradation of downstream DELLA protein, which encourages up-regulation of a transcription factor (GA-MYB) that promotes α-amylase gene expression through binding with GA response elements, GARE (Gocal et al. 2001; Ueguchi-Tanaka et al. 2005; Xia et al. 2015). ABA, an antagonist of GA action, can block GA-induced α-amylase synthesis directly by repressing GA-regulated genes (Hoecker et al. 1995) and indirectly by repressing GA-MYB expression (Gómez-Cadenas et al. 2001).
3.3 ROS, an Inevitable Player – Signaling and/or Direct Action in Growth
For a long period, reactive oxygen species (ROS) have been traditionally considered as cytotoxic agents that cause oxidative damage of lipids, DNA and protein ultimately leading towards cell death (Garg and Manchanda 2009). However, extensive research over the last few decades has brought about a paradigm shift in outlook of plant redox biology studies, particularly exploring the monumental beneficial roles of ROS in plant life (Kocsy et al. 2013; Singh et al. 2016). Among the most studied varieties of ROS viz. superoxide radical (O2˙ˉ), hydroxyl radical (˙OH), hydrogen peroxide (H2O2) and singlet oxygen (1O2), H2O2 is the most stable one (half-life of 1 ms; Bienert et al. 2006) whereas ˙OH is regarded as the most reactive form (cleaves wall polysaccharides; Schweikert et al. 2000). Numerous plant processes ranging from momentary phenomena e.g. chloroplast movements (Majumdar and Kar 2016, 2020) to plastic developmental events e.g. root growth (Gapper and Dolan 2006; Tsukagoshi 2016) have been identified to be mediated by ROS. The divergence of ROS-intervened plant processes justifies the wide distribution of ROS generators throughout the plant body in different intracellular organelles (e.g. chloroplast, mitochondria, peroxisome etc.) or plasma membrane and apoplast (Kar 2015). The strict adherence of ROS signaling events to their localization indicates presence of delicate communication systems among different subcellular components giving rise to a complex ROS-network (Shapiguzov et al. 2012). Since the basic requirements of plant growth i.e. cell divisions (Livanos et al. 2012) as well as cell elongations (Huang et al. 2019) are found to be regulated by ROS homeostasis, involvement of ROS in plant growth and development is inevitable. Parallel to other plant events, ROS have been reported to be crucially involved in seed germination starting from the very early stage of after-ripening and acceleration of loss of dormancy to the weakening of endosperm cap and radicle protrusion (Schopfer et al. 2001; Bailly et al. 2008; Müller et al. 2009a; Gomes et al. 2014; Bailly 2019). Concomitantly, imbibition of seeds with exogenous ROS (H2O2) promoted germination in many species of monocot e.g. Oryza sativa (Hemalatha et al. 2017), Triticum aestivum (Wahid et al. 2007), Hordeum vulgare (Bahin et al. 2011), Andropogon gerardii (Sarath et al. 2007) and dicot plants e.g. Arabidopsis thaliana (Leymarie et al. 2012), Pisum sativum (Barba-Espín et al. 2011), Vigna radiata (Chaudhuri et al. 2013).
As metabolism is nearly stalled in a dry (desiccated) mature seed, enzymatic ROS production is greatly reduced and the limited amount of available ROS may come from non-enzymatic reactions e.g. lipid peroxidation (El-Maarouf-Bouteau and Bailly 2008; Gomes and Garcia 2013). However, in a hydrated germinating seed, mitochondria [through respiratory electron transport chain (ETC)] and peroxisomes (including specifically, glyoxysomes) are the most active cellular organelles involved in ROS production (both O2˙ˉ and H2O2) at high rates (Bailly 2004; El-Maarouf-Bouteau and Bailly 2008). Among the enzymatic sources of ROS, plasma membrane (PM) NADPH oxidase (NOX) [or respiratory burst oxidase homologs (RBOHs); homologs of gp91phox subunit of mammalian NOX complex] is the most prominent one which is almost universal in distribution in plants and functions as the prime source of ROS (Sagi and Fluhr 2006). Its importance is even more pronounced in skotomorphogenic organs (e.g. seed, root etc) which are devoid of photosynthetic electron transport chains (pETC) (operating in chloroplasts; one of the most active ROS producers in plants) (Li et al. 2017). NOX produces O2˙ˉ by one electron reduction of O2 (Fluhr 2009), which is readily converted to H2O2 either spontaneously or by the activity of superoxide dismutase (SOD) enzyme. Treatment with DPI (specific NOX inhibitor) results in almost complete inhibition of germination and axis growth e.g. in Arabidopsis thaliana (Leymarie et al. 2012), Vigna radiata (Singh et al. 2014) and Oryza sativa (Li et al. 2017) highlighting the necessity of NOX-dependent ROS formation for successful completion of germination (Hu et al. 2020). Apart from NOX, cell wall located class III peroxidase (Prx) also plays key role in regulation of germination as it is the major enzymatic source of ˙OH radical that relaxes the cell wall by cleaving wall polysaccharides (Schweikert et al. 2000; Singh et al. 2015). It is well reported that onset and progress of germination is accompanied by production of ˙OH radicals (Schopfer et al. 2001; Müller et al. 2009a, b; Richards et al. 2015). Accordingly, the level of cellular Prx activity reached its peak at the time of axis emergence by rupturing the seed coat, whereas treatment with Prx inhibitors viz. salicylhydroxamic acid (SHAM) and ˙OH scavenger (sodium benzoate) suppressed V. radiata germination (Singh et al. 2015). Other sources of ROS that may mediate germination are also being explored. Chen et al. (2016) have reported that polyamine oxidase (PAO) regulates O. sativa seed germination by producing H2O2 and after studying gene expression patterns they have identified OsPAO5 as the gene that encodes most of the PAO activity during germination. Expression and activity of apoplastic germin-like oxalate oxidase (gl-OXO) enzyme has been identified in germinating T. aestivum embryo (Caliskan and Cuming 1998). The authors suggested that H2O2 originating from gl-OXO activity is involved in “cell wall-restructuring” through cross-linking of wall polymers.
Among the different ROS forms, H2O2 appears to be responsible for most of the ROS signaling owing to its structural and chemical properties. Unlike O2˙ˉ, 1O2 or ˙OH, H2O2 is freely diffusible through aquaporins (Mubarakshina and Ivanov 2010; Bienert and Chaumont 2014) thereby being able to cross membranes. Thus it can accumulate either at apoplast or protoplast and function irrespective of the site of production (e.g. mitochondria, peroxisome or NOX). However, O2˙ˉ and ˙OH also perform specific functions during germination in close proximity to their origin. Various modes of action are involved in ROS-mediated seed germination which are spatiotemporally differentiated. The accumulation of O2˙ˉ and H2O2 during after-ripening leads to protein carbonylation which has been suggested to underlie alleviation of dormancy (Oracz et al. 2007; Müller et al. 2009a; Bahin et al. 2011). Dormancy breaking and onset of germination are greatly dependent on the interactions between ROS and phytohormones. Thus, ROS signals are perceived by the nucleus and alterations in hormone metabolism take place following modified nuclear gene expression patterns. The germination-stimulatory role of H2O2 indeed involves promotion of GA biosynthesis and catabolism of ABA leading towards the establishment of low ABA/high GA content ratio necessary for germination (Liu et al. 2010; Gomes et al. 2014; Bailly 2019). The role of ˙OH in relaxation of cell wall by cleaving wall polysaccharides is well established (Schopfer et al. 2002; Liszkay et al. 2004; Müller et al. 2009b). This allows the mechanically weakened cell walls (with relaxed tension) to stretch in response to turgor pressure which essentially results in cell expansion (Fry 1998; Gomes et al. 2014). ROS are also involved in reserve mobilization by mediating oxidative break down of stored polysaccharides, DNA, RNA, proteins and fatty acids (Schweikert et al. 2002; Buetler et al. 2004; Job et al. 2005) which provides nutrients to the growing embryo. In a strictly GA-favored (and ABA-inhibited) manner, ROS carry out programmed cell death (PCD) of aleurone layer cells stimulating the release of amylase and protease enzymes that facilitates the mobilization of stored materials (Fath et al. 2001, 2002; Gomes et al. 2014).
3.4 Cross-Talk Between Hormone and ROS During Seed Germination
Interactions between ROS and phytohormones are well known to underlie a large number of plant processes encompassing different growth and stress tolerance responses (Baxter et al. 2014; Xia et al. 2015). For instance, inhibition of NOX by treatment with dipheneyelne iodoinium (DPI; specific NOX inhibitor) resulted in impairment of ABA-induced stomatal closure (Zhang et al. 2001) which was further corroborated by obtaining similar effects from Atrboh f single mutant as well as Atrboh d/f double mutants (Kwak et al. 2003; Mignolet-Spruyt et al. 2016). Regulation of plastic root system architecture by H2O2 has been suggested to be dependent on modulation of polar auxin transport by H2O2 which results into alteration of auxin accumulation and redistribution (Su et al. 2016). Moreover, heat stress specific systemic acquired acclimation (SAA) has been reported to be regulated by spatio-temporal interactions between ROS and ABA in Arabidopsis (Suzuki et al. 2013). Interestingly, proteins (transcription factors, kinases, phosphatases etc) that are specifically involved in hormone signaling have been found to act also as ROS signaling factors thereby integrating the two different signaling pathways (Mignolet-Spruyt et al. 2016; Oracz and Karpiński 2016).
It is evident that seed germination is a complex process which involves intense hormonal regulation along with pivotal roles played by ROS. Efforts are being made since long to identify any possible cross-talks among the two signaling systems during germination (as already found in case of different plant responses) (Oracz and Karpiński 2016). As ABA and GA are the primary phytohormones that antagonistically regulate seed germination, responses of ROS to alteration in ABA/GA balance are crucial for alleviation of dormancy and onset of germination. As such, the typical ROS “burst” in the seed coat and embryo was inhibited by ABA in both seed parts (and inhibited germination) whereas GA reversed the inhibitory effect of far-red light on ROS production and maintained the ROS level during germination in dark (Schopfer et al. 2001). Direct interaction of H2O2 with ABA is greatly studied in stomatal closure where ABA induces ROS production and H2O2 stimulates ABA-dependent signaling (Zhang et al. 2001; Kwak et al. 2003; Taiz et al. 2015). However, on the contrary to stomatal movement regulation, ABA reduces ROS production in seeds (especially embryo) and inhibits germination (Ye et al. 2012) probably by activating antioxidant enzymes viz. catalase, ascorbate peroxidase (Fath et al. 2001; Xia et al. 2015). Inhibition of germination under ABA treatment has often been found to be reversed/overcome by H2O2 e.g. in Panicum virgatum (Sarath et al. 2007), Vigna radiata (Chaudhuri et al. 2013). This can be explained by the findings of Liu et al. (2010) that imbibition with H2O2 significantly increases the expression of four ABA catabolism genes encoding. ABA 8′-hydroxylase (CYP707A1, CYP707A2, CYP707A3, CYP707A4) and promote germination. In addition they also showed that treatment with DPI reduces the expression of those genes and inhibits germination, suggesting a role of NOX in the process. In Nicotiana tabacum plants, H2O2 suppressed the expression of ABA biosynthesis genes encoding 9-cis-epoxycarotenoid dioxygenase (NCED1 and NCED3) and promoted CYP707A1 and CYP707A2 gene expression (Li et al. 2018). Similar effects were obtained on application of exogenous GA. Expression of ABA insensitive 3 and 5 [ABI3 and ABI5; central ABA signaling components] were significantly downregulated by H2O2 and GA whereas DPI and Uniconazole (Uni; GA biosynthesis inhibitor) promoted them (Li et al. 2018) depicting the synergistic effect of ROS and GA on repression of ABA signaling. Confirming the suggested involvement of NOX in ABA-dependent signaling in germination, Chaudhuri et al. (2013) reported that NOX activity was indeed repressed under the treatment of ABA and was stimulated when seeds were treated with ABA biosynthesis inhibitor (fluridone). Barba-Espín et al. (2011) has suggested that H2O2 may impair ABA transport from cotyledons to the embryo thereby promoting germination. Interestingly, Ishibashi et al. (2012) reported that H2O2 suppressed the expression and autophosphorylation of an ABA-responsive Ser/Thr protein kinase (PKABA) which is involved in inhibition of GAmyb expression (Gómez-Cadenas et al. 2001). In turn, reduced expression and activity of PKABA results in induction of α-amylase expression and promotes germination. Thus, the mode of action of H2O2 in antagonizing the ABA-dependent germination inhibition appears to correlate with GA signaling pathway. It is indeed observed that while ABA suppresses expression of GA biosynthesis genes e.g. GA3ox, H2O2 stimulated their expression and rescued germination (Liu et al. 2010).
Interactions between ROS and GA are found in different plant tissues which mostly involve the negative regulatory role played by DELLA proteins (Xia et al. 2015). It is reported by Achard et al. (2008) that DELLA proteins’ content increases in ga1-3 (GA deficient) mutant which promotes the expression and activity of SOD and catalase enzymes, resulting in inhibition of ROS accumulation. Extensive involvement of ROS in enhancement of GA biosynthesis and signaling during germination has also been reported (Leymarie et al. 2012; Xia et al. 2015; Li et al. 2018). During germination in N. tabacum, H2O2 promoted the expression of GA insensitive dwarf protein (GID1 and GID2; a receptor of GA signaling) which would facilitate the formation of GA-GID-DELLA complex and would release transcription factors from DELLA-mediated suppression (Xia et al. 2015; Li et al. 2018). The GA-mediated PCD of aleurone cells (leading to mobilization of storage reserve) involves key roles played by ROS e.g. damage to membrane lipids (resulting in loss of membrane integrity), DNA and cellular proteins (Bethke and Jones 2001; Fath et al. 2001). GA reduces the rate of activity of ROS-metabolizing enzymes e.g. catalase, ascorbate peroxidase, SOD and, in effect, makes the aleurone layer cells progressively more sensitive to H2O2 which accelerates PCD and promotes germination. Stimulation of H2O2 production has also been found to be induced by GA in wheat aleurone cells (Wu et al. 2014). Conforming to this, inhibition of germination under treatment of GA biosynthesis inhibitor (paclobutrazole, PAC) was counteracted and reversed by H2O2 in Vigna radiata (Chaudhuri et al. 2013). On the other hand, imbibition with H2O2 enhanced the transcription level of five GA biosynthesis genes viz.GA20ox1, GA20ox2, GA20ox3 (encoding GA 20-oxidase enzyme) and GA3ox1, GA3ox2 (encoding GA 3-oxidase enzyme) during early seed germination (Liu et al. 2010; Li et al. 2018). Nonetheless, reduced expression of GA catabolism gene viz.GA2ox3 (encoding GA 2-oxidase enzyme) was inflicted by H2O2 treatment (Bahin et al. 2011). Interestingly, ABA suppressed the expression of GA3ox genes. In an ABA catabolism mutant (cyp707a2), GA3ox expression was greatly reduced whereas in its overexpression line i.e. CYP707A2-OE, increased level GA3ox expression was obtained. Although treatment with DPI reduced the expression of all the five GA biosynthesis genes, exogenous H2O2 was able to successfully reverse the DPI-mediated reduction in gene expression (Liu et al. 2010). Different combinations of treatments were utilized by Li et al. (2018) and it was found that H2O2 + Uni and GA + DPI could overcome the inhibition of germination caused by individual treatments of DPI and Uni. From the inhibitory role of DPI it can be assumed that NOX is involved in GA signaling during germination. Corroborating to the proposal, Chaudhuri et al. (2013) reported that in the germinated axes of PAC treated seeds, reduction in NOX activity were detected in native PAGE assay indicating the enzyme’s positive involvement in GA signaling. Isocitrate lyase (ICL), a key enzyme of glyoxylate cycle that catalyzes irreversible aldol cleavage of isocitrate to glyoxylate and succinate, enhances mobilization of storage during germination. Treatment with DPI or Uni led to inhibition of ICL expression as well as activity which were efficiently counteracted by H2O2 and GA (Li et al. 2018).
Interestingly, expression of two GA-regulated proteins which are involved in cell wall loosening and cell expansion viz. xyloglucan endotransglucosylase (XTH5) and expansin (EXP2, EXP11) has been found to be upregulated by H2O2 (Yamauchi et al. 2004; Thiel et al. 2008; Liu et al. 2010; Bahin et al. 2011). Both the genes are down-regulated under DPI and c-PTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; NO scavenger] treatments (Liu et al. 2010). This clearly depicts the interaction or co-activity among GA and NOX-produced ROS in the cell elongation process leading to seed germination. Another ROS form, ˙OH radical mediates cell wall relaxation by cleaving wall polysaccharides e.g. pectin (Airianah et al. 2016). The ˙OH radical can be important for mediation of germination as it can weaken the endosperm cap which would result in less force being needed for the radicle to protrude through the cap (Müller et al. 2009b). Indeed, Schopfer et al. (2001) have reported an increase in ˙OH production in seed coat and embryo co-occurring during radish seed germination. The DPI treatment-induced reduction in ˙OH production is mimicked by ABA, whereas H2O2, GA and ethylene promotes ˙OH radical production and counteracts ABA signaling (Graeber et al. 2010; Barba-Espín et al. 2011; Richards et al. 2015).
Apart from ABA and GA, ethylene also plays important role in germination. Involvement of ethylene in germination is based on its cross-talks with ABA and GA (Oracz et al. 2008, 2009; Linkies et al. 2009). When seeds were imbibed with GA and AgNO3 (ethylene action inhibitor), germination could commence, while a combined treatment of PAC and ethrel could not restore germination depicting that ethylene alone cannot signal germination when GA is absent (Chaudhuri et al. 2013). Supportive molecular evidences show that GA could induce germination even in etr1 mutant seeds; however ethylene was unable to do the same in gib-1 mutant (Groot and Karssen 1987; Bleecker et al. 1988). It has been reported that ethylene counteracts ABA effects and promotes germination (Kucera et al. 2005). On the other hand, interaction of ethylene with ROS has also been observed during seed germination indicating towards a common signaling system involving ROS, ethylene and GA. Treatment with ethylene biosynthesis inhibitor repressed germination, which could be reversed when supplemented with exogenous H2O2 (Chaudhuri et al. 2013). However, when germination was stalled under treatment of propyl gallate (PG; general ROS scavenger), addition of ethylene was little effective (Chaudhuri and Kar 2008). In Helianthus annus, ethylene and cyanide release the seeds from dormancy and the mechanism involves cyanide-dependent stimulation of ethylene response factor1 (ERF1) expression which was sensitive to DPI treatment (Corbineau et al. 1990; Oracz et al. 2008, 2009). The cyanide-dependent dormancy alleviation was found to be mimicked by ROS generators e.g. MV (methyl viologen) and menadione and was counteracted by ROS scavengers e.g. Tiron (O2˙ˉ scavenger), DMTU (H2O2 scavenger), sodium benzoate (˙OH scavenger), ascorbic acid. Moreover, MV induced the expression of ETR2 and ERF1 genes significantly (Oracz et al. 2008, 2009). Supporting results were reported by Ishibashi et al. (2013) where a cross-talk among ROS and ethylene signaling pathways was reported during germination in Glycine max. Treatment with N-acetylcysteine (NAC, an antioxidant) counteracted the effects of ROS and suppressed germination. In addition, NAC treatment lowered cellular ethylene content by reducing the expression of ACC synthase genes viz. GmACS2e and GmACS6a. Addition of ethophen (which is converted to ethylene) reverses the effects of NAC on germination. Interestingly, the expression of ACS genes have been found to be promoted by H2O2 in both Vigna radiata (VrACS1 and VrACS6; Song et al. 2007) and Glycine max (GmACS6a; Ishibashi et al. 2013) during germination. Therefore it is evident that coordinated action of ROS and ethylene is underlying seed germination process.
3.5 ROS – PM H+-ATPase – Hormones: Extension of the Signaling Network
Being an electrogenic proton (H+) pump in nature, PM H+-ATPase is primarily responsible for conduction of H+ from cytosol across the PM to the apoplastic space utilizing the energy released from hydrolysis of ATP. The trans-membrane electrochemical gradient (negative at cytosolic side, positive at apoplastic side) arising from active H+-transport along with tight regulation of pH homeostasis (high at intracellular and low at extracellular sides) is instrumental in the apoplastic events including the activity of several enzymes and proteins like expansin involved in cell wall relaxation (Falhof et al. 2016). Besides, they also provide driving force for ion and metabolites transport (Palmgren 2001; Gévaudant et al. 2007). Consequently, involvement of PM H+-ATPase in several plant processes is known for decades which includes, but not limited to, dormancy alleviation and seed germination (De Bont et al. 2019), organ growth (Janicka-Russak 2011), stomatal responses (Kinoshita et al. 2003) and stress tolerance (Zhang et al. 2017).
One of the most important growth promoting roles of PM H+-ATPase is its ability to enable cell elongation mediated by cell wall loosening (Hager 2003; Janicka-Russak 2011). As germination is defined by radicle protrusion through seed coat which mostly depends on embryo cell elongation rather than cell division (Gimeno-Gilles et al. 2009; Sliwinska et al. 2009), PM H+-ATPase activity is indispensable for the process (Obroucheva 2017; Obroucheva et al. 2018). Interestingly, selective localization of the enzyme at certain regions of embryo has been observed during germination. These regions are predominantly involved in either secondary nutrient transport or cell elongation (Enríquez-Arredondo et al. 2005). Analyzing the effects of Vanadate (specific PM H+-ATPase inhibitor) and fusicoccin (a toxin that activates PM H+-ATPase) treatments on seed germination, De Bont et al. (2019) suggested crucial involvement of PM H+-ATPase in dormancy alleviation. Reduced germination (and root growth thereafter) of aged seeds has been co-related with inhibition of PM H+-ATPase activity too (Sveinsdóttir et al. 2009). Tissue-specific expression patterns of different isoforms of the enzyme have been studied and AHA10 (Arabidopsis PM H+-ATPase 10) has been found to be expressing exclusively in developing seeds (in the integument tissues surrounding the embryo sac) (Harper et al. 1994). Disruption of AHA10 gene resulted in severe reduction of production of proanthocyanidin in the seed coat endothelium in Arabidopsis (Baxter et al. 2005). However, AHA1 an AHA2 are expressed almost in every tissue and organs demonstrating the importance of the enzyme in different plant processes (Janicka-Russak 2011). Overexpression of PM H+-ATPase gene commonly results in enhancement of the enzyme activity. Gévaudant et al. (2007) selectively excluded last 103 amino acids (corresponding to the C-terminal auto inhibitory domain) from NpPMA4 (Nicotiana plumbaginifolia PM H+-ATPase 4) and created a constitutively active ΔPMA4. Under salt stress, overexpression of the ΔPMA4 (ectopically) in tobacco (N. tabacum) plants exhibited higher seed germination in contrast to the wild type PMA4, further corroborating the efficiency of H+-ATPase in promotion of germination.
Although operating through different modes of action, both apoplastic ROS and PM H+-ATPase enable cell elongation by loosening/relaxing the cell wall thereby playing pivotal roles in early seed germination (radicle emergence; depends on cell elongation only). The relationship between ROS and PM H+-ATPase is a matter of curiosity and significance and is being studied under different physiological conditions. Recently, involvement of PM H+-ATPase in a functional synchronization with NOX (initiator of apoplastic ROS cascade) has recently been identified during root growth in Vigna radiata (Majumdar and Kar 2018). This is in complete agreement with earlier studies exhibiting positive interplays between these enzymes. PM H+-ATPase activity was promoted, under different physiological conditions, by application of exogenous H2O2 and was repressed under the treatment of NOX inhibitor e.g. DPI or ROS scavengers (Zhang et al. 2007; Li et al. 2011; Zhao et al. 2015). Conversely, inhibition of PM H+-ATPase was found to be detrimental for NOX activity (Majumdar and Kar 2018). Moreover, H2O2 can promote activity as well as gene expression of PM H+-ATPase (Janicka-Russak et al. 2012) and NOX (via MAPK cascade pathway) (Yoshioka et al. 2016; Liu and He 2017; Hu et al. 2020). Since Ca+2 can activate both NOX (Sagi and Fluhr 2006; Kurusu et al. 2015) and PM H+-ATPase (Lang et al. 2014), threshold [Ca+2]cyt also serves as a potent mediator, apart from H2O2, of the enzymatic loop (Majumdar and Kar 2018). The hyperpolarization-activated Ca+2 channels (HACC) are crucial gates for Ca+2 entries into the cytosol. By definition HACCs require membrane hyperpolarization caused by PM H+-ATPase activity, whereas their activation depends on H2O2 (Michelet and Boutry 1995; Demidchik et al. 2003, 2007; Foreman et al. 2003; Mori and Schroeder 2004). Apparently, Ca+2influxes across PM into the cytosol are regulated by both ROS and PM H+-ATPase. Therefore, it can be presumed that during germination, PM H+-ATPase and NOX work co-operatively in a Ca+2-regulated manner and maintain PM electrical (charge) balance while mediating cell expansion. Interestingly, enzymatic production of apoplastic H2O2 by SOD is dependent on activities of both NOX and PM H+-ATPase simultaneously as the products of the latter enzymes (O2˙ˉ and H+, respectively) are substrates of SOD (Majumdar and Kar 2019). Thus a feed-forward relationship is established among the three enzymes as H2O2, being produced by SOD, activates both NOX and PM H+-ATPase either directly or through facilitating Ca+2 entry into the cell. Furthermore, cell wall located class III peroxidase (Prx) utilizes apoplastic H2O2 as substrate and produces ˙OH radical which cleaves wall polysachharides and relaxes cell wall (Schweikert et al. 2000; Liszkay et al. 2004; Müller et al. 2009b; Airianah et al. 2016). It has been found that H2O2 coming from the NOX-PM H+-ATPase-SOD loop is crucial for Prx activity since inactivation of the enzymatic loop inhibits Prx too (Majumdar and Kar 2019). Therefore, it appears that PM H+-ATPase is necessary for production of ˙OH radical. Additional support comes from Liszkay et al. (2004) who demonstrated that fusicoccin (a stimulator of PM H+-ATPase) could increase the production of ˙OH radical in Zea mays root.
On the other hand, varying relationships of PM H+-ATPase with different phytohormones have been documented time and again. Strikingly, the responses have been found to be location (tissue/organ) or condition (normal growth/stressful) dependent. The best example of such is ABA-PM H+-ATPase. ABA-induced inhibition of PM H+-ATPase in guard cells has been extensively reported (Taiz et al. 2015). In Arabidopsis, ABA induces the attachment of VAMP711 (vesicle-associated membrane protein; a R-SNARE family protein) to the C-terminal auto-inhibitory domain of AHA1 and AHA2 and inhibits the enzyme (Xue et al. 2018). On the contrary, ABA has been found to stimulate PM H+-ATPase in developing apple fruit, especially in phloem cells (Peng et al. 2003). Moreover, a specific CDPK viz. ABA-stimulated calcium-dependent protein kinase (ACPK1; ABA stimulates autophosphorylation and kinase activity) is identified in grape berry mesocarp, which acts on the C-terminal domain of PM H+-ATPase and activates the enzyme by phosphorylation (Yu et al. 2006). However during germination, ABA appears to inhibit PM H+-ATPase activity. Gimeno-Gilles et al. (2009) reported that ABA targets the process of cell wall loosening to restrict germination and inhibits the expression of several genes that are crucial for wall loosening (and resultant cell expansion) e.g. alpha-expansin, extensin, xyloglucan endotransglycosylase, cellulose synthase etc. Concomitantly, during seed germination in Arabidopsis, prominent non-transcriptional inhibition of PM H+-ATPase (AHA2) was induced by ABA via activation of SnRK2.2 kinase which phosphorylates unidentified amino acids at C-terminal domain of the enzyme (Planes et al. 2015). On the other hand, IAA-induced activation of PM H+-ATPase is known for long (Rayle and Cleland 1992; Hager 2003) and it is crucial for elongation of plant cells following increase in plastic extensibility of cell wall. IAA has been reported to stimulate PM H+-ATPase activity either by inducing phosphorylation of Thr947 (Chen et al. 2010; Takahashi et al. 2012; Haruta et al. 2015) or by indirectly inhibiting dephosphorylation of the enzyme [by inhibiting protein phosphatases (PP2C-D)]. IAA accelerates gene expression of SAUR19 (Small Auxin Up-RNA) which, in turn, interacts and inhibits PP2C-D (Spartz et al. 2014). Auxin up regulates PM H+-ATPase gene expression and increases exocytosis of the enzyme too (Hager et al. 1991; Du et al. 2020), which eventually results in an increased density of the enzyme at the PM (Xia et al. 2019). Although GA increased phosphorylation of the conserved Thr947, thereby promoting AHA1 and AHA2 activity, jasmonic acid (JA) and kinetin (cytokinin) significantly dephosphorylated the site (Chen et al. 2010). Additionally in Solanum tuberosum stolons, GA treatment was found to induce expression of PHA1 and PHA2 (potato PM H+-ATPase) (Stritzler et al. 2017). While exogenous polyamines (spermine and spermidine) have been found to promote 14-3-3 binding to PM H+-ATPase thereby increasing the enzyme activity (nearly two-fold), treatment with polyamine synthesis inhibitor reduced the enzyme’s activity (Reggiani et al. 1992; Garufi et al. 2007). The role of ethylene in promotion of H+-efflux and activation of expansin protein leading to enhanced petiole elongation in completely submerged Rumex palustris (Vreeburg et al. 2005) may be considered as its stimulatory effect on PM H+-ATPase. In agreement to this, Waters et al. (2007) have reported that ethylene induces enhancement of PM H+-ATPase (CsHA1) gene expression in Fe-deficient Cucumis sativus plants, whereas treatments with ethylene inhibitors [Co and AOA (aminooxyacetic acid)] resulted in inhibition of the gene’s expression and reduced extracellular acidification.
Considering the large cross-talks between ROS/hormones, ROS/PM H+-ATPase and hormones/PM H+-ATPase, it seems justifiable to presume a common signaling system working among them that mediates seed germination. Conforming to the hypothesis, De Bont et al. (2019) have suggested that the observed dormancy alleviation under treatments of ethylene and MV (ROS generator) may involve promotion of PM H+-ATPase activity (as indicated by membrane hyperpolarization) by both the agents. Delayed germination under treatment of ROS scavengers was accompanied by membrane depolarization indicating inhibition of PM H+-ATPase activity. Thus it is evident that a close relationship of PM H+-ATPase with ROS and phytohormones exists and regulation of seed germination process is governed by the signaling system.
3.6 Reactive Nitrogen Species (RNS): Another Potential Candidate to Play for Signaling
Apart from ROS, different other types of free radicals are also generated in living systems which can be broadly classified as reactive nitrogen species (RNS), reactive chlorine species (RCS), reactive bromine species (RBS) etc. Among these, RNS have been reported to play vast signaling roles in different aspects of plant life under both physiological and adverse conditions (Gupta and Igamberdiev 2015). As a collective term, RNS incorporates both free radicals [e.g. nitric oxide (˙NO), nitrogen dioxide (˙NO2), nitrate radical (˙NO3)] and non-radicals [e.g. nitrous acid (HNO2), nitrosyl cation (NO+), nitroxyl anion (NO−) etc] (Halliwell and Gutteridge 2015). Involvement of ˙NO in cellular signaling has been identified long back (Palmer et al. 1987; Laxalt et al. 1997) and extensive research is being carried out since then to further explore its functions and modes of operation. Consequently, ˙NO is now established to mediate diverse plant processes ranging from seed germination to stress tolerance (Šírová et al. 2011; Hancock and Neill 2019; Kohli et al. 2019).
In germinating seeds, ˙NO may be produced by both enzymatic [e.g. nitrate reductase (NR) and nitric oxide synthase (NOS)] and non-enzymatic sources (Corpas et al. 2009; Moreau et al. 2010; Šírová et al. 2011). It has been observed that treatment of seeds with sodium nitroprusside (SNP; a potent NO˙ donor) promotes early seed germination in Lupinus luteus. Moreover, SNP could counteract the negative effects of heavy metals (e.g. Pb and Cd) and NaCl and reinstate germination (Kopyra and Gwóźdź 2003). Conforming to this, Bethke et al. (2004, 2006a) have reported that exogenous application of NO˙ donors break dormancy of Arabidopsis seeds. On the other hand, treatment with c-PTIO [˙NO scavenger; 2-[4-carboxyphenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide] enhanced seed dormancy (Liu et al. 2010). The signalling process through which ˙NO regulates germination is complex and involves cross-talks with hormones and ROS. In different studies, SNP promoted germination by enhancing the positive effect of norflurazon (ABA synthesis inhibitor) while cPTIO negated the effects of fluridone and prevented germination (Bethke et al. 2006b; Piterková et al. 2012; Arc et al. 2013). ˙NO antagonizes the ABA-induced dormancy maintenance by promoting post-translational degradation of ABI5 (ABA insensitive 5) protein through S-nitrosylation of Cys153 residue (Albertos et al. 2015). As found in case of H2O2, treatment with SNP also induces the expression of ABA catabolism gene CYP707A2. Interestingly, SNP could reverse the DPI-induced reduction of gene expression; however, H2O2 was unable to overcome the inhibitory effects of cPTIO (Liu et al. 2010). On the other hand, expressions of GA biosynthesis genes (GA20ox and GA3ox) are enhanced by SNP and down-regulated by cPTIO. While SNP reversed the down-regulation induced by DPI, H2O2 was able to counteract the negative effects of cPTIO and enhance GA synthesis leading to germination. Exogenous H2O2 could also reverse the negative effects of cPTIO on expressions of two GA-regulated genes viz. XTH5 and EXP2 which are involved in cell wall loosening and cell expansion (Liu et al. 2010). Thus, it is evident that seed germination involves intrinsic cross-talks among ROS, ˙NO (or RNS), and hormones.
Interestingly, ˙NO has been found to be involved in regulation of ion homeostasis in a ethylene-dependent manner, which is mediated through the stimulation of PM H+-ATPase activity (Wang et al. 2009). While NaCl repressed PM H+-ATPase activity in Arabidopsis callus both individually as well as in combined treatments with AOA [aminooxyacetic acid; ethylene biosynthesis inhibitor] and L-NNA [Nω-nitro-L-arginin; nitric oxide synthase (NOS) inhibitor], the inhibitions could be overcome with treatments of ACC (1-aminocyclopropane-1-carboxylic acid; an ethylene precursor) and SNP. Nonetheless, the SNP-mediated stimulation of PM H+-ATPase was abolished by both PTIO [˙NO scavenger; 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide] and AOA treatments, depicting an interplay between ˙NO and ethylene that precedes PM H+-ATPase promotion. Further, Zhang et al. (2007) have reported that SNP-induced promotion of PM H+-ATPase activity in Populas euphratica callus could be eliminated by DPI whereas the positive effect of H2O2 on PM H+-ATPase was not reversed under treatments of NMMA (NG-monomethyl-L-Arginine acetate; NOS inhibitor) and PTIO. Thus, it appears that ˙NO promotes PM H+-ATPase activity through ethylene and (NOX-dependent) ROS homeostasis. Since PM H+-ATPase is closely associated with ROS/hormone cross-talks that regulate seed germination, it can be hypothesized that a functional ROS – ˙NO – PM H+-ATPase – hormone signalling network is governing the process.
3.7 Conclusion
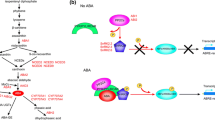
Seeds, if otherwise not in a dormant state, experience the first spell of growth upon germination (under a set of congenial environmental conditions) by radical protrusion as dictated by subtle levels of hormonal combination mainly dominated by GA. Ethylene has also been demonstrated to promote the process while ABA is recognized for inhibition of germination. These hormones exert their action through specific signaling pathways with purported crosstalk. ROS (generated through NOX-dependent apoplastic cascade) integrates with such signaling network along with a transmembrane H+ gradient inflicted by PM H+-ATPase. NO˙ modulates GA/ABA ratio and PM H+-ATPase activity in a ROS and ethylene-dependent way and thereby regulates seed germination process. A working model portraying the combined mode of action of hormones, ROS, ˙NO and PM H+-ATPase during seed germination has been depicted in the Fig. 3.1.
An integrated working model demonstrating the cross-talks between hormonal signaling network, ROS homeostasis and PM H+-ATPase activity during seed germination. NOX-dependent ROS production cascade is the major source of apoplastic ROS in plants. The O2˙ˉ being generated from NOX activity is readily converted (disproportionated) to H2O2 either by spontaneous reactions or by SOD activity. Co-activity of PM H+-ATPase with NOX ensures adequate supply of H+ at the apoplast, which is essential for SOD activity. De novo generated H2O2 is utilized by class III Prx enzyme and is converted to ˙OH radical that cleaves wall polysaccharides thereby mediating cell wall relaxation. Other apoplastic enzymes viz. polyamine oxidase and oxalate oxidase also produce H2O2 during germination. H2O2, from all the sources, diffuses across the plasma membrane and accumulates into cytosol. Mitochondrial respiratory electron transport chain (ETC) and peroxisome are the primary intracellular sources of ROS (both O2˙ˉ and H2O2) in germinating seeds and they contribute effectively in building a cytosolic ROS pool. PM H+-ATPase mediated hyperpolarization of plasma membrane and H2O2 together activate HACC channels that facilitate Ca+2influxes into cytosol from apoplast. Threshold [Ca+2]cyt, in turn, stimulates both the enzymes either by directly binding to the EF-hand motifs (of NOX) or by phosphorylating different amino acids. The cytosolic ROS signal is perceived by nucleus and alterations in gene expression pattern take place. ROS specifically up-regulate GA synthesis and ABA catabolism genes whereas down-regulate ABA synthesis and GA catabolism genes. Thus, a low ABA/high GA concentration ratio is established which stimulates germination. While ABA-induced inhibition of NOX is reduced in this condition, high GA concentration activates NOX and simultaneously deactivates antioxidant enzymes. Ethylene synthesis and response genes are also up-regulated by ROS. In a GA-dependent manner, ethylene promotes NOX activity and increases ROS production. ROS mobilize the storage reserves by breaking down polysaccharides, DNA, RNA, proteins and fatty acids and facilitating the release of amylase and protease enzymes and, in effect, promote seed germination
References
Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P (2008) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18:656–660
Airianah OB, Vreeburg RAM, Fry SC (2016) Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method. Ann Bot 117:441–455
Albertos P, Romero-Puertas MC, Tatematsu K, Mateos I, Sánchez-Vicente I, Nambara E, Lorenzo O (2015) S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat Commun 6:8669
Arc E, Galland M, Godin B, Cueff G, Rajjou L (2013) Nitric oxide implication in the control of seed dormancy and germination. Front Plant Sci 4:346
Arteca RN (1996) Plant growth substances: principles and applications. Chapman and Hall, New York
Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, Leymarie J (2011) Crosstalk between reactive oxygen species and hormonal signalling pathways regulates grain dormancy in barley. Plant Cell Environ 34:980–993
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Bailly C (2019) The signalling role of ROS in the regulation of seed germination and dormancy. Biochem J 476:3019–3032
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signalling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Barba-Espín G, Diaz-Vivancos P, Job D, Belghazi M, Job C, Hernández JA (2011) Understanding the role of H2O2 during pea seed germination: a combined proteomic and hormone profiling approach. Plant Cell Environ 34:1907–1919
Barroco RM, Van Poucke K, Bergervoet JHW, De Veylder L, Groot SPC, Inze D, Engler G (2005) The role of the cell cycle machinery in resumption of postembryonic development. Plant Physiol 137:127–140
Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci U S A 102:2649–2654
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Bentsink L, Kooenneef M (2002) Seed dormancy and germination. In: Somerville CR, Meyerowitz EM (eds) The Arabidopsis book. American Society of Plant Biologists, Rockville
Bethke PC, Jones RL (2001) Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J 25:19–29
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Bethke PC, Libourel IG, Reinöhl V, Jones RL (2006a) Sodium nitroprusside, cyanide, nitrite, and nitrate break Arabidopsis seed dormancy in a nitric oxide-dependent manner. Planta 223:805–812
Bethke PC, Libourel IG, Jones RL (2006b) Nitric oxide reduces seed dormancy in Arabidopsis. J Exp Bot 57:517–526
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840:1596–1604
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003
Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241:1086–1089
Buetler TM, Krauskopf A, Ruegg UT (2004) Role of superoxide as a signalling molecule. News Physiol Sci 19:120–123
Caliskan M, Cuming AC (1998) Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo development. Plant J 15:165–171
Chaudhuri A, Kar RK (2008) Inhibition of seed germination by propyl gallate, a free radical scavenger and recovery of germination by hydrogen peroxide and ethylene in Vigna radiata. World J Agri Sci 4:914–921
Chaudhuri A, Singh KL, Kar RK (2013) Interaction of hormones with reactive oxygen species in regulating seed germination of Vigna radiata (L.) Wilczek. J Plant Biochem Physiol 1:1–5
Chen Y, Hoehenwarter W, Weckwerth W (2010) Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. Plant J 63:1–17
Chen BX, Li WY, Gao YT, Chen ZJ, Zhang WN, Liu QJ, Chen Z (2016) Involvement of polyamine oxidase-produced hydrogen peroxide during coleorhiza-limited germination of Rice seeds. Front Plant Sci 7:1219
Corbineau F, Bagniol S, Côme D (1990) Sunflower (Helianthus annuus) seed dormancy and its regulation by ethylene. Isr J Bot 39:313–325
Corpas FJ, Palma JM, del Rio LA, Barroso JB (2009) Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol 184:9–14
Davies PJ (2010) The plant hormones: their nature, occurrence and functions. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action! Springer, Dordrecht, pp 1–15
De Bont L, Naim E, Arbelet-Bonnin D, Xia Q, Palm E, Meimoun P, Mancuso S, El-Maarouf-Bouteau H, Bouteau F (2019) Activation of plasma membrane H+-ATPases participates in dormancy alleviation in sunflower seeds. Plant Sci 280:408–415
Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116:81–88
Demidchik V, Shabala SN, Davies JM (2007) Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J 49:377–386
Du M, Spalding EP, Gray WM (2020) Rapid Auxin-mediated cell expansion. Annu Rev Plant Biol 71:379–402
El-Maarouf-Bouteau H, Bailly C (2008) Oxidative signalling in seed germination and dormancy. Plant Signal Behav 3:175–182
Enríquez-Arredondo C, Sánchez-Nieto S, Rendón-Huerta E, González-Halphen D, Gavilanes-Ruıíz M, Díaz-Pontones D (2005) The plasma membrane H+-ATPase of maize embryos localizes in regions that are critical during the onset of germination. Plant Sci 169:11–19
Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9:323–337
Fath A, Bethke PC, Jones RL (2001) Enzymes that scavenge reactive oxygen species are down-regulated prior to Gibberellic acid-induced programmed cell death in Barley aleurone. Plant Physiol 126:156–166
Fath A, Bethke P, Beligni V, Jones R (2002) Active oxygen and cell death in cereal aleurone cells. J Exp Bot 53:1273–1282
Fluhr R (2009) Reactive oxygen-generating NADPH oxidases in plants. In: Rio LA, Puppo A (eds) Reactive oxygen species in plant signalling. Springer, Berlin, pp 1–23
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Fry SC (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332:507–515
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Garufi A, Visconti S, Camoni L, Aducci P (2007) Polyamines as physiological regulators of 14-3-3 interaction with the plant plasma membrane H+-ATPase. Plant Cell Physiol 48:434–440
Gévaudant F, Duby G, Stedingk EV, Zhao R, Morsomme P, Boutry M (2007) Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance. Plant Physiol 144:1763–1776
Gimeno-Gilles C, Lelièvre E, Viau L, Malik-Ghulam M, Ricoult C, Niebel A, Leduc N, Limami AM (2009) ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: modifying enzymes and structural proteins in Medicago truncatula embryo axis. Mol Plant 2:108–119
Gocal GFW, Sheldon CC, Gubler F, Moritz T, Bagnall DJ, MacMillan CP, Li SF, Parish RW, Dennis ES, Weigel D, King RW (2001) GAMYB-like genes, flowering, and gibberellins signaling in Arabidopsis. Plant Physiol 127:1682–1693
Gomes MP, Garcia QS (2013) Reactive oxygen species and seed germination. Biologia 68:351–357
Gomes MP, Smedbol É, Lima MM, Carneiro C, Garcia QS, Juneau P (2014) Reactive oxygen species and plant hormones. In: Ahmad P (ed) Oxidative damage to plants. Academic Press, San Diego, pp 65–88
Gómez-Cadenas A, Zentella R, Walker-Simmons MK, Ho THD (2001) Gibberellin/abscisic acid antagonism in barley aleurone cells: site of action of the protein kinase PKABA1 in relation to gibberellins signaling molecules. Plant Cell 13:667–679
Graeber K, Linkies A, Müller K, Wunchova A, Rott A, Leubner-Metzger G (2010) Cross-species approaches to seed dormancy and germination: conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol Biol 73:67–87
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:e311
Groot SP, Karssen CM (1987) Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta 171:525–531
Gupta KJ, Igamberdiev AU (2015) Reactive oxygen and nitrogen species signaling and communication in plants. Springer, Cham
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin induced elongation growth: historical and new aspects. J Plant Res 116:483–505
Hager A, Debus G, Edel HG, Stransky H, Serrano R (1991) Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 185:527–537
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press
Hancock JT, Neill SJ (2019) Nitric oxide: its generation and interactions with other reactive signaling compounds. Plan Theory 8:41
Harper JF, Manney L, Sussman MR (1994) The plasma membrane H+-ATPase gene family in Arabidopsis: genomic sequence of AHA1O which is expressed primarily in developing seeds. Mol Gen Genet 244:572–587
Haruta M, Gray WM, Sussman MR (2015) Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr Opin Plant Biol 28:68–75
Hemalatha G, Renugadevi J, Eevera T (2017) Studies on seed priming with hydrogen peroxide for mitigating salt stress in rice. Int J Curr Microbiol App Sci 6:691–695
Hoecker U, Vasil IK, McCarty DR (1995) Integrated control of seed maturation and germination programs by activator and repressor functions of Viviparous-1 of maize. Genes Dev 9:2459–2469
Hu CH, Wang PQ, Zhang PP, Nie XM, Li BB, Tai L, Liu WT, Li WQ, Chen KM (2020) NADPH oxidases: the vital performers and central hubs during plant growth and signalling. Cell 9:1–41
Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Ishibashi Y, Tawaratsumida T, Kondo K, Kasa S, Sakamoto M, Aoki N, Zheng SH, Yuasa T, Iwaya-Inoue M (2012) Reactive oxygen species are involved in Gibberellin/Abscisic acid signaling in Barley aleurone cells. Plant Physiol 158:1705–1714
Ishibashi Y, Koda Y, Zheng SH, Yuasa T, Iwaya-Inoue M (2013) Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Annu Bot 111:95–102
Janicka-Russak M (2011) Plant plasma membrane H+-ATPases in adaptation of plants to abiotic stresses. In: Shanker A (ed) Abiotic stress response in plants–physiological, biochemical and genetic perspectives. InTech, pp 197–218
Janicka-Russak M, Kabała K, Wdowikowska A, Kłobus G (2012) Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J Plant Res 125:291–300
Job C, Rajjou L, Lovigny Y, Belghazi M, Job D (2005) Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol 138:790–802
Kar RK (2015) ROS signalling: relevance with site of production and metabolism of ROS. In: Gupta DK, Palma JM, Corpas FJ (eds) Reactive oxygen species and oxidative damage in plants under stress. Springer, Cham, pp 115–125
Kinoshita T, Emi T, Tominaga M, Sakamoto K, Shigenaga A, Doi M, Shimazaki KI (2003) Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol 133:1453–1463
Kocsy G, Tari I, Vanková R, Zechmann B, Gulyás Z, Poór P, Galiba G (2013) Redox control of plant growth and development. Plant Sci 211:77–91
Kohli SK, Khanna K, Bhardwaj R, Abd Allah EF, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants (Basel) 8:641
Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellins sensitive lines of Arabidopsis thaliana L. heynh. Theor Appl Genet 61:385–393
Kopyra M, Gwóźdź EA (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 41:1011–1017
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Kudo T, Kiba T, Sakakibara H (2010) Metabolism and long-distance translocation of cytokinins. J Integ Plant Biol 52:53–60
Kurusu T, Kuchitsu K, Tada Y (2015) Plant signalling networks involving Ca+2 and Rboh/Nox-mediated ROS production under salinity stress. Front Plant Sci 6:427
Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22:2623–2633
Lang V, Pertl-Obermeyer H, Safiarian MJ, Obermeyer G (2014) Pump up the volume - a central role for the plasma membrane H+ pump in pollen germination and tube growth. Protoplasma 251:477–488
Laxalt AM, Beligni MV, Lamattina L (1997) Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur J Plant Pathol 103:643–651
Lee JW, Jo IH, Kim JU, Hong CE, Kim YC, Kim DH, Park YD (2018) Improvement of seed dehiscence and germination in ginseng by stratification, gibberellin, and/or kinetin treatments. Hortic Environ Biotech 59:293–301
Leymarie J, Vitkauskaité G, Hoang HH, Gendreau E, Chazoule V, Meimoun P, Corbineau F, El-Maarouf-Bouteau H, Bailly C (2012) Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol 53:96–106
Li J, Chen G, Wang X, Zhang Y, Jia H, Bi Y (2011) Glucose-6-phosphate dehydrogenase-dependent hydrogen peroxide production is involved in the regulation of plasma membrane H+-ATPase and Na+/H+ antiporter protein in salt-stressed callus from Carex moorcroftii. Physiol Plant 141:239–250
Li WY, Chen BX, Chen ZJ, Gao YT, Chen Z, Liu J (2017) Reactive oxygen species generated by NADPH oxidases promote radicle protrusion and root elongation during rice seed germination. Int J Mol Sci 18:1–18
Li Z, Gao Y, Zhang Y, Lin C, Gong D, Guan Y, Hu J (2018) Reactive oxygen species and gibberellin acid mutual induction to regulate tobacco seed germination. Front Plant Sci 9:1279
Linkies A, Leubner-Metzger G (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31:253–270
Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CS, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Liszkay A, Van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2˙ˉ, H2O2 and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Liu Y, He C (2017) A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol 11:192–204
Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61:2979–2990
Livanos P, Apostolakos P, Galatis B (2012) Plant cell division: ROS homeostasis is required. Plant Signal Behav 7:771–778
Lozano-Elena F, Planas-Riverola A, Vilarrasa-Blasi J, Schwab R, Caño-Delgado AI (2018) Paracrine brassinosteroid signaling at the stem cell niche controls cellular regeneration. J Cell Sci 131:jcs204065
Majumdar A, Kar RK (2016) Integrated role of ROS and Ca+2 in blue light-induced chloroplast avoidance movement in leaves of Hydrilla verticillata (L.f.) Royle. Protoplasma 253:1529–1539
Majumdar A, Kar RK (2018) Congruence between PM H+-ATPase and NADPH oxidase during root growth: a necessary probability. Protoplasma 255:1129–1137
Majumdar A, Kar RK (2019) Orchestration of Cu-Zn SOD and class III peroxidase with upstream interplay between NADPH oxidase and PM H+-ATPase mediates root growth in Vigna radiata L. Wilczek J Plant Physiol 232:248–256
Majumdar A, Kar RK (2020) Chloroplast avoidance movement: a novel paradigm of ROS signalling. Photosynth Res 144:109–121
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase. Plant Physiol 108:1–6
Mignolet-Spruyt L, Xu E, Idänheimo N, Hoeberichts FA, Mühlenbock P, Brosché M, Breusegem FV, Kangasjärvi J (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot 67:3831–3844
Moreau M, Lindermayr C, Durner J, Klessig DF (2010) NO synthesis and signalling in plants – where do we stand? Physiol Plant 138:372–383
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca+2 channels. A signaling mechanism in polar growth, hormone transduction, stress signalling and hypothetically mechanotransduction. Plant Physiol 135:702–708
Mubarakshina MM, Ivanov BN (2010) The production 7and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol Planta 140:103–110
Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G (2009a) The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol 184:885–897
Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Luebner-Metzger G (2009b) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150:1855–1865
Obroucheva NV (2017) Participation of plasma membrane H+-ATPase in seed germination. Int J Cell Sci Mol Biol 2:64–67
Obroucheva NV, Lityagina SV, Sinkevich IA (2018) Plasma membrane H+-ATPase during embryo dormancy and dormancy release in horse chestnut seeds. Int J Cell Sci Mol Biol 4:55–58
Oracz K, Karpiński S (2016) Phytohormones signalling pathways and ROS involvement in seed germination. Front Plant Sci 7:864
Oracz K, El-Maarouf-Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as a novel mechanism of seed dormancy alleviation. Plant J 50:452–465
Oracz K, El-Maarouf-Bouteau H, Bogatek R, Corbineau F, Bailly C (2008) Release of sunflower seed dormancy by cyanide: crosstalk with ethylene signalling pathway. J Exp Bot 59:2241–2251
Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signalling during germination. Plant Physiol 150:494–505
Palmer RMJ, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845
Peng YB, Lu YF, Zhang DP (2003) Abscisic acid activates ATPase in developing apple fruit especially in fruit phloem cells. Plant Cell Environ 26:1329–1342
Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L (2008) The gibberellic acid signaling repressor rhl2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell 20:2729–2745
Piterková J, Luhová L, Hofman J, Turečková V, Novák O, Petřivalský M, Fellner M (2012) Nitric oxide is involved in light-specific responses of tomato during germination under normal and osmotic stress conditions. Annl Bot 110:767–776
Planes MD, Niñoles R, Rubio L, Bissoli G, Bueso E, García-Sánchez MJ, Alejandro S, Gonzalez-Guzmán M, Hedrich R, Rodriguez PL, Fernández JA, Serrano R (2015) A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J Exp Bot 66:813–825
Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99:1271–1274
Reggiani R, Zaina S, Bertani A (1992) Plasmalemma ATPase in rice coleoptiles: stimulation by putrescine and polyamines. Phytochemistry 31:417–419
Richards SL, Wilkins KA, Swarbreck SM, Anderson AA, Habib N, Smith AG, McAinsh M, Davies JM (2015) The hydroxyl radical in plants: from seed to seed. J Exp Bot 66:37–46
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Sarath G, Hou G, Baird LM, Mitchell RB (2007) Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 226:697–708
Sawada Y, Katsumata T, Kitamura J, Kawaide H, Nakajima M, Asami T, Nakaminami K, Kurahashi T, Mitsuhashi W, Inoue Y, Toyomasu T (2008) Germination of photoblastic lettuce seeds is regulated via the control of endogenous physiologically active gibberellin content, rather than of gibberellin responsiveness. J Exp Bot 59:3383–3393
Schopfer P, Plachy C, Frahry G (2001) Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol 125:1591–1602
Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A (2002) Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 214:821–828
Schweikert C, Liszkay A, Schopfer P (2000) Scission of polysaccharides by peroxidase-generated hydroxyl radicals. Phytochemistry 53:565–570
Schweikert C, Liszkay A, Schopfer P (2002) Polysaccharide degradation by Fenton reaction - or peroxidase-generated hydroxyl radicals in isolated plant cell walls. Phytochemistry 61:31–35
Seo M, Nambara E, Choi G, Yamaguchi S (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69:463–472
Shapiguzov A, Vainonen JP, Wrzaczek M, Kangasjärvi J (2012) ROS-talk– how the apoplast, the chloroplast, and the nucleus get the message through. Front Plant Sci 3:1–9
Shu K, Liu XD, Xie Q, He Z (2016) Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9:34–45
Singh KL, Chaudhuri A, Kar RK (2014) Superoxide and its metabolism during germination and axis growth of Vigna radiata (L.) Wilczek seeds. Plant Signal Behav 9:e29278
Singh KL, Chaudhuri A, Kar RK (2015) Role of peroxidase activity and Ca+2 in axis growth during seed germination. Planta 242:997–1007
Singh R, Singh S, Parihar P, Mishra RK, Tripathi DK, Singh VP, Chauhan DK, Prasad SM (2016) Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Front Plant Sci 7:1299
Šírová J, Sedlářová M, Piterková J, Luhová L, Petřivalský M (2011) The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci 181:560–572
Sliwinska E, Bassel GW, Bewley JD (2009) Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyls. J Exp Bot 60:3587–3594
Song YJ, Joo JH, Ryu HY, Lee JS, Bae YS, Nam KH (2007) Reactive oxygen species mediate IAA-induced ethylene production in mungbean (Vigna radiata L.) hypocotyls. J Plant Biol 50:18–23
Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26:2129–2142
Stritzler M, García MNM, Schlesinger M, Cortelezzi JI, Capiati DA (2017) The plasma membrane H+-ATPase gene family in Solanum tuberosum L. Role of PHA1 in tuberization. J Exp Bot 68:4821–4837
Su C, Liu L, Liu H, Ferguson BJ, Zou Y, Zhao Y, Wang T, Wang Y, Li X (2016) H2O2 regulates root system architecture by modulating the polar transport and redistribution of auxin. J Plant Biol 59:260–270
Suzuki N, Miller G, Salazar C, Mondal HA, Shulaev E, Cortes DF, Shuman JL, Luo X, Shah J, Schlauch K, Shulaev V, Mittler R (2013) Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25:3553–3569
Sveinsdóttir H, Yan F, Zhu Y, Peiter-Volk T, Schubert S (2009) Seed ageing-induced inhibition of germination and post-germination root growth is related to lower activity of plasma membrane H+-ATPase in maize roots. J Plant Physiol 166:128–135
Taiz L, Zeiger E (2010) Plant physiology, 5th edn. Sinauer Associates Inc., Sunderland
Taiz L, Zeiger E, Møller IM, Murphy A (2015) Plant physiology and development. Sinauer Associates, Inc., Sunderland
Takahashi K, Hayashi KI, Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159:632–641
Thiel J, Weier D, Sreenivasulu N, Strickert M, Weichert N, Melzer M, Czauderna T, Wobus U, Weber H, Weschke W (2008) Different hormonal regulation of cellular differentiation and function in nucellar projection and endosperm transfer cells: a microdissection-based transcriptome study of young barley grains. Plant Physiol 148:1436–1452
Thummel CS, Chory J (2002) Steroid signalling in plants and insects–common themes, different pathways. Genes Dev 16:3113–3129
Tsukagoshi H (2016) Control of root growth and development by reactive oxygen species. Curr Opin Plant Biol 29:57–63
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T, Hsing YC, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellins. Nature 437:693–698
Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, Staal M, Elzenga TM, Staals RHJ, Darley CP, McQueen-Mason SJ, Voesenek LACJ (2005) Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J 43:597–610
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pre-treatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wang YH, Irving HR (2011) Developing a model of plant hormone interactions. Plant Signal Behav 6:494–500
Wang HH, Liang XL, Wan Q, Wang XM, Bi YR (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Freidit Frey N, Leung J (2008) An update on Abscisic acid signalling in plants and more. Mol Plant 1:198–217
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, Alcántara E, Pérez-Vicente R (2007) Ethylene involvement in the regulation of the H+-ATPase CsHA1gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45:293–301
Weitbrecht K, Müller K, Leubner-Metzger G (2011) First off the mark: early seed germination. J Exp Bot 62:3289–3309
Weyers JDB, Paterson NW (2001) Plant hormones and the control of physiological processes. New Phytol 152:375–407
Wu M, Li J, Wang F, Li F, Yang J, Shen W (2014) Cobalt alleviates GA-induced programmed cell death in wheat aleurone layers via the regulation of H2O2 production and heme oxygenase-1 expression. Int J Mol Sci 15:21155–21178
Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66:2839–2856
Xia L, Marquès-Bueno MM, Bruce CG, Karnik R (2019) Unusual roles of secretory SNARE SYP132 in plasma membrane H+-ATPase traffic and vegetative plant growth. Plant Physiol 180:837–858
Xue Y, Yang Y, Yang Z, Wang X, Guo Y (2018) VAMP711 is required for Abscisic acid-mediated inhibition of plasma membrane H+-ATPase activity. Plant Physiol 178:1332–1343
Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S (2004) Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16:367–378
Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, Shi L, Jia L, Zhang J (2012) Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J Exp Bot 63:1809–1822
Yoshioka H, Adachi H, Nakano T, Miyagawa N, Asai S, Ishihama N, Yoshioka M (2016) Hierarchical regulation of NADPH oxidase by protein kinases in plant immunity. Physiol Mol Plant Pathol 95:20–26
Yu XC, Li MJ, Gao GF, Feng HZ, Geng XQ, Peng CC, Zhu SY, Wang XJ, Shen YY, Zhang DP (2006) Abscisic acid stimulates a calcium-dependent protein kinase in grape berry. Plant Physiol 140:558–579
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song CP (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Zhang F, Wang Y, Yang Y, Wu H, Wang D, Liu J (2007) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populas euphratica. Plant Cell Environ 30:775–785
Zhang J, Wei J, Li D, Kong X, Rengel Z, Chen L, Yang Y, Cui X, Chen Q (2017) The role of the plasma membrane H+-ATPase in plant responses to Aluminum toxicity. Front Plant Sci 8:1757
Zhao N, Wang S, Ma X, Zhu H, Sa G, Sun J, Li N, Zhao C, Zhao R, Chen S (2015) Extracellular ATP mediates cellular K+/Na+ homeostasis in two contrasting poplar species under NaCl stress. Trees 30:825–837
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Majumdar, A., Kar, R.K. (2021). Seed Germination: Explicit Crosstalk Between Hormones and ROS. In: Gupta, D.K., Corpas, F.J. (eds) Hormones and Plant Response. Plant in Challenging Environments, vol 2. Springer, Cham. https://doi.org/10.1007/978-3-030-77477-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-77477-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77476-9
Online ISBN: 978-3-030-77477-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)