Abstract
Transformed root (“hairy root”) cultures have been shown to be a good model for the study of many secondary metabolites. However, economically important compounds such as asiaticoside and madecassoside are produced in insignificant amounts in the root of Centella asiatica (L.) Urban. To overcome this problem, C. asiatica was transformed using Agrobacterium rhizogenes strain R1000 that harbors pCAMBIA1302 encoding the hygromycin phosphotransferase (hpt) and green fluorescence protein (mgfp5) genes and the hairy culture was coupled with elicitation technique. Hairy roots were obtained at a frequency of up to 14.1% from a tissue junction between the leaf and petiole. Abundant hairy roots were observed when co-cultivation of the plant with A. rhizogenes was done for 7 days (36.1%). Transformation was confirmed by PCR and Southern blot analyses. Five weeks after inoculation, no asiaticoside was detected in the hairy root samples. However, when 0.1 mM methyl jasmonate (MJ) was applied as an elicitor to the culture medium for 3 weeks, a large quantity of asiaticoside was generated (7.12 mg/g, dry wt). In the case of gene expression, 12 h after MJ treatment the expression of the CabAS (C. asiatica putative β-amyrin synthase) gene in the hairy roots is significantly different from that of the control and this level of transcripts was maintained for 14 days. Our results showed that production of C. asiatica hairy roots could be optimized and the resulting cultures could be elicited with MJ treatment for enhanced production of asiaticoside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
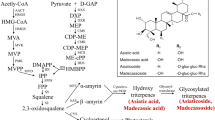
Centella asiatica (L.) Urban harbors several useful compounds, including centellasaponin, asiaticoside, madecassoside, and sceffoleoside (Kartnig and Hoffmann-Bohm 1992; Matsuda et al. 2001). Asiaticoside, one of the principal triterpene saponins found in leaves, is commercially utilized as a wound-healing agent, owing primarily to its potent anti-inflammatory effects (Pointel et al. 1987). Despite the economic value associated with asiaticoside generation, the increased production of this compound in genetically engineered plants is yet to be demonstrated.
Agrobacterium tumefaciens or A. rhizogenes-mediated transformation has been widely utilized for the introduction of functionally important genes into plants. In particular, culture systems using hairy roots induced by pRi genes have been shown to be a very useful tool for the study of secondary metabolites, principally due to the fact that hairy roots grow more rapidly than do other tissues (Flores et al. 1999; Srivastava and Srivastava 2007). In order to introduce exogenous genes into plants to increase the amount of target compounds or to gain insight into the functions of genes, the transformation conditions must first be optimized. Several protocols have thus far been developed for C. asiatica plant regeneration systems (Patra et al. 1998; Tiwari et al. 2000; Paramageetham et al. 2004). Plant regeneration of C. asiatica was achieved successfully via the culturing of embryogenic and organogenic calli, as well as micropropagation using axillary buds. Although several studies of plant regeneration have been conducted, none have involved the genetic transformation of C. asiatica with A. tumefaciens. The formation of A. rhizogenes-mediated transformed C. asiatica hairy roots has been previously reported (Beak 1997; Aziz et al. 2007). However, in this study a highly efficient protocol for the genetic transformation of C. asiatica was not achieved, and the asiaticoside content of the hairy roots was quite low (0.26 mg/g, dry wt) or not detectable. To the best of our knowledge, the transformation conditions of C. asiatica have not yet been optimized.
Some compounds in several plants have been shown to be tissue specific. For example, avencin (found in Avena strigosa) is tissue specific, and is biosynthesized only in the roots (Haralampidis et al. 2001). Our previous results have shown that asiaticoside biosynthesis is tissue specific to the leaf, and that the level of asiaticoside content is quite low in the roots of whole plants (Kim et al. 2004; Kim et al. 2005). Methyl jasmonate (MJ) treatments as elicitor have been previously applied in order to overproduce triterpene saponins from cultured Panax ginseng cells (Lu et al. 2001), Bupleurum falcatum root fragments (Aoyagi et al. 2001), and C. asiatica whole plants (Mangas et al. 2006). The results of these studies compelled us to seek more specific information regarding asiaticoside production capacity in hairy root tissues, in which the compound is synthesized in low amounts.
The primary objective of the current study was to establish a stable genetic transformation system for C. asiatica using the R1000 strain of A. rhizogenes. A protocol was established to increase the yield of C. asiatica hairy roots via the control of the co-cultivation period and the inoculation region of the explants. We have also described the use of hygromycin phosphotransferase (hpt) and green fluorescence protein (mgfp5) as selection markers for the generation of antibiotic-resistant hairy roots. Finally, the asiaticoside production capacity of the hairy root cultures was determined via HPLC analysis, and we also conducted RT-PCR to evaluate the transcript levels of a gene (putative β-amryrin synthase, CabAS) associated with asiaticoside biosynthesis in hairy roots treated with methyl jasmonate, in an attempt to determine whether or not hairy roots are an appropriate material for use in the study of the triterpenoid pathways.
Materials and methods
Plant materials
Whole plant cultures of C. asiatica were established from node segments as previously described by Kim et al. (2004). Four node segments per petri dish (90 × 20 mm) were cultured on MS basal medium (Murashige and Skoog 1962) supplemented with 3% sucrose and 0.8% agar at 23 ± 2°C with 16 h light period. After 2 weeks of cultivation, the leaves and petioles were used as explants for the induction of hairy roots.
Transformation
A single clone of the disarmed A. rhizogenes strain R1000 harboring the pCAMBIA1302 binary vector consisting of the hygromycin phosphotransferase (hpt) gene as a selectable marker and the green fluorescence protein (mgfp5) under the control of the CaMV 35S promoter was selected and cultured in YEP medium (20 ml) supplemented with 50 mg/L kanamycin sulfate (Sigma-Aldrich, St. Louis, MO) in darkness at 28°C for 16 h at 220 rpm. The bacterial cells were pelleted by centrifugation followed by washing twice with liquid half-strength MS medium. This suspension was employed for A. rhizogenes infection. All antibiotics were filter-sterilized and added to autoclaved medium.
In order to induce hairy roots with Agrobacterium, we tested three types of explants, namely, leaves and petioles. As shown in Fig. 1, the sample leaf was cut into half. Explants, including petioles, were submerged in the bacterial suspension for 40 min, washed with 50 ml sterilized water, and blot-dried on sterile filter paper. Finally, the petioles and the lower parts of the leaf tissues were placed in half-strength MS medium supplemented with 3% sucrose, 0.8% agar, and 50 μM acetosyringone at 19 ± 2°C in darkness. For the optimization of the length of co-cultivation, only the B region in the leaf was used as explants. After the co-culture period, the explants were washed in sterile distilled water and transferred to half-strength MS medium supplemented with 3% sucrose, 0.8% agar, and 300 mg/l of cefotaxime (Bioworld, Dublin, OH). After the culture period, with subculturing to fresh medium at 3-week intervals, induced roots of more than 5 cm in length were excised from the parental tissues and transferred to a selectable medium with 300 mg/l of cefotaxime and 20 mg/l of hygromycin (Duchefa, Haarlem, Netherland) to screen for putative transformed roots until the residue bacteria had been completely killed.
Liquid hairy root cultures were established with 10-mm-long tips from 1-month-old hairy roots. Ten root tips were inoculated in 100-mL Erlenmeyer flasks containing 30 mL of half-strength MS medium supplemented with 3% sucrose without plant growth regulators. The roots were grown at 25°C on a rotary shaker (set at 100 rpm) in darkness.
In the methyl jasmonate (MJ) elicitation experiment, hairy roots were pre-cultured for 4 weeks and then treated with 0.1 mM MJ. For asiaticoside content analysis, cultured hairy roots were freeze-dried under vacuum at −50°C. For molecular work, cultured tissues were collected by filtration, frozen with liquid nitrogen and stored at −80°C.
DNA analysis in transgenic hairy roots
Genomic DNAs of the non-transformed leaves and hairy roots were isolated via the cetyldimethylethylammonium bromide method (Doyle and Doyle 1987). DNA was dissolved in autoclaved water and stored at −20°C until use. PCR was conducted in order to detect the hpt, rolB, and virC genes in both the T-DNA of the Agrobacterium plasmid and the genomic DNAs of the non-transformed leaves and hairy roots. The plasmid DNA of the A. rhizogenes R1000 strain containing pCAMBIA1302 was employed as a positive control. PCR primers were designed according to the DNA sequences of the hpt, rolB, and virC genes. The primer pair employed for the hpt gene amplification of an 713-bp fragment was as follows: 5′-GCCTGACCTATTGCATCTCC-3′ and 5′-TTCTACACAGCCATCGGTCC-3′; the pair employed for the rolB gene amplification of an 858-bp fragment was as follows: 5′-CTTATGACAAACTCATAGATAAAGGTTG-3′ and 5′-TCGTAACTATCCAACTCACATCAC-3′; and for the VirC gene amplification of an 730-bp fragment, the primer pair used was: 5′-ATCATTTGTAGCGACT-3′ and 5′-AGCTCAAACCTGCTTC-3′. The PCR mixture consisted of DNA, 0.4 mM of each primer, 0.2 mM of each dNTP, 2.5U of Taq DNA polymerase (5 U/μl, Takara, Shiga, Japan), and 10 μl of 10× buffer in a final volume of 100 μl. The PCR was conducted under the following conditions: initial denaturation for 5 min at 94°C; denaturation for 1 min at 94°C; primer annealing for 1 min at 56°C; elongation for 1 min at 72°C, 30 cycles; a final extension step at 72°C for 10 min. The amplification PCR products were evaluated via electrophoresis on a 1.5% (w/v) agarose gel.
For Southern analysis, the DNAs (10 μg) were digested with HindIII, electrophoresed on 0.8% agarose gel, then transferred to a positively charged nylon membrane (Boehringer, Mannheim, Indianapolis, IN) via capillary blotting (Sambrook et al. 1989). For hybridization, PCR-amplified hpt gene products were labeled with digoxigenin (DIG)-dUTP (PCR DIG Probe Synthesis kit, Roche, Basel, Switzerland) as the probe. The DIG-labeled nucleic acid was hybridized and detected using DIG Easy Hyb solution and DIG Nucleic Acid Detection kits (Roche). DNA cross-linked positively nylon membranes were incubated for 12 h at 48°C with the probe for hybridization, then washed twice at 25°C, each wash for 5 min, in 2× SSC, 0.1% SDS, followed by two washes for 15 min each, in 0.5× SSC, 0.1% SDS at 68°C.
GFP expression was visualized with a Leica MZ6 stereo fluorescence microscope (Leica Instruments Pty, North Ryde, Australia). The microscope was equipped with a Leica GFP Plus filter (480/40 nm excitation filter) and a narrow bandpass interference filter (S550/100NP) which blocks the red autofluorescence of chlorophyll. GFP expression in the hairy root was monitored after 5 days of induction from the explants.
RT-PCR analysis
RT-PCR analyses were conducted using the AccessQuick RT-PCR System (Promega, Madison, WI). One microgram of total RNA was used as a template. For normalization, an actin fragment amplified by the primer designed from Panax ginseng was employed as an internal standard. The forward and reverse sequences used for PCR amplification of actin were 5′-GATGACATGGAAAAGATTTGGCATC-3′ and 5′-TGTTGTACGACCACTAGCATACAGG-3′, respectively, and those used for amplification of CabAS gene (GenBank, accession number: AY520818) were 5′-TGCACAGCATCAATAATAGCAGCT-3′ and 5′-TCAATTGGAGAGCCACAAGCGTTT-3′. The RT-PCR conditions for both actin and CabAS were as follows: one cycle for 45 min at 45°C, followed by 30 cycles at 95°C for 1 min, 58°C for 1 min, 72°C for 1 min, and a final 5 min extension step at 72°C. The sizes of the PCR products were determined via agarose gel (1.5%) electrophoresis.
HPLC analysis of asiaticoside
Asiaticoside extraction was conducted by the method established by Bonfill et al. (2006). Quantitative determinations of asiaticoside contents were achieved via HPLC (Agilent 1100 series equipped with an auto sampler, a diode array detector, and a quaternary pump) using a Capcell-pak C18 UG (4.6 × 250 mm, 5 μm, 120 Å) column (Shiseido, Tokyo, Japan). The HPLC conditions for asiaticoside isolation were as follows: gradient elution, the eluents being acetonitrile (ACN) and water (ACN: 0–40 min, 20–100%); flow rate, 1 ml/min; column temperature, 30°C; detector wave-length, 214 nm. The asiaticoside standards were purchased from ROTH (Karlsruhe, Germany). The concentration of asiaticoside for spiking was adjusted to 0.1 mg/ml and a total volume of 20 μl of asiaticoside:hairy root extract (1:1, v/v) was injected.
Results and discussion
Explant source and co-cultivation periods
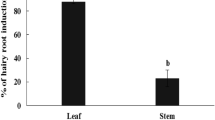
The explants of choice for hairy root induction after infection with Agrobacterium rhizogenes constitute the most salient of the integrated factors for a successful root transformation. Plant transformation efficiency differs significantly according to the source of the explant (Damgaard and Rasmussen 1991; Shi and Kintzios 2003; Alpizar et al. 2006). Kang et al. (2006) showed the importance of explant choice by producing more hairy roots of Aralia elata on the root segment than on the petiole explant. It has been reported in several previous studies that the morphological patterns and hairy root production characteristics of infected tissues differed substantially (Bercetche et al. 1987; Ottani et al. 1990). These differences in hairy root production may depend upon differences between species, plant organs, or sites of infection. Thus, in order to compare explant sources for transformation, we evaluated three regions of C. asiatica shoot explants, with regard to their capacity to induce hairy roots via transformation (Fig. 1). After 5 days of co-cultivation, a few putative hairy roots were obtained from all regions over a 4-week culture period. We estimated the transformation rates from each of the explants after 3-month culture on selection medium containing 20 mg/l hygromycin. Hygromycin-resistant hairy roots were induced from tissue micro-calli grown on half-strength MS medium, and hairy roots were also directly acquired from the cut edges of the leaf and petiole tissues. In particular, putative transformed hairy roots were induced at a high rate at the tissue junction between the leaf and petiole (B region). The high transformation rate observed in this region may be attributed to the fact that the larger midveins in this area permitted an elevated level of bacterial-host cell contact. These results indicated that the transformation rates from the B-region of leaf were 4.4-times as high as those observed in other regions (Table 1). In conclusion, the use of explants containing the B region is optimally efficient with regard to the production of hairy roots from C. asiatica.
After Agrobacterium infection, the plant tissues and bacteria are cultivated for a few days. Important events occur during co-cultivation; plant cells and bacteria divide further, and T-DNA is transferred from the bacteria to the plant cells (Komari et al. 2004). The length of the co-cultivation period also influences transformation efficiency (Mihaljevic et al. 1996; Niu et al. 2000; Tao and Li 2006). Therefore, the effects of co-cultivation length on the induction of hairy roots were assessed. As shown in Table 2, significant differences were determined to exist among different co-culture periods (3, 7, 14, 21, and 28 days). A co-cultivation period of more than 7 days with Agrobacterium resulted in a significant increase in the number of produced hairy roots, which are capable of growth under hygromycin selection conditions. Likewise, prolonged co-cultivation (over 21 days) with Agrobacterium would induce damage to explants. However, neither Agrobacterium overgrowth nor explant damage was observed in this study. This may be attributable to the fact that anti-bacterial compounds may be generated in the leaves of C. asiatica which harbor significant quantities of pharmacologically active triterpenoids, including asiaticosides, madecassosides, asiatic acides, and madecassic acid. A low transformation efficiency was observed over 21 days of co-cultivation. Thus, the optimum co-culture period was found to be 7 days. When the explants after co-cultivation were cultured on medium harboring hygromycin and cefotaxime, no root formation was observed, and necrosis occurred in the explants (data not shown). After 1 month of cefotaxime treatment to eliminate bacteria, a combined treatment of hygromycin with cefotaxime should be applied to the medium for hairy root selection. When such treatment is applied, hairy roots can be generally induced from explants. When selected hairy roots were cultured on media supplemented with 40 mg/L of hygromycin, general growth without necrosis was observed (Fig. 2a). The expression of GFP as a reporter gene was observed 5 days after the induction of hairy roots (Fig. 2b). GFP gene expression was clearly visible in the hairy root tips of C. asiatica transformed with pCAMBIA1302, but no expression was observed in the wild-type roots (data not shown).
Confirmation of hairy roots via molecular analysis
In order to verify the integration of the A. rhizogenes plasmid T-DNA into the genome of C. asiatica, we conducted PCR analysis using primers designed in accordance with the sequences of the hpt and rolB genes. Using DNAs from the hairy roots (T1, T2, T3, T6, and T7 lines) and the non-transformed leaves (N line) as templates, fragments with lengths of 700 and 858 bp, corresponding to hpt and rolB, respectively, were detected from the hairy roots, but not detected from non-transformed plants (Fig. 3). In order to check for residual Agrobacterium in the transformed tissues, we conducted additional PCR reactions with primers designed to amplify regions outside of the T-DNA (Hamill et al. 1991). Negative results from the attempted amplification of the VirC gene in the root DNA suggested that no contamination with Agrobacterium had occurred. Southern analysis was conducted to further verify the introduction of the hpt gene into the C. asiatica genome (Fig. 4). Different numbers of hpt gene bands were detected in the genomic DNA from three hairy roots (in T1, T6, and T7 lanes), whereas no hybridization band was observed in the non-transformed plant (in the N lane). Southern blot analysis of the HindIII-digested genomic DNA from C. asiatica hairy roots confirmed the presence of the hpt gene.
PCR analysis of the hpt (H), rolB (R), and VirC (V) genes in C. asiatica hairy roots. Lanes: M molecular size markers; P plasmid DNA isolated from A. rhizogenes R1000 strain as a positive control; T1, T2, T3, T6, T7 DNA isolated from the hairy roots of transformed lines; N DNA isolated from non-transformed leaves as a negative control
Asiaticoside production capacity in the hairy roots
Recently, several reports have demonstrated that the overproduction of useful compounds from plant tissues can be achieved via the genetic engineering of medicinal plants (Chen et al. 2000; Lee et al. 2004). The full-length cDNAs related to triterpene biosynthesis, including squalene synthase, squalene epoxidase, and β-amyrin synthase in plants have been cloned and determined to be transcriptionally induced in cultured cells and tissues in response to treatment with MJ (Suzuki et al. 2002; Hayashi et al. 2003). Until now, it has been demonstrated that the squalene synthase gene is an important key enzyme, which affects the upregulation of triterpene compound production (Lee et al. 2004). However, no functions have been elucidated with regard to other genes contributing to triterpene biosynthesis.
Asiaticoside is biosynthesized principally in the leaf, and this compound has been shown to be tissue specific to the leaf in C. asiatica (Kim et al. 2004). In addition, the transcripts of the CabAS (putative β-amyrin synthase) gene, which are ssociated with asiaticoside biosynthesis, have been detected only in the leaf tissues of whole plants (Kim et al. 2005). In a previous paper, the hairy roots of C. asiatica were reported to synthesize very low level of asiaticoside and madecassoside (Beak 1997). It is similar to recent results that the hairy roots and undifferentiated cells of C. asiatica have no capacity to produce asiaticoside (Aziz et al. 2007). However, Nath and Buragohin (2005) reported the interesting fact that sizeable quantities of asiaticosides are biosynthesized in suspension-cultured cells and undifferentiated calli of C. asaitica originated from India. We applied MJ as elicitor to cultures of C. asiatica hairy roots in order to gain greater insights into asiaticoside production, using HPLC and RT-PCR analysis techniques. We also analyzed the asiaticoside contents of 5-week-cultured hairy roots in an attempt to determine major secondary metabolite production capacity. As shown in Fig. 5a, b, the HPLC spectrum indicated that the isolated asiaticoside of the standard achieved a peak at 14.75 min, and this signal was not detected in the hairy roots. However, after 3 weeks of treatment with 0.1 mM MJ, a large quantity of asiaticoside (7.12 mg/g dry wt) was detected and quantified in the hairy roots (Fig. 5c). To confirm the peak indicated asiaticoside, we also co-injected (spiking) internal asiaticoside standard. Figure 5d shows that only the peak corresponding to asiaticoside increased.
HPLC analysis of asiaticoside. Four chromatograms of HPLC analysis, an authentic asiaticoside (a), the extract isolated from hairy roots not treated with elicitor (b), and the extract isolated from hairy roots treated with 0.1 mM MJ for 3 weeks (c), indicate the increase of asiaticoside content with MJ elicitation. Two diagrams were made overlapping before or after co-injection with an authentic asiaticoside and the extract of hairy roots treated with MJ (d). The retention time of asiaticoside is 14.75 min under a wavelength of 214 nm
Expression levels of the CabAS gene associated with asiaticoside biosynthesis were monitored for a 21-day period, in which the hairy root cultures were subjected to MJ treatment in the growth phase. In the semi-quantitative RT-PCR analysis, CabAS gene expression was detectable at significant levels within 12 h of MJ treatment, and was maintained for 14 days (Fig. 6a). Interestingly, the up-regulation of transcripts of CabAS gene for beta-amyrin synthase was sustained for a longer period than those of other plants such as Medicago truncatula (Suzuki et al. 2002) and Glycyrrhiza glabra (Hayashi et al. 2003) whose transcripts were maintained for 48 h and 7 days, respectively, after MJ elicitation. These data suggest that CabAS gene might be affected by other systems regulating its transcripts in response to MJ treatment. Figure 6b shows the continuous accumulation of asiaticoside in hairy roots for 3 weeks of MJ treatment. An observation similar to our results has shown that in in vitro-cultured roots of whole plants treated with MJ, asiaticoside contents were about five times that of the controls (Mangas et al. 2006). Based on these results, the detection of the high levels of CabAS mRNA and the substantial asiaticoside contents observed in the hairy roots can be considered to demonstrate that the gene, which evidenced specific transcripts in the leaf, can be directly associated with asiaticoside biosynthesis. In conclusion, our RT-PCR and HPLC results show clearly that the developed hairy root culture systems can be used for the study of asiaticoside production.
Time course of CabAS gene transcripts and asiaticoside contents in C. asiatica hairy roots. After treatment with 0.1 mM MJ, the levels of CabAS mRNA (a) and asiaticoside contents (b) in the hairy roots were detected via RT-PCR and HPLC analysis, respectively. An actin fragment generated by primers designed from P. ginseng was amplified as an internal loading control. Bars represent standard errors
We established an efficient transformation system for asiaticoside production in C. asiatica, using the R1000 strain of A. rhizogenes. Our results indicate that the integration of T-DNA into the plant genome was verified via PCR analysis and Southern hybridization. The establishment of an efficient genetic transformation protocol for C. asiatica will unequivocally facilitate biotechnological applications for the enhancement of target compound yields. Also, the production capacity of asiaticoside in these tissues shows that our transformation protocol may prove a valuable tool for the study of the regulation of genes associated with triterpene biosynthetic enzymes.
Abbreviations
- CabAS :
-
Centella asiatica putative beta-amyrin synthase
- GFP:
-
Green fluorescence protein
- HPLC:
-
High-performance liquid chromatography
- hpt :
-
Hygromycin phosphotransferase
- MJ:
-
Methyl jasmonate
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
References
Alpizar E, Dechamp E, Espeout S, Royer M, Lecouls AC, Nicole M, Bertrand B, Lashermes P, Etienne H (2006) Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Rep 25:959–967
Aoyagi H, Kobayashi Y, Yamada K, Yokoyama K, Kusakari K, Tanaka H (2001) Efficient production of saikosaponins in Bupleurum falcatum root fragments combined with signal transducers. Appl Microbiol Biotechnol 57:482–488
Aziz ZA, Davey MR, Power JB, Anthony P, Smith RM, Lowe KC (2007) Production of asiaticoside and madecassoside in Centella asiatica in vitro and in vivo. Biol Plant 51:34–42
Baek YW (1997) Micropropagation of Centella asiatica (L.) Urban by in vitro cultures and production of triterpene glycosides. PhD thesis, Chonnam University, Gwangju
Bercetche J, Chriqui D, Adam S, David C (1987) Morphogenetic and cellular reorientation induced by Agrobacterium rhizogenes (strains 1855, 2659 and 8196) on carrot, pea and tobacco. Plant Sci 52:195–210
Bonfill M, Mangas S, Cusidó RM, Osuna L, Piñol MT, Palazón J (2006) Identification of triterpenoid compounds of Centella asiatica by thin-layer chromatography and mass spectrometry. Biomed Chromatogr 20:151–153
Chen DT, Ye HC, Li GF (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Damgaard O, Rasmussen O (1991) Direct regeneration of transformed shoots in Brassica napus from hypocotyls infections with Agrobacterium rhizogenes. Plant Mol Biol 17:1–8
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Flores HE, Vivanco JM, Loyola-Vargas VM (1999) Radicle biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4:220–226
Hamill JD, Rounsley S, Spencer A, Todd G, Rhodes MJC (1991) The use of the polymerase chain reaction in plant transformation studies. Plant Cell Rep 10:221–224
Hayashi H, Huang PY, Inoue K (2003) Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol 44:404–411
Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001) A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci USA 98:13431–13436
Kang HJ, Anbazhagan VR, You XL, Moon HK, Yi JS, Choi YE (2006) Production of transgenic Aralia elata regenerated from Agrobacterium rhizogenes-mediated transformed roots. Plant Cell Tissue Organ Cult 85:187–196
Kartnig T, Hoffmann-Bohm K (1992) Centella. In: Hänsel R, Keller K, Rimpler H, Schneider G (eds) Hager’s handbuch der pharmazeutischen praxis. Springer, Berlin
Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B (2004) Stimulation of asiaticoside production from Centella asiatica whole plant cultures by elicitors. Plant Cell Rep 23:339–344
Kim OT, Kim MY, Huh SM, Bai DG, Ahn JC, Hwang B (2005) Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep 24:304–311
Komari T, Ishida Y, Hiei Y (2004) Plant transformation technology: Agrobacterium-mediated transformation. In: Christou P, Klee H (eds) Handbook of plant biotechnology. Wiley, England
Lee MH, Jeong JH, Seo JW, Shin CG, Kim YS, In JG, Yang DC, Yi JS, Choi YE (2004) Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol 45:976–984
Lu MB, Wong HL, Teng WL (2001) Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep 20:647–677
Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM, Piñol MT, Palazón (2006) The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry 67:2041–2049
Matsuda H, Morikawa T, Ueda H, Yoshikawa M (2001) Medicinal foodstuffs. XXVII. Saponin constituents Gotu Kola (2): Structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponin B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chem Pharm Bull 49:1368–1371
Mihaljevic S, Stipkovic S, Jelaska S (1996) Increase of root induction in Pinus nigra explants using agrobacteria. Plant Cell Rep 15:610–614
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nath S, Buragohain AK (2005) Establishment of callus and cell suspension cultures of Centella asiatica. Biol Plant 49:411–413
Niu X, Li P, Veronese P, Bressan RA, Weller SC, Hasegawa PM (2000) Factors affecting Agrobacterium tumefaciens-mediated transformation of peppermint. Plant Cell Rep 19:304–310
Ottani MP, Schel JHN, Hänisch ten Cate Ch H (1990) Variation in structure and plant regeneration of Agrobacterium rhizogenes transformed and control roots of the potato cv. Bintje. Plant Cell Tissue Organ Cult 20:25–34
Paramageetham Ch, Prasad Babu G, Rao JVS (2004) Somatic embryogenesis in Centella asiatica L. an important medicinal and neutraceutical plant of India. Plant Cell Tissue Organ Cult 79:19–24
Patra A, Rai B, Rout GR, Das P (1998) Successful plant regeneration from callus culture of Centella asiatica (L.) Urban. Plant Growth Regul 24:13–16
Pointel JP, Boccalon H, Cloarec M, Ledebehat C, Joubert M (1987) Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 38:46–50
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloing: a laboratory manual, 2ndedn. Cold Spring Harbor Laboratory Press, Plainview
Shi HP, Kintzios S (2003) Genetic transformation of Pueraria phaseoloides with Agrobacterium rhizogenes and puerarin production in hairy roots. Plant Cell Rep 21:1103–1107
Srivastava S, Srivastava AK (2007) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27:29–43
Suzuki H, Achnine L, Xu R, Matsuda SPT, Dixon RA (2002) A genomic approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J 32:1033–1048
Tao J, Li L (2006) Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. S Afr J Bot 72:211–216
Tiwari KN, Sharma NC, Tiwari V, Singh BD (2000) Micropropagation of Centella asiatica (L.), a valuable medicinal herb. Plant Cell Tissue Organ Cult 63:179–185
Acknowledgments
We thank Prof. Lourdes B. Cardenas, Institute of Biological Sciences, University of the Philippines Los Baños, for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. R. Liu.
Rights and permissions
About this article
Cite this article
Kim, OT., Bang, KH., Shin, YS. et al. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) Urban elicited by methyl jasmonate. Plant Cell Rep 26, 1941–1949 (2007). https://doi.org/10.1007/s00299-007-0400-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0400-1