Abstract
This work centered on developing an efficient hairy root induction system to achieving a high production capacity of specialized metabolites in Calotropis procera (Aiton) W.T. Aiton. Accordingly, three strain types of Agrobacterium rhizogenes, two different explants (leaf and internodal stem segment), and inoculation suspension containing ½MS medium with 3 and 6% sucrose were studied. Besides, inoculation methods including injection and immersion with or without wounding as well as two inoculation times (5 and 30 min) were examined. Afterwards, the hairy roots were treated with different concentrations of synthesized Fe3O4 nanoparticles (Fe3O4 NPs) and salicylic acid (SA) to improve growth and productivity. The high frequency of transformation was observed in pre-pricked leaf explants immersed in the suspension consisting A4 strain and half-strength MS with 6% sucrose. Also, the result illustrated that the application of NPs had a positive effect on increasing growth, soluble sugars, total proteins and antioxidant enzymes and reduction H2O2 and MDA levels. This effect was significantly greater in the hairy roots treated with both Fe3O4 NPs and SA compared to those exposed to these elicitors individually. In addition, the comparison between the essential oil components of intact plant and the transformed hairy root of C. procera confirmed that hairy root culture, especially supplemented with Fe3O4 NPs and SA, has potential ability to greater production of essential oil. This study suggests that Fe3O4 NPs and SA together can be used as powerful elicitors to improve growth and yield in hairy roots.
Key message
This study demonstrates a valuable method in hairy root induction system for Calotropis procera and improvement of their growth and physiological characteristics by Fe3O4 nanoparticles and salicylic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, producing secondary metabolites by developing hairy root cultures in valuable, rare, threatened, or endemic medicinal species due to their economic and technological advantages has gained an increasing interest. It is well established that the yield of secondary metabolites can be improved by utilization of techniques like elicitation in hairy root cultures (Hedayati et al. 2020; Thilip et al. 2019). One of stimuli that are being recently used in plant cultures is nanoparticle. Compared with native bulk compounds, nanoparticles have created a new generation of effective elicitors in metabolic engineering approaches due to their very small diameter and a large specific surface area. Previous studies show that nanoparticles are able to activate secondary metabolism and increase antioxidant activities (Tawfik et al. 2021; Javed et al. 2018). In addition, they have been shown to promote development of plants by enhancing elemental uptake and use of nutrients and increase resistance to various abiotic and biotic stresses (Khan et al. 2021). Among different nanoparticles, iron oxide nanoparticles (Fe3O4 NPs) have received special attention due to their magnetic characteristics, potent catalytic capacity and surface absorption capability. Iron is an essential microelement affected on plant growth and development, photosynthesis, enzymatic pathways, auxin activity, RNA synthesis and DNA transcription (Sheykhbaglou et al. 2018). Studies have shown that Fe3O4 NPs coated with magnetic particles have some advantages in biodegradability and biocompatibility. A number of investigations have been indicated also that they have a beneficial effect on plant growth, physiological traits and biochemical properties (Feng et al. 2022; Tawfik et al. 2021). Recently, they were widely used as a novel elicitor to enhance bioactive compounds in the hairy root cultures of medicinal plants (Alcalde et al. 2022; Nourozi et al. 2019). However, the impact of nanoparticles on plants relies on the size and surface charge of particles as well as the susceptibility of the plant species and optimization of elicitor concentration is essential to synthesize the optimum amount of secondary metabolites.
Furthermore, many studies have showed that exogenous application of salicylic acid (SA) is able to boost the plant metabolism because of its signaling role in regulating the expression of critical genes of the stress defense pathway and maintaining reactive oxygen species at an optimal level (Koo et al. 2020). Hence, in this study, SA was used an abiotic elicitor along with Fe3O4 NPs to promote the physiological and biochemical attributes of Calotropis procera, as well as minimize possible adverse effects of Fe3O4 NPs.
Calotropis procera (Aiton) W.T.Aiton which is referred to as stabragh, belongs to the Apocynaceae family, mainly grown in arid and semi-arid regions. It is a branching perennial shrub with toxic milky sap and a multipurpose plant that is used for medicine, fiber, fodder, phytoremediation, synthesis of nanoparticles and fuel purposes (Kaur et al. 2021). It is widely used in traditional medicine as an anti-diarrheal, anti-inflammatory, antimicrobial, anti-asthmatic, and analgesic substance. The industrial and medicinal attributes of C. procera can be ascribed to secondary metabolites. Owing to socio-economic importance of C. procera, the development of new protocols for producing a higher amount of secondary metabolites than in intact plants seems to be necessary. It is believed that hairy root culture along with techniques like elicitation can be an eco-friendly alternative for the extraction of phytotherapeutic compounds. However, there have been few published reports on hairy root induction in Calotropis species up until the present time. For instance, Sun et al. (2012) developed a hairy root protocol for C. gigantean to improve cardenolide production.
As far as we know, the impact of any elicitor on physiological and biochemical properties of hairy roots of C. procera has not been investigated so far. Hence, the aim of the present study was to set up C. procera hairy root culture and improve growth, physiology and production of essential oils of the hairy roots by iron oxide nanoparticle and salicylic acid. For this purpose, in the first part of this study, establishment of C. procera hairy root and optimization of its growth conditions was examined. To assess whether hairy root culture can be an alternative system for highly efficient production of desirable compounds in large quantities, a comparison of essential oils between intact parent and the hairy roots of C. procera were also performed. In another part of this study, to improve growth and physiological parameters of the hairy roots, the hairy roots were treated with different concentrations of Fe3O4 NPs and SA. Then, the effects of the Fe3O4 NPs and SA on growth, nutritional elements, accumulation of H2O2, malondialdehyde level, total protein content, activity of antioxidant enzymes and main essential oils accumulation in C. procera hairy roots were evaluated.
Materials and methods
Establishment of hairy root cultures
Agrobacterium rhizogenes culture
Three strains of Agrobacterium rhizogenes including k 599 (cucumopine-type), A4 and ATCC 15,834 (agropine-type) were employed for transformation experiments. A single clone of each A. rhizogenes strain was selected and cultured on 10 mL Luria–Bertani (LB) medium, supplemented by 50 mg L−1 rifampicin. All cultures were simultaneously shaken and incubated at 28 °C for 24 h by a shaking incubator at 110 rpm. After growth of bacteria in liquid medium, 200 µl of the resulting suspension was add to 40 mL LB medium and incubated again in the mentioned conditions for 48 h (OD600 = 0.6). Afterwards, the resulting suspension was centrifuged at 4 °C with 4000 ×g for 10 min and bacterial pellets were collected. The pellets were resuspended in 20 mL MS, and half-strength MS liquid media composed of different concentrations of sucrose (3 and 6%) and shaken on a rotary shaker for 1 h before inoculation into explants.
Hairy root induction
In this experiment, 21-day-old C. procera seedlings grown under in vitro conditions were used for hairy root induction. For this purpose, Calotropis procera seeds, collected from wild-growing plant populations in Shahdad (Kerman province, Iran) were surface sterilized with 70% (v/v) ethanol for 20 s and a 20% (v/v) of commercial bleach solution for 10 min. After washing several times with sterile water, the sterilized seeds were cultured in one-fourth Murashige and Skoog nutrient medium (MS; Murashige and Skoog 1962) containing 7.5 g L−1 sucrose (Merck, Darmstadt, Germany). The cultures were maintained for 21 days in the growth chamber at 25 °C with a16-h light/8-h dark photoperiod provided by white fluorescent lamps at a light intensity of 90 µmol m− 2 s−1 (Osram Sylvania,Versailles, KY).
To induce hairy roots, the leaf and internodal stem segments of the seedlings were aseptically cut into small sections and infected with the aforesaid bacterial suspension by injection and immersion in two ways. In the injection method, the explants were injected with bacterial suspension directly by sterile syringes. Regarding immersion method, the explants were either immersed in the inoculation suspension after wounding by sterile needle and shaken on the rotary shaker for 5 and 30 min or were floated in the bacterial solution without wounding for 30 min. After drying with sterile filter paper, all samples were placed on hormone free MS medium set at pH 5.7–5.8 containing 30 g L−1 sucrose and 8 g L−1 agar–agar (Merck, Darmstadt, Germany) and maintained under dark conditions at 25 ± 2 °C for 48 h. The samples were washed with sterile water consisting 600 mg L−1 cefotaxime antibiotic and transferred to the MS medium fortified with 600 mg L−1 cefotaxime to remove the bacteria. After incubation of co-cultured explants in dark at 25 ± 2 °C for 48 h, the explants were sub-cultured in fresh MS medium every week. The antibiotic concentration was progressively decreased in succeeding subcultures from 600 to 500, 400, 300, 200, 100 mg L−1 until all bacteria were finally eliminated. Control cultures were also treated in a similar way without bacteria under similar temperature and light conditions.
Establishment of hairy root clones
Roots that appeared from the wound sites were individually excised and transferred to the solid MS medium supplemented with appropriate antibiotic (cefotaxime, 100 mg L−1). They were incubated in dark at 25 ± 2 °C and then sub-cultured in fresh medium every 2 weeks in order to avoid browning. The cefotaxime level was gradually reduced to 50 and 25 mg L−1 during the second and third subculture, respectively. Subsequently, root cultures were maintained in the solid MS medium free of the antibiotic. In addition, the root pieces about 2 cm, obtained from four weeks old hairy roots of C. procera were transferred to liquid MS medium and incubated on a shaker at 120 rpm under dark condition at 25 ± 2 °C. After four weeks of culture, the hairy roots were harvested for extraction of genomic DNA subjected to polymerase chain reaction (PCR).
PCR analysis for confirmation of rol B gene integration
In order to detect the rol B gene integrated to the plant genome, PCR was performed by rolB specific primers (787 pb, GenBank accession number k03313). The primers designed to amplify rolB were forward primer: 5′ATGGATCCCAAATTGCTATTCC3′ and reverse primer: 5′TTAGGCTTCTTTCTTCAGGTTT3′. Genomic DNA was first extracted from individual hairy roots and untransformed plants according to a modified CTAB method (Rogers et al. 1985). Next, the DNA samples were amplified by PCR mixture (20 µl) including 1 µl of each primer, 2 µl of DNA, 10 µl of master mix (Sinna Gen, Iran), and 6 µl of distilled H2O. Once assembled, the reaction was placed in a thermal cycler (CG1-96 model, Palmcycler, India). The PCR programme were initial denaturation at 95 ̊C for 5 min, 35 cycles of 95 ̊C for 45 s, 62 ̊C for 90 s and 72 ̊C for 1 min and a final extension at 72 ̊ C for 10 min. Finally, the resulting amplified products were detected by 1% agarose gel electrophoresis (EH-705 model, Fanavaran Akhtarian, Iran) and visualized by gel documentation systems (Uvitec, UK).
Hairy roots treatment by abiotic elicitor
Elicitor preparation
In this study, synthesized iron oxide nanoparticles (Fe3O4 NPs) in average size about 11 nm by co-precipitation method using leaf extract of C. procera were used (Adabavazeh et al. 2022a). Salicylic acid (SA) was also purchased from Merck (Merck, Darmstadt, Germany).
To prepare liquid MS media containing Fe3O4 NPs (0, 50, 100, 150, and 200 mg L−1) NPs and SA (0 and 0.01 mM), SA was first added to the MS media. Then, the pH of the media was adjusted to 5.7–5.9 with 1 M NaOH or HCl. All media were sterilized by autoclaving at 121 °C with a pressure of 103 kPa for 20 min. In the next stage, after the media were cooled, different concentrations of sterilized Fe3O4 NPs were added to the media. For this purpose, Fe3O4 NPs were sterilized using UV under laminar air-flow hood for 30 min and then, a solution of 1000 mg L−1 of Fe3O4 NPs was prepared in sterile water as previously described by Adabavazeh et al. (2022b).
Hairy roots treatment
The hairy root pieces about 2 cm were transferred in 50 mL of MS liquid medium fortified with different concentrations Fe3O4 NPs (0, 50, 100, 150, and 200 mg L−1) and SA (0, 0.01 mM). After incubation for 72 h on an orbital shaker under continuous darkness at 28 °C, all samples were washed several times with sterile water and transferred into elicitor-free MS liquid medium. All cultures were kept in aforementioned condition for 21 days. Then, dry and fresh weight, nutritional elements, the amount of H2O2, lipid peroxidation, soluble sugar content, proteins, activities of antioxidant enzymes and the main essential oils in hairy roots of C. procera were measured.
Determination of fresh and dry weight of C. procera hairy roots
Dry and fresh weight of hairy roots was recorded 21 days after treatment. The hairy roots per each treatment were taken out from the culture vessel, blotted dry and weighed to determine the fresh weight. Then, the dry weight of roots was defined after putting the samples in an oven for 24 h at 70 °C.
Determination of hydrogen peroxide (H2O2)
The amount of H2O2 was analyzed in treated hairy roots according to Oktay et al. (2003). In this method, a portion (0.2 g) of fresh hairy root was extracted with 3 mL of 0.1% (v/v) TCA (trichloroacetic acid) in a cooled mortar. After centrifugation of the homogenate at 10,000 ×g and 4 °C for 20 min, 0.5 mL of supernatant was mixed with 0.5 mL of phosphate buffer (10 mM, pH = 7) and 1 mL of 1 M KI (potassium iodide) solution. Afterward, the mixture was incubated at 25 °C for 15 min, followed by the measurement of absorbance at 390 nm. To calculate hydrogen peroxide contents, a standard curve was developed using a series of H2O2 standards.
Measurement of Malondialdehyde (MDA)
The MDA content was determined by adapting the method reported by Heath and Packer (1968). A portion (0.2 g) of hairy root was homogenized in 3 mL of 0.1% (v/v) TCA. The extraction was centrifuged at 10,000 ×g for 20 min at 4 °C. Then, 0.5 ml of the supernatant was added to 2 mL of 20% (v/v) TCA containing 0.5% (v/v) thiobarbituric acid (Merck). The mixture was first incubated at 95 °C for 30 min and then, cooled quickly in an ice bath. After centrifuging at 10,000 ×g at 4 °C for 10 min, the supernatant absorbance was measured at 532 and 600 nm. The MDA content was calculated by drawing on an extinction coefficient of 155 mM−1 cm 1 and expressed as nmol g−1 fresh weight.
Measurement of soluble sugar
Soluble sugar content in the treated hairy roots was determined employing a modified anthrone reagent according to method of Fales (1951). For this purpose, 0.1 g of fresh tissue was first homogenized in 2.5 mL of 80% (v/v) ethanol at 95 °C for 60 min. Next, the extraction was centrifuged at 3000 ×g for 10 min, and the supernatant was collected, followed by evaporation to remove the extracting solvent. After suspending of the solid residue in 2.5 mL of deionized water, 0.5 mL of this mixture was blended with 5 mL of anthrone reagent and the reaction mixture was warmed for 17 min at 90 °C. Absorption intensity of extractions was measured at 625 nm. Anthrone reagent was prepared by dissolving 0.4 g of anthrone in 200 ml of sulfuric acid and then adding the acid solution to a flask containing 60 ml of distilled water and 15 ml of 95% ethyl alcohol. A standard curve was developed using a series of glucose standards and the results were reported according to mg g−1 fresh weight.
Protein and enzyme assay
For protein and enzyme extraction, 0.1 g of hairy root was homogenized with 2 mL of 25 mM sodium phosphate buffer (pH 7.8). The homogenate was centrifuged at 15,000 ×g for 20 min at 4 °C. The supernatant was used for measurement of protein concentration and antioxidant enzyme activities, described as follows.
Measurement of protein concentratio
The total amount of protein was analyzed according to the procedure of Bradford (1976) with slight modification (Olson and Markwell 2007). The absorbance of the extract was measured at 595 nm. A standard curve was drawn by standard concentrations of bovine serum albumin and the results were reported as mg g−1 fresh weight.
Superoxide dismutase (SOD) activity
The SOD activity was determined by adding 100 µL of the extracts to 1900 µL of reaction mixture containing 50mM sodium phosphate buffer (pH 7.8), 50 mM Na2CO3, 12 mM L-methionine, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1 µM riboflavin, and 75 µM p-nitro blue tetrazolium chloride (NBT) in dark conditions, based on the Giannotolitis and Ries (1997) protocol. The mixtures were lighted for 10 min under fluorescent lamp at a light intensity of 45 µmol m− 2s−1 and blank was incubated in the darkness. The absorbance was measured by the formation of formazan at 560 nm. In the described conditions, the absorbance in the sample without enzyme extract was assumed to be 100% and the enzyme activity was calculated by determining the percentage of NBT inhibition per minute. One unit of SOD was identified as the amount of enzyme needed to prevent 50% (w/v) NBT photo-reduction rate in 1 min (Beauchamp and Fridovich 1971), and the activity was reported as unit mg−1 protein.
Catalase (CAT) activity
The CAT activity was estimated based on rate of H2O2 decomposition (extinction coefficient 36 mM−1 cm−1) and consequently the reduction of absorbance at 240 nm in 120 s (Aebi 1974). The reaction mixture consisted of 800 µl of 50 mM sodium phosphate buffer (pH 7), 100 µL of enzyme extract, and 100 µL of 37% (v/v) H2O2. The activity was expressed as unit mg−1 protein. One unit of CAT activity was defined as 1 µmol H2O2 decomposed per 1 min at 25 °C.
Ascorbate peroxidase (APX) activity
The activity of APX was estimated according to the reduction in absorbance due to oxidation of ascorbic acid (AsA) at 290 nm in 60 s (extinction coefficient 2.8 mM−1 cm−1), following the procedure of Nakano and Asada (1981). Of the supernatant, 100 µL was added to a reaction mixture composed of 50 mM sodium phosphate buffer (pH 7.0) comprising 0.2 mM EDTA, 250 mM H2O2, and 0.5 mM ascorbic acid. The APX activity was reported as unit mg−1 protein. One unit of APX activity was defined as the amount of enzyme oxidising 1 µmol of AsA per 1 min.
Guaiacol peroxidase (GPX) activity
The activity of GPX was estimated by adding 50 µL of the extracts to a mixture of 50 mM phosphate buffer (pH = 7) containing 19 mM H2O2 and 9 mM guaiacol, following the method of Lin and Kao (1999). The increase in absorbance of the solution at 470 nm was measured, and the activity of GPX was stated as unit mg−1 protein. One unit of GPX activity was identified as the amount of enzyme that caused the formation 1µmol tetraguaiacol per min.
Element analysis by ICP-OES
Here, we used plasma optical emission spectrometry (ICP-OES) to evaluate whether Fe3O4 NPs and SA have an influence on the accumulation of essential elements such as iron, potassium, calcium, and magnesium. Prior to analysis, the treated hairy roots were dried for 24 h at 70 °C and then, were digested with nitric acid (Sagner, 1998). After that, the samples were quantified by ICP-OES.
Determination of essential oils
To compare the essential oil components between intact parent and the hairy roots, we collected leaf and flower from wild-growing plant populations in Shahdad (Kerman province, Iran). Identification of essential oil components in C. procera leaf, flower and hairy root was determined according to the gas chromatography-mass spectrometry (GC/MS). For this purpose, a Hewlett–Packard 5890 GC (Hewlett Packard, Waldbronn, Germany), equipped with a flame ionization detector (HP-5970 mass-selective detector) and a 50 m × 0.20 mm HP-5 (cross-linked phenyl–methyl silicon) column with 0.25 μm film thickness was used. The FID was maintained at 250 °C and the temperature program was 100–250 °C with changes of 4 °C min−1. Additionally, ionization energy was 70 eV. Helium was used as a carrier gas, the flow through the column was 1 mL min−1, and the split ratio was set to 100:1. Identification was based on sample retention time and mass recorded (Li et al. 2009; Davies 1990). Moreover, to evaluate whether Fe3O4 NPs and SA have an influence on the accumulation of main essential oils in hairy roots, the important essential oil constituents of hairy root treated with Fe3O4 NPs and SA were also measured by GC/MS.
Statistical analysis
All experiments were conducted in a 2*5 factorial in a completely random design involving two levels of SA treatment and five levels of Fe3O4 NPs. Each treatment contained one explant; three replicates were performed for each treatment. One-way analysis of variance (ANOVA) with Duncan’s tests was used to determine significant differences between multiple groups of data at the 5% level. Results were given as averages ± standard deviations.
Result
Establishment of hairy root
The effect of explant type, A. rhizogenes strain and inoculation suspension, inoculation method and time on hairy root induction
Hairy root induction took place in the leaf explants about 10 days and the stem explants about 14 days post-infection from wounded sites only with strain A4 of A. rhizogenes; the leaf explants experiencing higher transformation efficiency than the stem explants (Fig. 1). In term of morphology, hairy roots induced from leaf explant were white and slender, while hairy roots induced from stem explant were thick, brittle and branched, and they gradually changed from white to brown (Fig. 2).
The remarkable increase in rate of transformation frequency (88.3%) was observed on the leaf explants using bacterial inoculation suspension consisting half-strength MS with 6% sucrose (Fig. 3). Likewise, Fig. 3 showed that of the different methods of infection employed immersion with pre-pricking was most effective and inoculation time was an important parameter in increasing the efficiency of the transformation process. In this study, long time exposure (30 min) of explants to the bacterial suspension resulted in the maximum rate of root induction.
In addition, our findings indicated that the hairy root cultures formed in MS liquid medium showed a more rapid growth compared to hairy roots in solid medium (Fig. 4).
Confirmation of transgenic roots
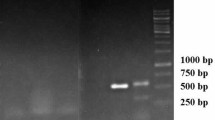
PCR reaction confirmed rolB gene integration in the hairy root genome, while the amplification of rolB gene was not observed in PCR product of the untransformed plants, as negative control (Fig. 5). The amplicon size (787 bp), observed from bacterial plasmid DNA, exactly matched the hairy root which conforming the transgenic nature of the hairy roots.
Effect of Fe3O4 nanoparticles and salicylic acid on fresh and dry weight of C. procera hairy roots
Analysis of variance of data to assess the impacts of Fe3O4 NPs, SA and their interaction on growth, physiological and biochemical parameters investigated was represented in Table 1. The effects of different concentrations of Fe3O4 NPs and SA on the fresh and dry weight of hairy roots of C. procera were presented in Table 2. Compared to the control, all treatments tested increased significantly the fresh and dry weight of hairy roots. The highest weight of the roots was obtained at the 200 mg L−1 of Fe3O4 NPs, which was 1.5 times higher than that of control samples.
Effect of Fe3O4 nanoparticles and salicylic acid on soluble sugar, protein contents, H2O2, and MDA
Data presented in Table 2 reveal that soluble sugars and protein contents were increased by all treatments tested. The hairy roots received 200 mg L−1 Fe3O4 NPs combined with 0.01 SA showed the highest content of soluble sugars by 191% and protein content by 66% when compared to controls.
The obtained results presented in Table 2 also showed that the Fe3O4 NPs combined with SA had a positive influence on reduction in the level of H2O2, MDA and protein oxidation. The highest increase in the H2O2 content was obtained at 50 mg L−1 Fe3O4 NPs, while the lowest content was recorded in the hairy root treated with 200 mg L−1 Fe3O4 NPs and 0.01mM SA. Also, the statistical analysis for the MDA content showed significant differences among hairy roots treated with Fe3O4 NPs combined with SA and control sample. As indicated in Table 2, the application of different concentrations of Fe3O4 NPs alone or with SA decreased MDA content. The highest and the lowest value of the MDA were seen in the control and sample treated with 200 mg L−1 Fe3O4 NPs in combination with 0.01 mM SA, respectively.
Effect of Fe3O4 nanoparticles and salicylic acid on antioxidant enzyme activity
The data presented in Table 2 showed the significant differences for enzyme activities among the different levels of individual and combined treatment of Fe3O4 NPs and SA. Relative to the control, SOD activity were progressively increased with rising Fe3O4 NPs concentrations up to 150 mg L−1 then decreased with 200 mg L−1 treatment. The same table also showed that 0.01 mM SA either alone or with Fe3O4 NPs decreased SOD activity, compared to the control. As seen in Table 2, CAT activity increased by all treatments applied in hairy roots relative to the control. However, 100 mg L−1 Fe3O4 NPs combined with 0.01 mM SA resulted in the highest activity in CAT up to 399%. As compared to control sample, 200 mg L−1 Fe3O4 NPs combined with 0.01 mM SA prompted a notable increase in APX activity by 121%. Of the different concentrations of Fe3O4 NPs employed, 50 mg L−1 Fe3O4 NPs increased GPX activity about 3.5-fold compared to the control. In addition, GPX activity increased when SA was applied alone or with Fe3O4 NPs.
Effect of Fe3O4 nanoparticles and salicylic acid on nutritional elements
The effect of Fe3O4 NPs and SA on iron, magnesium, potassium, and calcium contents was presented in Table 3. In comparison with control, 200 mg L−1 Fe3O4 NPs either alone or with 0.01 mM SA increased significantly iron content, whereas SA alone had no significant effect on this content. The data also showed a similar trend in potassium content. The same table also indicated that magnesium and calcium contents were increased by individual and combined treatment of 200 mg L−1 Fe3O4 NPs and 0.01 mM SA.
Determination of essential oils
The essential oil components within the intact parent leaf and flower and the transformed hairy roots of C. procera were identified by GS/MS as shown in Table 4. The GC/MS analysis of essential oil of C. procera leaves showed 41 constituents that the major components were geranial, alpha-terpinolene, alpha-pinene, a-phellandrene, 1,8-cineole, citronellal, camphor, terpinen-4-ol, and citronellol. The same table also revealed that of 39 identified compounds from flower, linalool, benzaldehyde, α-terpineol, nonanal, 3-hexanal, β-ocymene, and α-furole made up the major components of the flower oil. Moreover, the data presented in Table 4 confirmed the presence of 43 compounds in hairy root oil. Most of the identified phytocompounds in hairy root oil were the same as those usually synthesized in intact parent leaf and flower, with the exception of camphene, limonene, thymoquinone, decanal, carvacro, alpha- longipinene, borneol, cis-pulegone oxide, myrtenal, octanol, alpha-terpinen which were detected in hairy root extract only. According to the GC/MS results, hairy root culture induced a significant increase in the yield of essential oil as compared to intact parent leaf and flower. For instance, as compared to the flower essential oil, alpha-pinene by 262.5%, alpha-thujone by 130%, 1,8-cineole by 19%, cinnamene by 39%, citronellal by 1375%, p-cymene by 162.5%, a-phellandrene by 13%, geranial by 17.6% and germacrene-D by 94.4% were increased by hairy root induction. Likewise, C. procera hairy root had large capacity to accumulate linalool, germacrene-D, alpha-thujone, β-ocymene, phenyl acetaldehyde, and benzyl acetate, relative to the intact parent leaf.
It is worthwhile to mention that the positive impact of Fe3O4 NPs and SA on the important essential oil constituents of C. procera hairy roots has been described in detail in our previous study (Adabavazeh et al. 2022b). Briefly, as presented in Tables 5 and 200 mg L−1 Fe3O4 NPs with 0.01 mM SA had the most effect on the enhancing the yield of linalool up to 115%, alpha-thujone up to 194%, alpha-terpinolene up to 61.5%, germacrene-D up to 122%, phenyl acetaldehyde up to 20%, camphene and carvacrol up to 73, in comparison to control; while citronellol and alpha-terpinen under 200 mg L−1 Fe3O4 NPs and geranial under 0.01 mM SA alone increased by 36%, 17%, and 5%, respectively.
Discussion
Hairy root culture is currently one of the most widely utilized techniques, leading to production of high amounts of important natural products compared to the intact plants. The evaluation and determination of Agrobacterium strains, plant factors, and growth medium are prerequisites for the successful establishment of hairy root culture. Selection of a proper A. rhizogenes strain to infect plant species is an important parameter which affects transformation efficiency (Sahu et al. 2013). It is well known that Agropine-type strains (A4, ATCC 15,834, LBA 9402, and HRI) are the most infectious and often used in hairy root induction (Lee et al. 2010). The disparity in chromosomal virulence genes (chv D-E) may be the main reason for the differential degree of infectivity among different strains of A. rhizogenes (Tiwari et al. 2007). In addition, explant type, cultivar, genotypes, and culture conditions are the important plant factors that play an important role in the successful transformation (Colling et al. 2010). Previous studies have been well established the specificity of A. rhizogenes strains for different plant species and explant types. For instance, in our experiments, the strain A4 had better performance and proved to be more competent than the others strains employed; while the strain LBA 9402 was more competent for infecting leaf explants of Calotropis gigantean (Sun et al. 2012). Here, the highest transformation frequency was observed in C. procera leaf explants at 88.3%, infected with A4 strain after 10 days; whereas, A4 strain induced rooting in shoot explants14 days post-infection by 23%. The variation observed among plant species and explant type could be due to the sucrose and/or auxin level (Nilsson and Olsson 1997).
The inoculation medium such as full and half-strength MS media consisting 3 and 6% sucrose using the Agrobacterium strains employed was also viewed as another important parameter affecting the efficiency of transformation. In this study, it was observed that decrease of MS medium strength increased transformation efficiency; this was in conformity with previous findings (Wu et al. 2008; Sharafi et al. 2014) also reported that the transformation efficiency can be significantly improved by decreasing of mineral components. According to the obtained results, half-strength MS with 6% sucrose using A4 strain had the potential ability to greater production of hairy root.
The effects of inoculation methods and duration of plant tissue-bacterial suspension on the efficiency of hairy roots induction have also been well confirmed. In C. procera, successful transformation was achieved using immersing pre-pricked explants in bacterial suspension for 30 min. Similarly, numerous studies revealed that the highest transformation efficiency was achieved using explants which had been wounded with a sterile needle and inoculated with bacterial suspension, regardless of Agrobacterium strains employed (Sahu et al. 2013; Swain et al. 2012; Rao et al. 2012). Finally, in this study, a reproducible protocol for hairy root induction of C. procera was successfully established, which could be utilized as a platform for the mass production of specific metabolites.
After successful hairy root induction, the effects of the Fe3O4 NPs and SA on growth, nutritional contents, physiological traits and concentration of main essential oils in the hairy roots were evaluated. This study confirmed that the treatments tested could elevate growth of C. procera hairy roots. Of all treatments tested, the additions of synthesized Fe3O4 NPs at 200 mg L−1 had the most impact on fresh and dry weight of C. procera hairy roots. A similar finding was observed by Moharrami et al. (2017), who reported the highest hairy root fresh and dry weights in the medium supplemented with 900 mg L−1 Fe NPs. The enhanced hairy roots growth with Fe3O4 NPs could be related to the Fe NPs potential in absorption and transport of soluble nutrients such as Fe, Mg, Ca and K, necessary minerals with the highest demand in the plant growth and development (Abdi et al. 2008), which was in good line with the results obtained in this study.
The increase in soluble sugars and protein contents could be another possible reason for the enhanced weight of C. procera hairy roots under the treatments applied. Based on previous studies, iron as an enzyme cofactor, which plays an important role in electron transfer and catalysis can affect all plant metabolisms. In this regard, Askary et al. (2017) indicated that nano-compounds can be more rapidly and completely absorbed by plants as compared with their native bulk compounds, which makes plants able to use the nutrients in them and grow more prolifically. Accordingly, it seems that Fe3O4 NPs could make available sufficient Fe2+ required for enzymatic reactions involved in sugars and nitrogen metabolism, and increase the production of sugars and protein. Similarly, increase in sugars and protein contents in response to Fe3O4 NPs have also been reported by various workers (Sheykhbaglou et al. 2018; Vadivel et al. 2012). However, in this study, nanoparticles were substantially more effective when applied with SA. A number of studies have shown that the activity of sucrose phosphate synthase, the major enzyme involved in sucrose synthesis (Dong et al. 2011) and nitrate reductase, the key enzyme for nitrogen assimilation (Fariduddin et al. 2003) was up-regulated by SA treatment.
One of significant achievements in this research is a reduction in H2O2 and MDA contents of C. procera hairy roots treated with Fe3O4 NPs and SA. Here, H2O2 content showed a significant decrease in treatment with 200 mg L−1 Fe3O4 NPs combined with 0.01 SA, whereas all other treatments had shown no significant difference with control. Interestingly, MDA content decreased significantly upon exposure to all treatments. These results indicated that the use of Fe3O4 NPs, especially in combination with SA didn’t cause any membrane damage in treated hairy roots; rather they reduced the extent of membrane damage in comparison to control. These results were also consistent with the results reported by Rawat et al. (2017), who showed that treatment of 4 ppm of iron sulfide NPs decreased MDA and H2O2 content. It is speculated that the Fe3O4 NPs may have potentially lowered the oxidative damage based on the following evidence. First of all, Fe3O4 NPs have intrinsic enzyme peroxidase-like activity, as presented by Pariona et al. (2016) and Wang et al. (2010). It has been suggested that the peroxidase-like activity of Fe3O4 NPs could reduce internal ROS levels. Secondly, Fe3O4 NPs activate the cellular antioxidant system via H2O2 signaling pathway, including antioxidant enzymes and modulators of H2O2 metabolism (Trujillo-Reyes et al. 2014). Besides the positive effect of Fe3O4 NPs on reduction of MDA content, SA also increased antioxidant activity and polyamines, which help to inhibit membrane injury by retarding lipid peroxidation and preserving membrane integrity (Németh et al. 2002).
In the current experiments, antioxidant enzymes were significantly affected by Fe3O4 NPs and SA treatments. SOD activity decreased under 200 mg L−1 Fe3O4 NPs alone or with 0.01 SA, while CAT activity showed an increase relative to the untreated hairy roots. Moreover, the highest concentration of Fe3O4 NPs combined with SA prompted a notable increase in APX and GPX activities, whereas 200 mg L−1 Fe3O4 NPs alone had no significant effects on the activity of these enzymes. In the same way, Moharrami et al. (2017) have also mentioned similar response of Hyoscyamus reticulatus hairy roots towards iron oxide NPs. Further, Rawat et al. (2017) have also reported that Brassica juncea plants treated with sulfide NPs showed significant increase in APX, CAT and GPX activities in comparison to untreated plants. It seems that in addition to the activity of Fe3O4 NPs peroxidase-like activity, antioxidant enzymes, especially CAT, have also contributed to H2O2 regulation and cellular redox homeostasis, and thus improved growth of the treated hairy roots. Furthermore, SA also plays a key role in the accumulation and production of polyamines, which can improve antioxidant ability by increasing the activity of SOD, CAT, APX, and POD.
Another important finding of this research was greater production of important products of C. procera using hairy root culture. According to the literature published to date, hairy root cultures lead to mass production of secondary metabolites and important products compared to the normal plants (Garagounis et al. 2020; Hedayati et al. 2020,), which was consistent with our findings. For instance, in C. procera hairy roots, the most important components such as alpha-thujone, germacrene-D, alpha-pinene, citronellal, cinnamene, p-cymene, linalool, and geranial was higher than the normal plant. Moreover, numerous studies have referred to the presence of biotic and abiotic components in hairy root media to improve the performance of hairy roots in increasing plant metabolite production (Moradi et al. 2020; Rashidi Asl et al. 2019; Moharrami et al. 2017). In line with these studies, our results revealed that elicitation with the highest Fe3O4 NPs concentration in combination to SA could enhance the production of important essential oil constituents of C. procera hairy roots. Here, an improved linalool, alpha-thujone, germacrene-D, camphene and carvacrol contents was obtained in hairy roots after elicitation with 200 mg L−1 Fe3O4 NPs with 0.01 mM SA. The redox homeostasis of the treated hairy roots could possibly be the reason for this reported increase in growth and essential oil yield. Also, it seems that these elicitors stimulate the expression of genes involved in the biosynthetic pathway of the target secondary metabolite.
Overall, this study highlights the optimization of hairy root induction in C. procera and improvement of growth, physiological and biochemical characteristics of the hairy roots by Fe3O4 NPs and SA treatment. Our data suggest that the immersing pre-pricked leaf explants in A. rhizogenes A4 strain suspension consisting half-strength MS with 6% sucrose is the best condition for hairy root induction. Based on the observation, hairy root culture can act as an effective alternative to enhance the synthesis of essential oils and pharmacological constituents of C. procera. We propose that growth rate and essential oil production could be promoted by 200 mg L−1 Fe3O4 NPs with 0.01 mM SA. Our work provides a new insight to future research on the production of the medicinal compounds. The application of Fe3O4 NPs with SA in hairy root culture will help design strategies to improve desirable bioactive compounds in other medicinal species.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- APXAscorbate peroxidase:
-
Ascorbate peroxidase
- AsA:
-
Ascorbic acid
- CAT:
-
Catalase
- EDTA:
-
Ethylenediaminetetraacetic acid
- Fe3O4 NPs:
-
iron oxide nanoparticles
- GC/MS:
-
Gas Chromatography/Mass Spectrometry
- GPX:
-
Guaiacol peroxidase
- KI:
-
Potassium iodide
- (LB) medium:
-
Luria–Bertani medium
- MDA:
-
Malondialdehyde
- MS:
-
Murashige and Skoog nutrient medium
- PCR:
-
Polymerase chain reaction
- ROSs:
-
reactive oxygen species
- SA:
-
Salicylic acid
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
References
Abdi G, Salehi H, Khosh-Khui M (2008) Nano silver: a novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol Plant 30(5):709–714. https://doi.org/10.1007/s11738-008-0169-z
Adabavazeh F, Nadernejad N, Pourseyedi S, Razavizadeh R, Mozafari H (2022a) Synthesis of magnetic nanoparticles and their effects on growth and physiological parameters of Calotropis procera seedlings. Environ Sci Pollut Res 29:59027–59042. https://doi.org/10.1007/s11356-022-19660-7
Adabavazeh F, Pourseyedi S, Nadernejad N, Razavizadeh R, Mozafari H (2022b) Evaluation of synthesized magnetic nanoparticles and salicylic acid effects on improvement of antioxidant properties and essential oils of Calotropis procera hairy roots and seedlings. Plant Cell Tiss Organ Cult 151:133–148. https://doi.org/10.1007/s11240-022-02338-w
Aebi H (1974) Catalases. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684. https://doi.org/10.1016/b978-0-12-091302-2.50032-3
Alcalde MA, Perez-Matas E, Escrich A, Cusido RM, Palazon J, Bonfill M (2022) Biotic Elicitors in Adventitious and Hairy Root cultures: a review from 2010 to 2022. Molecules 27(16):5253. https://doi.org/10.3390/molecules27165253
Askary M, Talebi SM, Amini F, Dousti A, Bangan B (2017) Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija 63:65–75. https://doi.org/10.6001/biologija.v63i1.3476
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assay applicable to acrylamide gels. Ann Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteindye binding. Annu Rev Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Colling J, Groenewald JH, Makunga NP (2010) Genetic alterations for increased coumarin production lead to metabolic changes in the medicinally important Pelargonium sidoides DC (Geraniaceae). Metab Eng 12(6):561–572. https://doi.org/10.1016/j.ymben.2010.08.001
Davies NW (1990) Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methylsilicon and carbowax 20 M phases. J Chromatogr A 503:1–24. https://doi.org/10.1016/S0021-9673(01)81487-4
Dong CJ, Wang XL, Shang QM (2011) Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci Hortic 129(4):629–636. https://doi.org/10.1016/j.scienta.2011.05.005
Fales FW (1951) The assimilation and degradation of carbohydrates by yeast cells. J Bio Chem 193:113–124. https://doi.org/10.1016/S0021-9258(19)52433-4
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 41:281–284. https://doi.org/10.1023/B:PHOT.0000011962.05991.6c
Feng Y, Kreslavski VD, Shmarev AN, Ivanov AA, Zharmukhamedov SK, Kosobryukhov A, Yu Min, Allakhverdiev SI, Shabala S (2022) Effects of Iron Oxide Nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of Mineral Elements in Wheat (Triticum aestivum) plants. Plants 11:1894. https://doi.org/10.3390/plants11141894
Garagounis C, Beritza K, Georgopoulou M, Sonawane P, Haralampidis K, Goossens A, Aharoni A, Papadopoulou KK (2020) A hairy-root transformation protocol for Trigonella foenum-graecum L. as a tool for metabolic engineering and specialised metabolite pathway elucidation. Plant Physiol Biochem 154:451–462. https://doi.org/10.1016/j.plaphy.2020.06.011
Giannotolitis CN, Ries SK (1997) Superoxide dismutase: II. Purification and quantitative relationship with water-soluble protein in seedling. Plant Physiol 59:315–318. https://doi.org/10.1104/pp.59.2.315
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hedayati A, Hosseini B, Palazon J, Maleki R (2020a) Improved tropane alkaloid production and changes in gene expression in hairy root cultures of two Hyoscyamus species elicited by silicon dioxide nanoparticles. Plant Physiol Biochem 155:416–428. https://doi.org/10.1016/j.plaphy.2020a.07.029
Javed R, Yucesan B, Zia M, Gurel E (2018) Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech 20(2):194–201. https://doi.org/10.1007/s12355-017-0539-1
Kaur A, Batish DR, Kaur Sh, Chauhan BS (2021) An overview of the characteristics and potential of Calotropis procera from botanical, ecological, and economic perspectives. Front Plant Sci Rev. https://doi.org/10.3389/fpls.2021.690806
Khan AU, Khan T, Khan MA, Nadhman A, Aasim M, Khan NZ, Ali W, Nazir N, Zahoor M (2021) Iron-doped zinc oxide nanoparticles-triggered elicitation of important phenolic compounds in cell cultures of Fagonia indica. Plant cell Tiss Organ Cult 147:287–296. https://doi.org/10.1007/s11240-021-02123-1
Koo YM, Heo AY, Choi HW (2020) Salicylic acid as a safe plant protector and growth regulator. Plant Pathol J 36:1. https://doi.org/10.5423/PPJ.RW.12.2019.0295
Lee SY, Kim SG, Song WS, Kim YK, Park N, Park SU (2010) Influence of different strains of Agrobacterium rhizogenes on hairy root induction and production of alizarin and purpurin in Rubia akane Nakai. Rom Biotechnol Lett 15:5405–5409
Li XM, Tian SL, Pang ZC (2009) Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and system distillation. Food Chem 115:1114–1119. https://doi.org/10.1016/j.foodchem.2008.12.091
Lin CC, Kao CH (1999) NaCl induced changes in ionically bound peroxidase activity in roots of rice seedlings. Plant Soil 216(1–2):147–153. https://doi.org/10.1023/A:1004714506156
Moharrami F, Hosseini B, Sharafi A, Farjaminehad M (2017) Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell Dev Biol Plant 53:104–111. https://doi.org/10.1007/s11627-017-9802-0
Moradi A, Sharifi M, Mousavi A (2020) Induced production of tropane alkaloids, and expression of hyoscyamine 6β-hydroxylase (h6h) and putrescine N-methyl transferase (pmt2) genes in hairy roots and propagated plantlets of Atropa belladonna L. elicited by methyl jasmonate. South Afr J Bot 131:328–334. https://doi.org/10.1016/j.sajb.2020.01.042
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Németh M, Janda T, Hovarth E, Paldi E, Szali G (2002) Exogenous salicylic acid increases polyamine content but may decrease drought tolerance in maize. Plant Sci 162:569–574. https://doi.org/10.1016/S0168-9452(01)00593-3
Nilsson O, Olsson O (1997) Getting to the root: the role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol Plant 100:463–473. https://doi.org/10.1111/j.1399-3054.1997.tb03050.x
Nourozi E, Hosseini B, Maleki R, Mandoulakani BA (2019) Iron oxide nanoparticles: a novel elicitor to enhance anticancer flavonoid production and gene expression in Dracocephalum kotschyi hairy-root cultures. J Sci Food Agric 99(14):6418–6430. https://doi.org/10.1002/jsfa.9921
Oktay M, GülÒ«in İ, Küfrevioğlu Öİ (2003) Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci Technol 36:263–271. https://doi.org/10.1016/S0023-6438(02)00226-8
Olson BJSC, Markwell J (2007) Assays for determination of protein concentration. Curr Protoc Protein Sci 48:3.4.1-3.4.29
Pariona N, Martínez AI, Hernandez-Flores H, Clark-Tapia R (2016) Effect of magnetite nanoparticles on the germination and early growth of Quercus macdougallii. Sci Total Environ 575:869–875. https://doi.org/10.1016/j.scitotenv.2016.09.128
Rao K, Chodisetti B, Mangamoori LN, Giri A (2012) Agrobacterium- mediated transformation in Alpinia galanga (Linn.) Willd. for enhanced acetoxychavicol acetate production. Appl Biochem Biotechnol 168:339–347. https://doi.org/10.1007/s12010-012-9777-6
Rashidi Asl K, Hosseini B, Sharafi A, Palazon J (2019) Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng Life Sci 19(1):73–89. https://doi.org/10.1002/elsc.201800087
Rawat M, Nayan R, Negi B, Zaidi MGH, Arora S (2017) Physio-biochemical basis of iron-sulfide nanoparticle induced growth and seed yield enhancement in B. juncea. Plant Physiol Biochem 118:274–284. https://doi.org/10.1016/j.plaphy.2017.06.021
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76. https://doi.org/10.1007/BF00020088
Sahu L, Jena S, Swain SS, Sahoo S, Chand PK (2013) Agrobacterium rhizogenes-mediated transformation of a multi-medicinal herb, Boerhaavia diffusa L.: optimization of the process and anti-microbial activity against bacterial pathogens causing urinary tract infections. Front Life Sci 7(3–4):197–209. https://doi.org/10.1080/21553769.2013.879266
Sharafi A, Sohi HH, Azadi P, Sharafi AA (2014) Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants 20:257–262. https://doi.org/10.1007/s12298-013-0217-z
Sheykhbaglou R, Sedghi M, Fathi-Achachlouie B (2018) The effect of ferrous nano-oxide particles on physiological traits and nutritional compounds of soybean (Glycine max L.) seed. Anais da Academia Brasileira de Ciências 90(1):485–494
Sun J, Xiao J, Wang X, Yuan X, Zhao B (2012) Improved cardenolide production in Calotropis gigantea hairy roots using mechanical wounding and elicitation. Biotechnol Lett 34:563–569. https://doi.org/10.1007/s10529-011-0804-4
Swain SS, Sahu L, Pal A, Barik DP, Pradhan C, Chand PK (2012) Hairy root cultures of butterfly pea (Clitoria ternatea L.): Agrobacterium × plant factors influencing transformation. World J Microbiol Biotechnol 28(2):729–739. https://doi.org/10.1007/s11274-011-0869-1
Tawfik MM, Mohamed MH, Sadak MS, Thalooth AT (2021) Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull Natl Res Cent 45:177. https://doi.org/10.1186/s42269-021-00624-9
Thilip C, Mehaboob VM, Varutharaju K, Faizal K, Raja P, Aslam A, Shajahan A (2019) Elicitation of withaferin-A in hairy root culture of Withania somnifera (L.) Dunal using natural polysaccharides. Biologia 74:961–968. https://doi.org/10.2478/s11756-019-00236-9
Tiwari RK, Trivedi M, Guang ZC, Guo GQ, Zheng GC (2007) Genetic transformation of Gentiana macrophylla with Agrobacterium rhizogenes: growth and production of secoiridoid glucoside gentiopicroside in transformed hairy root cultures. Plant Cell Rep 26:199–210. https://doi.org/10.1007/s00299-006-0236-0
Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2014) Exposure studies of coreeshell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263. https://doi.org/10.1016/j.jhazmat.2013.11.067
Vadivel N, Yuvakkumar R, Suriyaprabha R, Rajendran V (2012) Catalytic effect of iron nanoparticles on heterocyst, protein and chlorophyll content of Anabaena sp. Int J Green Nanotechnol 4(3):326–338. https://doi.org/10.1080/19430892.2012.706185
Wang N, Zhu L, Wang D, Wang M, Lin Z, Tang H (2010) Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason Sonochem 17:526–533. https://doi.org/10.1016/j.ultsonch.2009.11.001
Wu J, Kong J, Wang Y, Han Z, Xu X (2008) Agrobacterium rhizogenes mediated transformation and hairy root regeneration of Malus baccata (L.) Borkh. Acta Horticult Sinica 35:959–966. https://www.ahs.ac.cn/EN/Y2008/V35/I7/959
Acknowledgements
This study was supported by Shahid Bahonar University of Kerman, Iran.
Author information
Authors and Affiliations
Contributions
FA Investigation, data curation, formal analysis, writing - original draft and editing. SP Design of the study, reviewing and editing. NN Project administration, reviewing and editing. RR Supervision, reviewing and editing. All authors contributed to the study conception, read, and approved final version of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the author.
Additional information
Communicated by Mohammad Faisal.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adabavazeh, F., Pourseyedi, S., Nadernejad, N. et al. Hairy root induction in Calotropis procera and optimization of its phytochemical characteristics by elicitors. Plant Cell Tiss Organ Cult 155, 567–580 (2023). https://doi.org/10.1007/s11240-023-02481-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-023-02481-y