Abstract
An efficient transformation system for the medicinal plant Pueraria phaseoloides was established by using agropine-type Agrobacterium rhizogenes ATCC15834. Hairy roots could be obtained directly from the cut edges of petioles of leaf explants or via callus 10 days after inoculation with the bacteria. The highest frequency of explant transformation by A. rhizogenes ATCC15834 was about 70% after infection for 30 days. Hairy roots could grow rapidly on solid, growth regulator-free Murashige and Skoog medium and had characteristics of transformed roots such as fast growth and high lateral branching. Paper electrophoresis revealed that bacteria-free hairy roots of P. phaseoloides could synthesize agropine and mannopine. The polymerase chain reaction amplification of rooting locus genes showed that left-hand transferred DNA of the root inducing plasmid of A. rhizogenes was inserted into the genome of transformed P. phaseoloides hairy roots. The content of puerarin in hairy roots reached a level of 1.190 mg/g dry weight and was 1.067 times the content in the roots of untransformed plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The soil-borne bacterium Agrobacterium rhizogenes carries root-inducing (Ri) transferred DNA that induces the formation of hairy roots after its integration into the plant genome (White and Nester 1980; Tepfer 1984; Tepfer and Casse-Delbart 1987). Since the genetic transformation does not impair the natural root's synthetic capacities, hairy roots, which can often grow vigorously and produce high levels of secondary metabolites, have been recognized as a potential way of producing important pharmaceuticals as secondary compounds from medicinal plants (Hamill et al. 1987; Yoshikawa and Furuya 1987).
Pueraria roots prepared from Pueraria phaseoloides (Roxb.) Benth are one of the most important crude drugs in the pharmaceutical industry, especially in Chinese traditional medicine. Studies on the main active constituents of Pueraria roots, puerarin, showed the presence of hypothermic, spasmolytic, hypotensive and anti-arrhythmic activities (Takaya and Itokawa 1982; Chai et al. 1985; Song et al. 1988). To date, however, puerarin is still mainly extracted from roots of wild plants. Evidently, this practice cannot meet the increasing demands for puerarin from the pharmaceutical industry, due to the limited resources of wild plants, and will also lead to considerable reduction of the natural medicinal plant population and soil erosion. So establishment of Pueraria hairy root cultures will be an effective alternative way towards large-scale production of puerarin instead of using wild plants. However, there have been no reports of hairy root induction with A. rhizogenes in P. phaseoloides. In this paper we describe an efficient system for genetic transformation of P. phaseoloides with A. rhizogenes and production of puerarin by hairy root cultures.
Materials and methods
Plant material
Seeds of Pueraria phaseoloides (Roxb.) Benth were collected from the biological garden of the Institute of Life Science, South China Normal University, China. Seeds were surface-sterilized in 0.1% (w/v) aqueous HgCl2 solution containing 0.1% Tween 20 for 25 min, followed by washing 3 times with sterile water. They were then placed on water-wetted cotton in 150 ml-flasks to germinate at 25°C under a 14-h photoperiod with a light intensity of 35 μmol m-2 s-1. After 4 weeks, stems and petioles excised from the germinated seedlings were rapidly propagated at 25°C with the same photoperiod according to previously described methods (Shi 2000).
Agrobacterium culture
A single clone of A. rhizogenes, ATCC15834, harboring agropine-type plasmid pRiA4b, was selected and cultured into 40 ml yeast extract broth (YEB, yeast extract 1.0 g l-1, beef extract 5.0 g l-1, peptone 5.0 g l-1, sucrose 5.0 g l-1, MgSO4.7H2O 0.49 g l-1, pH 7.2) in the dark at 28°C on a rotary shaker at 160 rev/min. When OD600 was approximately 1.0, the bacterial solution was collected and centrifuged at 4,000 rev/min for 5 min, followed by re-suspension in growth regulator-free liquid MS medium (Murashige and Skoog 1962) for inoculation.
Induction and culture of P. phaseoloides hairy roots
Young leaves (second and third leaves from the apex) from the 2-month-old seedlings were cut into leaf explants with or without petioles and pre-cultured on solid, growth regulator-free MS medium for 24 h. The leaf explants were then infected by dipping them into an Agrobacterium suspension in MS medium for 20 min. Following infection, the leaf explants were washed once with sterile double distilled H2O and blotted with filter paper to remove excess Agrobacteria. After 2 days of co-cultivation at 28°C in the dark, the explants were transferred onto MS medium containing 500 mg l-1 carbenicillin (filter-sterile, added to the medium) and kept in an air-conditioned chamber at 25°C, under 14 h/day light (35 μE m-2 s-1) to induce hairy roots. Control explants were given the same treatment but were dipped in sterile YEB medium. Each treatment consisted of 30 explants (6 explants per 100 ml-Erlenmayer flask) and the infection experiments were repeated twice.
Thirty days after infection, hairy roots were excised from infected Pueraria leaf explants and cultured on growth regulator-free MS agar (1%) medium with 500 mg/l carbenicillin to eliminate the agrobacterium; after several days of culture, the elongating root tips were cut off and transferred to growth regulator-free MS agar medium without carbenicillin. This procedure was repeated 3–4 times until no colony of bacteria appeared. Sterile hairy root cultures were maintained at 25°C in the dark on growth regulator-free MS medium without antibiotic for confirmation.
Confirmation of hairy roots by opine detection
Opine synthesis is a firm indication that hairy roots are transformed (Hamill et al. 1987). Extraction and detection of opines (agropine and mannopine) in bacteria-free hairy roots were carried out according to the method of Petit et al. (1983). High voltage paper electrophoresis was performed at 400 V for 1.5 h.
Identification of transformed Pueraria hairy roots by polymerase chain reaction
For confirmation, isolation of total DNA from bacterium-free Pueraria hairy roots and natural (non-transformed) roots was conducted using established methods (Edwards et al. 1991). Polymerase chain reaction (PCR) identification of the rooting locus genes rolB and rolC was performed using DNAs from the hairy roots as template and the non-transformed roots as control, respectively. The following primers, which were designed totally according to Furner et al. (1986), were used for amplification of rolB and rolC sequences: rolB-1 (5′-GCTCTTGCAGTGCTAGATTT-3′); rolB-2 (5′-GAAGGTGCAAGCTACCTCTC-3′); rolC-1 (5′-CTCCTGACATCAAACTCGTC-3′); rolC-2 (5′-TGCTTCGAGTTATGGGTACA-3′). For amplification, the PCR parameters consisted of a denaturation step of 3 min at 94°C and 35 cycles (each consisting of 1 min at 94°C, 1 min at 53.5°C and 1 min at 72°C), followed by a final extension at 72°C for 6 min. The PCR products were fractionated by electrophoresis on a 0.8% agarose gel using Tris-acetate-EDTA buffer and photographed under a UV lamp at a wavelength of 260 nm.
Puerarin extraction and analysis by high performance liquid chromatography
The hairy roots subcultured on growth-regulator-free MS medium for 10 days were harvested and dried to constant weight; 200 mg dry weight of hairy roots were ground and extracted with 10 ml methanol at 4°C for 24 h. The extract was then filtered through a filter paper. The extraction procedure was repeated three times. Next, a total of 30 ml extracted solution was concentrated to dryness. Methanol was added to dissolve the residue to a final volume of 2.0 ml before analysis. Roots excised from untransformed plants were extracted as control by the same procedure.
The puerarin content was analysed by high performance liquid chromatography (HPLC) using a Beckman System Gold HPLC (Beckman Instruments, Fullerton, Calif., USA) equipped with a UV detector (System Gold 168) and with a System Gold 125 Solvent module. Sample solution (20 μl in methanol) was injected into an Elite Hypersil BDS C18 (Dalian Elite Scientific Instruments, Dalian, China) column (particle size 5 μm, 25 cm×4.6 mm) and eluted with a mixture of methanol and water (85/15, v/v). The flow rate was 1.0 ml/min throughout the analysis. The puerarin content was detected by UV absorbance at 248 nm. For quantitative analysis, the system was calibrated with authentic puerarin purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Standard curves were fitted by linear regression.
Results
Transformation and establishment of hairy root cultures
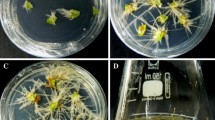
During initial experiments, cotyledon explants of P. phaseoloides were infected with A. rhizogenes ATCC15834. However, no hairy roots were obtained from cotyledon explants 2 months after inoculation. Consequently, further infection experiments were only performed with leaf explants with or without petioles. When leaf explants with petioles were inoculated with freshly grown A. rhizogenes suspensions in MS medium, hairy roots were induced from micro-calli of leaf petioles or directly from the cut edges of leaf petiole (Fig. 1), but the frequency of root induction (mean number of roots per explant) was higher from micro-calli than directly from the cut edges of leaf petioles. The first roots were visible 10 days after inoculation. With increasing incubation periods, the percentages of rooted leaf explants also increased. Thirty days after inoculation, the percentage of rooted leaf explants with petioles transformed by A. rhizogenes ATCC15834 was about 70%. When leaf explants without petioles were infected, hairy roots were only induced via callus from the leaf vein of cut edges of leaf explants 6–8 weeks after infection; the proportion of rooted leaf explants was about 40%. No roots were observed in non-infected leaf explants after approximately 8 weeks but a small callus was formed at the cut edges of leaf veins.
The induced hairy roots were excised and put onto fresh, growth regulator-free MS medium containing 500 mg/l carbenicillin to eliminate agrobacteria. After several days of culture, elongating root tips were cut off and transferred to growth regulator-free MS agar medium without carbenicillin. This procedure was repeated 3–4 times until no colony of bacteria appeared. When axenic hairy roots were transferred onto MS agar (1%) medium without antibiotic, they grew vigorously in the dark at 25°C and had characteristics of transformed roots, such as fast growth and high lateral branching (Fig. 2A). During incubation, hairy roots changed gradually from white to red-brown and some red-brown substance secreted from hairy roots after 3–4 weeks could be observed. In comparison with roots in solid culture, hairy roots cultured in liquid, growth regulator-free MS medium grew rapidly and had high lateral branching, but with increasing incubation time, the initial parts of the hairy roots and the culture medium turned brown (Fig. 2B). As a control, adventitious roots excised from P. phaseoloides sterile seedlings were cultured on solid, growth regulator-free MS medium, but these roots grew very slowly, did not branch and perished after 2–3 subcultures.
Evidence for transformation by opine detection
A. rhizogenes ATCC15834 is of the agropine-type. Both opines (agropine and mannopine) were detected by paper electrophoresis in extracts derived from Pueraria hairy roots but not in untransformed control roots (Fig. 3). The production of opines, along with rapid growth on growth regulator-free medium, was considered a proof of transformation.
Confirmation of P. phaseoloides hairy roots by PCR analysis
By using DNAs from the hairy roots as template and the non-transformed roots as control, PCR products amplified with rolB primers and rolC primers, respectively, could be detected (Fig. 4). It was demonstrated that two fragments, with lengths of 540 bp and 770 bp and corresponding to rolB and rolC, respectively, were amplified only from hairy root cultures but not from untransformed roots. These results indicated that the rolB and rolC genes from the Ri plasmid of A. rhizogenes ATCC15834 were integrated into the genome of P. phaseoloides hairy roots.
Production of puerarin in hairy roots
Hairy roots cultured onto growth regulator-free MS medium for 10 days were harvested and used for determination of puerarin contents with HPLC. As shown in Table 1 and Fig. 5, hairy roots could also produce puerarin, and the content of puerarin in P. phaseoloides hairy roots reached a level of 1.190 mg/g dry weight and was 1.067 times the puerarin content of the roots of untransformed plants.
Discussion
Hairy roots induced by the Ri plasmid of A. rhizogenes are widely used for the production of important pharmaceutical chemicals from many medicinal plants, such as Catharanthus roseus (Parr et al. 1988), Panax giseng (Yoshikawa and Furuya 1987), Datura candida (Christen et al. 1989), Salvia miltiorrhiza (Hu and Alfermann 1993) and Ocimum basilicum (Tada et al. 1996). So far, no protocols on the efficient transformation of P. phaseoloides with A. rhizogenes have been reported. In our study, we established an efficient transformation system for P. phaseoloides by using A. rhizogenes ATCC15834. Our results showed that P. phaseoloides is susceptible to A. rhizogenes ATCC15834 infection at a 70% frequency for explant transformation. This increased hairy root induction from petioles, as compared with cotyledons and leaf explants, may be due to the fact that petioles are more competent for transformation than cotyledons or leaf explants. Bercetche et al. (1987) reported that hairy roots could only be induced from calli of carrot tuber explants and stem segments of Nicotiana tabacum. After inoculation with A. rhizogenes, hairy roots could be induced directly from infected leaf and tuber explants, but only induced via callus from stem explants (Ottani et al. 1990). This suggests that differences in morphological patterns of hairy roots could be dependent upon different species and different plant organs or infected sites.
In our study, we obtained P. phaseoloides hairy roots with rapid growth on solid medium. Hairy roots could also be cultured in liquid medium but older parts of such roots become dark-brown, eventually darkening the culture medium. This might have been related to the effect of some metabolites released into the medium. Efficient culture of rapidly growing hairy roots in liquid medium is a key prerequisite for large-scale production of puerarin from P. phaseoloides. Some reports described effects of the medium composition and culture conditions on the growth rate of hairy roots and the production rate of secondary roots (Sauerwein et al. 1991). We have recently also succeeded in culturing Pueraria hairy roots in a 1.5 l airlift bioreactor, where root browning was not observed (manuscript under preparation).
The results presented here showed that P. phaseoloides hairy roots could produce puerarin, whose concentration is slightly higher than those in roots of untransformed plants and may be used as a useful system for large-scale production of puerarin. We are currently focusing our research on defining the optimal medium and culture conditions for the scale-up liquid culture of P. phaseoloides and puerarin production, as well as investigation various biochemical parameters (such as key metabolic enzymes, mitochondrial function and cell membrane potential) possibly related to both hairy root growth and puerarin production.
Abbreviations
- HPLC :
-
High performance liquid chromatography
- MS :
-
Murashige and Skoog medium
- PCR :
-
Polymerase chain reaction
- Ri :
-
Root inducing
- rolB:
-
Rooting locus B
- rolC:
-
Rooting locus C
- YEB :
-
Yeast extract broth
References
Bercetche J, Chriqui D, Adam S, David C (1987) Morphogenetic and cellular reorientation induced by Agrobacterium rhizogenes (strains 1855, 2659 and 8196) on carrot, pea and tobacco. Plant Sci 52:195–210
Chai XS, Wang ZX, Chen PP, Wang LY, Lü XR, Kang B (1985) Anti-arrhythmic action of puerarin. Acta Pharmacol Sin 6:166–168
Christen P, Roberts MF, Phillipson JD, Evans WC (1989) High-yield production of tropane alkaloids by hairy root cultures of a Datura candida hybrid. Plant Cell Rep 8:75–77
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349
Furner IJ, Huffman GA, Amasino RM, Garfinkel DJ, Gordon MP, Nester EW (1986) An Agrobacterium transformation in the evolution of the genus Nicotiana. Nature 319:422–427
Hamill JD, Parr AJ, Rhodes MJC, Robins RJ, Walton NJ (1987) New routes to plant secondary products. Biotechnology 5:800–804
Hu ZB, Alfermann AW (1993) Diterpenoid production in hairy root cultures of Salvia miltiorrhiza . Phytochemistry 32(2):699–703
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479
Ottani MP, Schel JHN, Hänisch ten Cate Ch H(1990) Variation in structure and plant regeneration of Agrobacterium rhizogenes transformed and control roots of the potato cv. Bintje. Plant Cell Tissue Organ Cult 20:25–34
Parr AJ, Peerless AC , Hamill JD, Walton NJ, Robins RJ, Rhodes MJC (1988) Alkaloid production by transformed root cultures of Catharanthus roseus. Plant Cell Rep 7:309–312
Petit A, David C, Dahl GA, Ellis IG, Guyon P, Casse-Delbart F, Tempe J (1983) Further extension of the opine concept: plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet 190:204–214
Sauerwein M, Yamazaki T, Shimomura K (1991) Hernandulcin in hairy root cultures of Lippia dulcis. Plant Cell Rep 9:579–581
Shi HP (2000) Plantlet regeneration from the petiole and stem of Pueraria phaseoloides (in Chinese with English abstract). Zhong Cao Yao (Chinese traditional and herbal drugs) 31:550–552
Song XP, Chen PP, Chai XS (1988) Effects of puerarin on blood pressure and plasma rennin activity in spontaneously hypertensive rats. Acta Pharmacol Sin 9:55–58
Tada H, Murakami Y, Omoto T, Shimomuro K, Ishimaru K (1996) Rosmarinic acid and related phenolics in hairy root cultures of Ocimum basilicum. Phytochemistry 42:431–434
Takaya K, Itokawa H (1982) Isoflavonoids and the other constituents in callus tissue of Pueraria lobata. Chem Pharm Bull 30:1496–1499
Tepfer D (1984) Genetic transformation of several species of higher plants by Agrobacterium rhizogenes: phenotypic consequences and sexual transmission of the transformed genotype and phenotype. Cell 37:959–967
Tepfer M, Casse-Delbart F(1987)Agrobacterium rhizogenes as a vector for transforming higher plants. Microbiol Sci 4:24–30
White FF, Nester EW (1980) Hairy root: plasmid encodes virulence traits in Agrobacterium rhizogenes. J Bacteriol 41:1134–1141
Yoshikawa T, Furuya T (1987) Saponin production by cultures of Panax ginseng transformed with Agrobacterium rhizogenes. Plant Cell Rep 6:449–453
Acknowledgements
The authors express their gratitude to Prof. Jacques Tempé, INRS, France, for kindly providing agropine and mannopine standards. This work was supported by the Ministry of Science and Technology of China and the Ministry of Development of Greece.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Dudits
Rights and permissions
About this article
Cite this article
Shi, HP., Kintzios, S. Genetic transformation of Pueraria phaseoloides with Agrobacterium rhizogenes and puerarin production in hairy roots. Plant Cell Rep 21, 1103–1107 (2003). https://doi.org/10.1007/s00299-003-0633-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0633-6