Abstract
IL-17 has a role in inflammation in RA, and its levels in joints correlate with disease severity. Multiple RCTs have been performed to study effects of anti-IL-17 agents. The objective of this study was to perform a systematic review and meta-analysis to analyze the efficacy and safety of anti-IL-17 agents in the management of RA. This work is based on a systematic review of studies retrieved by a sensitive search strategy in PubMed, EMBASE and Cochrane CENTRAL from inception through 9/7/15. Study selection criteria were the following: adult patients (age ≥ 18 years) with RAs, random selection of patients for anti-IL-17 therapy and treatment response compared to placebo. We performed systematic literature review per PRISMA guideline and two investigators independently selected seven randomized clinical trials (RCTs) for meta-analysis. We used random effect model calculating odds ratio (OR) and 95 % confidence interval (CI) to measure the efficacy with ACR20/50/70 responses and the safety with adverse events. Seven studies with total of 1226 patients including 905 in anti-IL-17 group and 321 in placebo were included in the meta-analysis. Anti-IL-17 was effective in achieving ACR20 and ACR50 compared to placebo (OR 2.47, 95 % CI 1.29–4.72, P = 0.006, I 2 77 % and OR 2.94, 95 % CI 1.37–6.28, P = 0.005, I 2 64 %, respectively). Data analysis for ACR70 showed a favorable trend toward anti-IL-17 (OR 2.62, 95 % CI 1–6.89, P = 0.05, I 2 15 %). Subgroup analysis of ACR20 for individual anti-IL-17 agents showed that ixekizumab was more effective than placebo, while secukinumab showed a trend toward achieving the ACR20 response. However, brodalumab was not effective compared to placebo. Safety analysis did not show increased risk of any or serious adverse effects by anti-IL-17 compared to placebo (OR 1.23, 95 % CI 0.94–1.61, P = 0.13, I 2 = 0 % and OR 1.28, 95 % CI 0.57–2.88, P = 0.55, I 2 = 0 %, respectively). This meta-analysis concludes that anti-IL-17 is effective in the treatment of RA without increased risk of any or serious adverse effects; however, the results are limited by significant heterogeneity and small duration of studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune and inflammatory disease affecting almost 1 % of adults worldwide [1]. It causes synovitis and cartilage damage which result in joint destruction and significant deformity. T cell activation leading to cytokine release is the pathophysiology of inflammation and tissue destruction. Th1-derived cytokines were initially thought to be a major player of tissue destruction in RA. However, a recent identification of a subset of CD4+ T helper cells has focused target of therapy beyond Th1/Th2 paradigm. This subset of T helper cells called Th17 is involved in pathogenesis of a wide range of autoimmune diseases, including RA [2, 3].
Th17 cells are derived from CD4+ cells after stimulation by TGF-β and IL-6 [2, 4, 5]. They produce IL-17A, IL-17F, IL-22, IL-26 and the chemokine CCL20 [5]. IL-17 is a pro-inflammatory cytokine [6] believed to be involved in both induction and expansion of a cytokine cascade in RA [7]. This ultimately results in initiation as well as amplification of joint destruction. Role of IL-17 in inflammatory arthritis has been tested in animal studies [8, 9]. These studies have shown association of Th17 and IL-17 levels with tissue and joint inflammation. A recent study found marked suppression of collagen-induced arthritis in IL-17-deficient mice [9]. Additionally, IL-17 blockage has shown to reduce inflammation and bone erosions [10]. Moreover, there is a lack of response to anti-TNF agents in patients with high baseline Th17 cell levels [11]. IL-17 is present in high concentration in synovial fluid of patients with arthritis [12, 13]. And increased concentration of IL-17A in synovial fluid is a marker of disease severity in RA [14–16]. Also, blockade of IL-17A can inhibit osteoclast activity in synovial tissue.
ACR 2012 guideline recommends conventional DMARDs, either alone or in combination as a first-line therapy for newly diagnosed cases of RA [17]. However, remission is achieved with DMARDs in 30–40 % cases only [18]. Recent advancement and identification of mediators of chronic inflammation in RA has shifted treatment focus to novel biological agents. Currently available biologics target monocyte derived pro-inflammatory cytokines including TNF, IL-1β or IL-6. However, 30 % of patients with TNF inhibitors have suboptimal response leading to further tissue damage [5, 18, 19] implicating a need to explore therapeutic options targeting IL-17 and other cytokines beyond current biologics.
Human trials have confirmed effectiveness of anti-IL-17 agents in treatment of some chronic inflammatory conditions [20, 21]. RCTs of anti-IL-17 therapy, however, have shown mixed results. To define the role of this novel therapeutic agent in the treatment of RA and explore its safety, we performed a systematic review and meta-analysis.
Methods
Data sources and search strategy
We searched electronic database of MEDLINE, EMBASE and Cochrane CENTRAL Register for Clinical Trials for publications from inception through September 2015 with search terms “Anti IL-17” OR “Interleukin 17” OR “IL-17 inhibitor” OR “Interleukin 17 inhibitor” OR “Secukinumab” OR “Ixekizumab” OR “Brodalumab” AND “Rheumatoid Arthritis” OR “RA” OR “arthritis” AND “Randomized Controlled Trial” OR “Randomly” OR “Randomized” OR “Controlled Clinical Trial” OR “Comparative Study.” Search strategy was designed by primary investigator (S.K.) and did not include MeSH terms. Search strategy, study selection and meta-analysis were guided by a written study protocol. Two investigators (S.K. and S.S.) independently performed the database search and agreed on final study selection. A manual search was performed for relevant references from the selected articles and published reviews.
Study selection
We included randomized placebo-controlled trials comparing all available anti-IL-17 agents to placebo in treatment of RA in adult population (age ≥18 years). We excluded abstracts without full text publications, nonrandomized designs and studies with pediatric patients and animals. Also excluded were abstracts of annual scientific meetings as our protocol prespecified inclusion of full text publications only.
Data extraction
Two authors (S.K. and S.S.) extracted data from the selected studies in duplicate using standardized data extraction table. Data were extracted on study characteristics (number of patients, study design, study location, name of the drug with dosing route and frequency, follow-up duration, inclusion/exclusion criteria, primary and secondary endpoints), patient characteristics [age, sex, body mass index (BMI), disease duration, disease severity at baseline, efficacy (ACR20/50/70 response) and adverse effects], ACR 20/50/70 responders and adverse events. The studies measured baseline severity with CRP, ESR, Disease Activity Score of 28 joints (DAS28), tender and swollen joint counts, Patient’s and Physician’s Global Assessment of disease activity and Health Assessment Questionnaire Disability Index (HAQ-DI).
Individual studies provided data under different subgroups (different doses) for the agent studied. We combined data from all subgroups from each study to get the final mean and SD for each baseline characteristics. For outcome analysis, total numbers of patients with specific outcomes were simply added together.
We extracted data for safety analysis from any or serious adverse events as well as the individual adverse events. They were reported only if occurred in more than 3–5 % of patients. Any fatal or life-threatening adverse events or events requiring (or prolonging) hospitalization or causing substantial disability or congenital anomaly were considered to be serious adverse effects. Serious adverse effects also included events that were considered by the investigators as medically important.
Major outcomes
The efficacy outcomes were measured with ACR20/50/70 response to anti-IL-17 therapy as compared to placebo, whereas the composite and individual adverse events including infections, headache, GI-related side effects, neutropenia, leukopenia, RA flare up and drug discontinuation were the safety outcomes.
Statistical analysis
We performed the meta-analysis using random effects model with the help of Review Manager (RevMan 5.2, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark) for statistical analyses. Categorical variables were pooled as odds ratio (OR) with 95 % confidence interval (CI). Crude events from each study were used to calculate odds ratio with 95 % confidence intervals when appropriate. The P value <0.05 (2 tailed) was considered statistically significant. Study heterogeneity was evaluated by Cochrane’s Q and I 2 index. We used Cochrane Collaborations’s tool for assessing risk of bias in individual studies which showed mostly unclear risk of bias.
Results
Description of individual studies
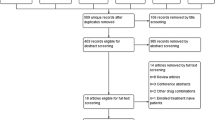
We retrieved 244 citations from electronic database and manual searches as shown in Fig. 1. After duplicate articles were removed, 226 were assessed for eligibility. We reviewed 25 citations for full text articles. Nine full text articles were then included for final review. Two studies were further excluded as they were not placebo controlled [22, 23]. Seven studies met eligibility criteria and were included in final analysis [1, 24–29]. One included study had two groups: group A was analyzed for safety outcome only, and group B was analyzed for both safety and efficacy outcomes [29]. Groups A and B were both included in meta-analysis for safety and safety/efficacy analysis, respectively.
There were total of 1226 patients in 7 included studies. A total of 905 patients were in anti-IL-17 group and 321 patients in placebo. Efficacy analysis was done on 1204 patients (888 in anti-IL-17 group and 316 in placebo). Safety analysis, however, was done for all 1226 patients. There were totally 233 (19 %) male and 993 (81 %) female patients with similar male/female distribution (anti-IL-17 group: male 19.2 % and female 80.8 %, placebo group: male 18.4 % and female 81.6 %). Baseline characteristics in all the studies were comparable in both groups. Patient and study characteristics in individual studies are shown in Tables 1 and 2, respectively.
Most of the studies included active RA as defined by American College of Rheumatology Criteria (diagnosed more than 3–6 months prior to screening). Active RA was defined by ≥6 swollen joints, ≥6 tender joints (≥8 tender joints in 2 studies) [1, 26] and CRP ≥ 15 mg/l or ESR ≥ 28 mm/h. Inclusion criteria in the studies were adults ≥18 years with active RA and requirement of a stable dose of methotrexate (7.5–25 mg/week) [1, 26, 27] or ≥1 DMARDs [24, 29] (methotrexate, sulfasalazine or hydroxychloroquine) prior to screening. Other two studies did not require but allowed methotrexate [28] and ≥1 DMARDs [25] (methotrexate, sulfasalazine and hydroxychloroquine). Oral prednisone was allowed by most of the studies except one [27], if was taken at a stable dose of 10 mg or less per day starting weeks prior to screening. Concomitant use of NSAIDs was also allowed by some. Exclusion criteria were stage 4 RA, recent or recurrent infection, use of prednisone at >10 mg/day, concomitant other rheumatologic or autoimmune diseases, malignancy and use of biological agents within 2–3 months prior to screening. However, one study allowed participation of the patients with prior use of biological agents after an appropriate washout period before randomization [28]. Another study involved patients with inadequate response to TNF inhibitors, however, did not permit use of TNF inhibitors within 1 month (anakinra, etanercept) to 12 months (rituximab) prior to baseline [24].
Efficacy outcome
Compared to placebo, anti-IL-17 agents were more effective in achieving ACR20 response [51 vs. 34 %; odds ratio (OR) 2.47; 95 % confidence interval (CI) 1.29–4.72; P = 0.006; I 2 = 77 %] and ACR50 response [24.3 vs. 7 %; OR 2.94; CI 1.37–6.28; P = 0.005; I 2 = 64 %]. For ACR70 response, anti-IL agents showed a trend toward efficacy but did not reach statistical significance [10.7 vs. 2.3 %; OR 2.62; CI 1–6.89; P = 0.05; I 2 = 15 %]. Because of significant heterogeneity observed in ACR20 and ACR50 as well as variation in effect estimates in the individual studies, sensitivity analysis was performed by excluding one study at a time to evaluate the effect of any individual study in the overall heterogeneity. We found that the Burmester et al. study contributed to significant heterogeneity. Sensitivity analyses after removal of Burmester et al. study found that the anti-IL-17 agents were effective for both outcomes with reduced heterogeneity: ACR20 (OR 1.7; CI 1.16–2.49; P = 0.006; I 2 = 31 %) and ACR50 (OR 2.2; CI 1.23–3.95; P = 0.008; I 2 = 29 %) (figures not shown).
Meta-analysis of ACR20 showed an increased response with ixekizumab [OR 2.32; CI 1.52–3.53; I 2 = 0 %, P < 0.0001] (2 studies, N = 525). Similarly, secukinumab showed a trend toward achieving ACR20 response [OR 4.06; CI 0.84–19.68; I 2 = 88 %; P = 0.08] (3 studies, N = 388) and heterogeneity resolved after removing Burmester et al. study [OR 1.66; CI 0.95–2.91; I 2 = 0 %; P = 0.08]. However, brodalumab was not effective in achieving ACR20 [OR 1; CI 0.57–1.75; I 2 = 0 %, P = 1.0] (2 studies, N = 278).
Safety outcome
The anti-IL agents, compared to placebo, did not increase the risks of any (56 vs. 51 %; OR 1.23; CI 0.94–1.61; P = 0.13; I 2 = 0 %) or serious adverse events (3.6 vs. 2.5 %; OR 1.28; CI 0.57–2.88; P = 0.55; I 2 = 0 %) or treatment discontinuation (3 vs. 2.5 %; OR 0.97; CI 0.34–2.74; P = 0.95; I 2 = 17 %; Fig. 3).
A total of 21.6 and 16.5 % reported infections in anti-IL-17 and placebo group, respectively. In the analysis of individual adverse events, anti-IL agents had significant increase in the risk of infections (OR 1.44; CI 1.01–2.04; P = 0.04; I 2 = 0 %), reduced the risk of headache (OR 0.52; CI 0.30–0.92; P = 0.03; I 2 = 0 %) and had no effect on leukopenia (OR 2.64; CI 0.48–14.58; P = 0.27; I 2 = 0 %), neutropenia (OR 2.16; CI 0.67–6.90; P = 0.2; I 2 = 0 %), UTI (OR 1.28; CI 0.57–2.88; P = 0.55; I 2 = 0 %), URI/nasopharyngitis (OR 1.76; CI 0.88–3.50; P = 0.11; I 2 = 0 %), GI-related adverse events (OR 1.35; CI 0.64–2.82; P = 0.43; I 2 = 0 %) and RA flare up (OR 0.87; CI 0.44–1.73; P = 0.69; I 2 = 0 %) as shown in Figs. 4 and 5. One patient (0.1 %) died in treatment group secondary to cardiopulmonary failure. There was no fatality in placebo group.
Serious adverse events are shown in Table 3. DVT was reported in 4 patients in anti-IL-17 group, and all other serious events were reported in 2 or less patients. Because of the small number of occurrence of individual serious adverse events, we did not perform subgroup analysis. In one study, six cases of neoplasms (two breast cancers and each of TCC of bladder, soft tissue neoplasm, melanocytic nevus, uterine leiomyoma) were reported in treatment group and 1 (uterine leiomyoma) in placebo [24]. These neoplasms were identified in less than 80 days except 1 case of breast cancer which was diagnosed 5.5 months after the last dose of anti-IL-17. Because these neoplasms were diagnosed within short period of time from exposure to anti-IL-17, it is less likely to have any causal relationship. Antidrug antibody was reported by three studies; however, there were no neutralizing antibodies [1, 26, 29]. Allergy and hypersensitivity were reported by two studies (anti-IL-17 and placebo) with two cases of type three reaction [24, 29]. One patient in anti-IL-17 group developed serum sickness, leading to discontinuation of the drug. All other cases were mild to moderate without need for discontinuation.
Discussion
This meta-analysis showed the superiority of anti-IL-17 therapy in achieving target of ACR20 and ACR50 and a trend toward ACR70 with acceptable safety profile in short-term follow-up compared to placebo. There were no increased risks of any adverse events, serious adverse events, drug discontinuation, leukopenia, neutropenia or RA flare up. However, anti-IL-17 increased the risk of infection and decreased the incidence of headache.
Meta-analysis of ACR20 and ACR50 showed significant heterogeneity, so the result should be viewed with caution. The heterogeneity in summary estimate was due to a single study, Burmester et al., which was likely due to a racial variation in patient selection (Russian—63 %) and high seropositivity (83 %) compared to other studies. Subgroup analysis by Burmester et al. showed much larger treatment differences between secukinumab and placebo in Russian subjects and the patients with seropositivity. DAS28-CRP least-squares mean (SEM) change from baseline was −2.32 (0.3) (P < 0.0001) and −0.38 (0.45) (P = 0.40) for Russian and EU/USA subgroups and −2.05 (0.28) (P < 0.0001) and 0.06 (0.60) (P = 0.92) for seropositive and seronegative subjects, respectively.
Currently available biologics are not effective enough to achieve remission in around one-third of the patients [18, 19]. Multiple randomized controlled trails have been done or are underway to explore newer biological agents based on newly identified cytokines and their transducers [30, 31]. Recent studies have shown effect of anti-IL-17 agents in treatment of chronic plaque psoriasis [20, 21], psoriatic arthritis [32] and other immune-mediated inflammatory diseases [33]. RCTs also have shown effectiveness of anti-IL-17 in RA patients [24, 28, 29]. A phase 2 trial of secukinumab in RA patients by Genovese et al. [22] showed improved clinical endpoints (ACR response and DAS28-CRP) at week 16 which sustained through week 52. Improved clinical endpoints have been studied and found to have association with patient-reported outcomes (PROs). A study by Strand et al. [23] found clinically meaningful improvement in physical function, fatigue and health-related quality of life (HRQoL) even from a small benefit in clinical endpoint (ACR20/50/70). Interestingly, this improvement in PRO was incremental when achieving higher threshold of clinical endpoint with anti-IL-17.
Despite its significant benefit in psoriatic arthritis and animal studies, some human studies did not show meaningful improvement from anti-IL-17 therapy in RA [1]. Koenders et al. [34] studied anti-IL-17 therapy in SCID mouse model which showed treatment response only to mice with CD3+ (source of IL-17)-rich synovium. Similar finding was observed by van Baarsen et al. [35] showing heterogenous expression patterns of IL-17A, IL-17F and their receptors. Limitation in clinical response has been suggested by low levels of IL-17 at the site of inflammation. Both studies proposed preselection based on IL-17 expression for maximum therapeutic effect.
The current meta-analysis showed improved ACR20/50/70 response with reasonable safety profile (Figs. 2, 3, 4, 5) pointing to a possible and exciting role of anti-IL 17 agents in the treatment of RA. The roles of these agents should be further elucidated by larger RCTs. In our analysis, we found a clear role of secukinumab and ixekizumab but not brodalumab, but because of limited number to perform subgroup analyses on the individual drug, we did not report the results.
This meta-analysis concludes that anti-IL-17 therapy is effective in treatment of active RA (stages 1, 2, 3) with small increment in infections. Larger RCTs are, however, needed to further analyze the efficacy and long-term safety of this interesting novel agent.
The major limitations of this meta-analysis are limited number of studies, small sample size, variable doses of anti-IL-17 agents and inability to analyze efficacy on Disease Activity Score as well as individual set components of ACR20 (due to insufficient data). Also, long-term safety data were not available due to short duration of follow-up. Significant heterogeneity was observed in ACR20 and ACR50 which was further explored with sensitivity analysis. Nevertheless, this meta-analysis is strengthened by inclusion of all available randomized trials on the role of these interesting agents in the management of RA and the absence of detectable heterogeneity in most of the outcomes.
References
Martin DA, Churchill M, Flores-Suarez L et al (2013) A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther 15:R164. doi:10.1186/ar4347
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Annu Rev Immunol 27:485–517. doi:10.1146/annurev.immunol.021908.132710
Ely LK, Fischer S, Garcia KC (2009) Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol 10:1245–1251. doi:10.1038/ni.1813
Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T cells. N Engl J Med 361:888–898. doi:10.1056/NEJMra0707449
Furst DE, Emery P (2014) Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatol Oxf Engl 53:1560–1569. doi:10.1093/rheumatology/ket414
Moseley TA, Haudenschild DR, Rose L, Reddi AH (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev 14:155–174
Miossec P (2000) Are T cells in rheumatoid synovium aggressors or bystanders? Curr Opin Rheumatol 12:181–185
Cai L, Yin JP, Starovasnik MA et al (2001) Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine 16:10–21. doi:10.1006/cyto.2001.0939
Nakae S, Nambu A, Sudo K, Iwakura Y (2003) Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol Baltim Md 1950 171:6173–6177
Lubberts E (2008) IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 41:84–91. doi:10.1016/j.cyto.2007.09.014
Alzabin S, Abraham SM, Taher TE et al (2012) Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann Rheum Dis 71:1741–1748. doi:10.1136/annrheumdis-2011-201024
Chabaud M, Durand JM, Buchs N et al (1999) Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum 42:963–970. doi:10.1002/1529-0131(199905)42:5<963:AID-ANR15>3.0.CO;2-E
Ziolkowska M, Koc A, Luszczykiewicz G et al (2000) High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol Baltim Md 1950 164:2832–2838
Tang C, Chen S, Qian H, Huang W (2012) Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology 135:112–124. doi:10.1111/j.1365-2567.2011.03522.x
van den Berg WB, Miossec P (2009) IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol 5:549–553. doi:10.1038/nrrheum.2009.179
Leipe J, Grunke M, Dechant C et al (2010) Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum 62:2876–2885. doi:10.1002/art.27622
Singh JA, Furst DE, Bharat A et al (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res 64:625–639. doi:10.1002/acr.21641
Storage SS, Agrawal H, Furst DE (2010) Description of the efficacy and safety of three new biologics in the treatment of rheumatoid arthritis. Korean J Intern Med 25:1–17. doi:10.3904/kjim.2010.25.1.1
Mewar D, Wilson AG (2011) Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol 162:785–791. doi:10.1111/j.1476-5381.2010.01099.x
Gordon KB, Leonardi CL, Lebwohl M et al (2014) A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis. J Am Acad Dermatol 71:1176–1182. doi:10.1016/j.jaad.2014.07.048
Xiong H-Z, Gu J-Y, He Z-G et al (2015) Efficacy and safety of secukinumab in the treatment of moderate to severe plaque psoriasis: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 8:3156–3172
Genovese MC, Durez P, Richards HB et al (2014) One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol 41:414–421. doi:10.3899/jrheum.130637
Strand V, Kosinski M, Gnanasakthy A et al (2014) Secukinumab treatment in rheumatoid arthritis is associated with incremental benefit in the clinical outcomes and HRQoL improvements that exceed minimally important thresholds. Health Qual Life Outcomes 12:31. doi:10.1186/1477-7525-12-31
Genovese MC, Greenwald M, Cho C-S et al (2014) A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol Hoboken NJ 66:1693–1704. doi:10.1002/art.38617
Burmester GR, Durez P, Shestakova G et al (2015) Association of HLA-DRB1 alleles with clinical responses to the anti-interleukin-17A monoclonal antibody secukinumab in active rheumatoid arthritis. Rheumatol Oxf Engl. doi:10.1093/rheumatology/kev258
Pavelka K, Chon Y, Newmark R et al (2015) A study to evaluate the safety, tolerability, and efficacy of brodalumab in subjects with rheumatoid arthritis and an inadequate response to methotrexate. J Rheumatol 42:912–919. doi:10.3899/jrheum.141271
Hueber W, Patel DD, Dryja T et al (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2:52ra72. doi:10.1126/scitranslmed.3001107
Genovese MC, Durez P, Richards HB et al (2013) Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis 72:863–869. doi:10.1136/annrheumdis-2012-201601
Genovese MC, Van den Bosch F, Roberson SA et al (2010) LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 62:929–939. doi:10.1002/art.27334
Maringwa J, Kågedal M, Hamrén UW et al (2014) Pharmacokinetic-pharmacodynamic modeling of fostamatinib efficacy on ACR20 to support dose selection in patients with rheumatoid arthritis (RA). J Clin Pharmacol. doi:10.1002/jcph.406
Lee YH, Bae S-C, Song GG (2015) Comparative efficacy and safety of tofacitinib, with or without methotrexate, in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Rheumatol Int. doi:10.1007/s00296-015-3291-4
Mease PJ, Genovese MC, Greenwald MW et al (2014) Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 370:2295–2306. doi:10.1056/NEJMoa1315231
Baeten D, Baraliakos X, Braun J et al (2013) Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl 382:1705–1713. doi:10.1016/S0140-6736(13)61134-4
Koenders MI, Marijnissen RJ, Joosten LAB et al (2012) T cell lessons from the rheumatoid arthritis synovium SCID mouse model: CD3-rich synovium lacks response to CTLA-4Ig but is successfully treated by interleukin-17 neutralization. Arthritis Rheum 64:1762–1770. doi:10.1002/art.34352
van Baarsen LGM, Lebre MC, van der Coelen D et al (2014) Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther 16:426. doi:10.1186/s13075-014-0426-z
Author contributions
Dr. Kunwar is guarantor of the study, had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of data analysis. Kunwar, Dahal and Sharma were involved in study concept and design. Kunwar, Dahal and Sharma were involved in data acquisition and analyzed and interpreted the data. Kunwar and Dahal drafted the manuscript. Dahal was involved in statistical analysis. Kunwar and Sharma supervised the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors SK, KD and SS declare no conflict of interest.
Ethical approval
This article does not contain any studies with animals or human participants performed directly by any of its authors. Authors of the included studies have declared in their published articles that their protocols were approved by institutional review boards or ethics committee at each participating site.
Informed consent
The authors of this article did not directly involve any human subjects; however, the individual studies have declared obtaining informed consent from the patients.
Rights and permissions
About this article
Cite this article
Kunwar, S., Dahal, K. & Sharma, S. Anti-IL-17 therapy in treatment of rheumatoid arthritis: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatol Int 36, 1065–1075 (2016). https://doi.org/10.1007/s00296-016-3480-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3480-9