Abstract
We conducted this systematic reviews and meta-analysis to investigate the safety and efficacy of ocrelizumab in patients with active rheumatoid arthritis (RA) who exhibited resistance or intolerance to methotrexate or biological therapy. We performed a web-based literature search of PubMed, Google Scholar, EBSCO, Scopus, Embase, and Web of science for studies that compared ocrelizumab plus methotrexate versus methotrexate plus placebo in RA patients. Data were extracted from eligible studies and pooled as risk ratios (RR), using RevMan software. Pooling data from four RCTs (2230 patients) showed that ocrelizumab plus methotrexate were superior to methotrexate plus placebo at 24 weeks in terms of improvement on the American college of rheumatology (ACR20, ACR50, and ACR70) criteria (p < 0.00001), disease activity score 28-ESR (RR = 3.77, 95% CI [2.47, 5.74], p < 0.00001), and Sharp/van der Heijde radiological score (RR = 1.63, 95% CI [1.43, 1.85], p < 0.00001). These effects were consistent among all ocrelizumab doses. The rates of serious adverse events were comparable between the ocrelizumab and placebo containing groups (RR = 1, 95% CI [0.78, 1.28], p = 0.98). However, infusion related reactions were significantly higher in ocrelizumab group (RR = 2.13, 95% CI [1.69, 2.68], p < 0.00001), compared to placebo group. The combination of ocrelizumab plus methotrexate was superior to methotrexate plus placebo on all clinical and radiographic improvement scales. The incidence of adverse events, including serious adverse events, was comparable between both groups. Future trials should investigate the efficacy of ocrelizumab alone and develop strategies to alleviate its related infusion reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disorder, characterized by symmetric progressive damage of affected joints, and it affects 0.5 to 1% of the global population with an estimated annual incidence of 0.02 to 0.05% [1, 2]. The resulting decline of productivity and increase in the cost of healthcare can become a significant burden to both individuals and societies [3]. Early management of RA with disease modifying anti-rheumatic drugs (DMARDS) as methotrexate (MTX) or biologic agents as tumor necrosis factor (TNF) inhibitor may efficiently control the disease activity and prevent further joint damage [4]. Recent studies have suggested a central role for B (CD20) cells in the pathogenesis of RA through auto-antibody formation, T cell activation, production of pro-inflammatory cytokines, and regulation of dendritic cell function [5–7]. Therefore, novel biologic anti-CD20 DMARDs have been recently introduced to improve therapeutic outcomes and disability prognosis in patients with insufficient response to conventional DMARDs.
Rituximab (RTX), a chimeric monoclonal anti-CD20 antibody, has demonstrated clinical efficacy and a long-term safety profile in RA patients with an inadequate response to conventional therapeutic agents [8, 9]. It has been recently approved for treatment of active RA in Europe and the United States. Ocrelizumab (OCR) (rhuMAb 2H7) is a humanized monoclonal antibody that selectively targets a different molecule on the B cell CD20 receptor. It is hypothesized that ocrelizumab may offer a higher tolerability than rituximab owing to its humanized nature, which may decrease associated immunogenicity [10]. Moreover, in vitro studies have demonstrated that OCR induced higher antibody-dependent cell mediated cytotoxicity (ADCC) and lower complement-dependent cytotoxicity (CDC) than rituximab [11].
Recently, several clinical trials have evaluated the tolerability and efficacy of ocrelizumab in RA patients who exhibited an inadequate response to conventional DMARDs or biologic therapy [12–15]. Therefore, we conducted this systematic review and meta-analysis to synthesize evidence from published randomized controlled trials (RCTs) regarding the safety and efficacy of ocrelizumab for patients with active RA.
Methods
We performed all steps in this study in a strict accordance with the Cochrane handbook of systematic reviews of interventions [16]. We also followed the PRISMA statement guidelines during reporting this systematic review and meta-analysis [17].
Search strategy
We searched six electronic databases: PubMed, Google Scholar, EBSCO, SCOPUS, Embase, and Web of science through March 2016 using the following query: [Ocrelizumab OR CD20 Antagonist AND Rheumatoid Arthritis]. No time or language restrictions were imposed. We also searched the reference lists of eligible articles to find relevant studies.
Study selection
We included randomized controlled trials (RCTs) that compared ocrelizumab plus methotrexate versus placebo plus methotrexate in patients with active RA who exhibited resistance or intolerance to DMARDs or TNF inhibitors. We excluded studies that compared ocrelizumab to other therapeutic regimens or enrolled treatment-naïve patients. We also excluded observational, non-randomized studies, and studies from which data could not be reliably extracted. Eligibility screening was conducted in two steps, each by two independent reviewers: (a) title and abstract screening for matching the inclusion criteria, and (b) full text screening for eligibility to quantitative analysis. Disagreements were resolved upon the opinion of a third reviewer to reach a final decision.
Main outcome variable
Two authors extracted the relevant data independently and another author resolved disagreements. The extracted data included the following: (a) characters of study design, (b) characteristics of study patients, (c) risk of bias domains, and (d) study outcomes including:
-
1.
Efficacy outcomes:
-
American College of Rheumatology (ACR) improvement score: represents a percentage of improvement of RA manifestations with fixed scores of 20, 50, and 70%. It is used to assess the improvement in tender or swollen joint counts, patient’s global assessment of disease activity, physician’s global assessment of disease activity, patient’s assessment of pain, functional disability questionnaire, and acute phase response [18].
-
Disease Activity Score 28 (DAS-28): a clinical index of RA disease activity that combines information from swollen joints, tender joints (out of 28 joints), acute phase response, and general health. A response is defined by achieving a score of less than 2.6 [19].
-
European League of Associations for Rheumatology (EULAR) improvement criteria: relies on the DAS 28 score to classify individual patients as non-, moderate, or good responders, depending on the extent of change and the level of disease activity reached [20].
-
Health Assessment Questionnaire- Disability Index: a quality of life questionnaire in which the patient reports the degree of experienced difficulty in performing common daily activities such as eating, dressing, and walking. A response is defined by achieving a meaningful reduction of 0.25 units or more [21].
-
Sharp/van der Heijde Score (SHS): a radiologic score of joint damage in which joint erosions are scored according to their number and their size in relation to the joint surface. A response was achieved if the progression of joint damage was less or equal to zero [22].
-
-
2.
Safety outcomes: Incidence of all adverse events (AEs), serious adverse events (cardiac, nervous, gastrointestinal, musculoskeletal, and hematological), adverse events leading to withdrawal, infections, and infusion related reactions (IRRs).
Risk of bias assessment
To assess the risk of bias within each included study, two independent reviewers used the Cochrane risk of bias (ROB) assessment tool, adequately described in Chap. 8.5 of the Cochrane handbook of systematic reviews of interventions 5.1.0 [16]. This tool can detect five types of bias including selection bias, performance bias, detection bias, attrition bias, and reporting bias. The authors classified included studies in each domain as low, high, or unclear risk of bias.
According to Egger’s and colleagues [23], publication bias cannot be assessed for less than ten included studies. Therefore, we were not able to check for publication bias using Egger’s funnel-plot-based methods due to the small number of included studies.
Data analysis
Data for dichotomous outcomes were extracted and pooled as risk ratios (RR) with 95% confidence interval in a fixed effect meta-analysis model, using the Mantel Haenszel (M–H) method. We used RevMan version 5.3 for windows. Heterogeneity was assessed by Chi-square test and its extent was measured by I-square test. In case of significant heterogeneity (Chi-square p < 0.1), the analysis was conducted under the random effects model and a sensitivity analysis was performed to resolve it.
A subgroup analysis by ocrelizumab dose was performed to precisely evaluate the effect of the two most commonly investigated doses (200 and 500 mg) on safety and efficacy outcomes. We also performed a sensitivity analysis to verify that none of the individual studies affected the results of our analysis.
Results
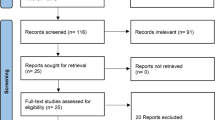
Our search retrieved 509 unique citations. Following title and abstract screening, 18 full text articles were retrieved and screened for eligibility. Of them, 14 articles were excluded and four RCTs [12–15] (n = 2230 patients) were included in this analysis (see PRISMA flow diagram) (Fig. 1). Reasons for exclusion of the 14 studies were as follows: eight full text articles were reviews [10, 24–30], two studies used other drug combinations [31, 32], one study enrolled treatment naïve patients [33], and three reports were conference abstracts [34–36]. For the included studies, a summary of their design and main results is shown in Table 1 and baseline characteristics of their patients are shown in Table 2.
All included studies were of a low risk of bias in terms of random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. However, only two of the four included studies adequately reported how they achieved allocation concealment. A summary of risk of bias assessment domains and authors’ judgments with justifications are shown in Supplementary file 1.
Efficacy analysis
-
3.
American college of Rheumatology (ACR) improvement criteria (week 24):
-
(i)
ACR 20: The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for ACR 20 response rate (RR = 1.72, 95% CI [1.52, 1.96], p < 0.00001); pooled studies were heterogeneous (p = 0.08; I² = 55%) (Fig. 2a). Heterogeneity was best resolved by excluding the study by Rigby et al. (RR = 2.08, 95% CI [1.69, 2.56], p < 0.00001); pooled studies were homogenous (p = 0.99; I 2 = 0%).
-
(ii)
ACR 50: The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for ACR 50 response rate (RR = 2.22, 95% CI [1.79, 2.75], p < 0.00001); pooled studies were homogenous (p = 0.59; I² = 0%) (Fig. 2b).
-
(iii)
ACR 70: The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for ACR 70 response rate (RR = 2.54, 95% CI [1.74, 3.70], p < 0.00001); pooled studies were homogenous (p = 0.95; I² = 0%) (Fig. 2c).
-
(i)
-
4.
Disease Activity Score 28-ESR (DAS 28-ESR) week 48: The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for DAS 28-ESR response rate (RR = 3.77, 95% CI [2.47, 5.74], p < 0.00001); pooled studies were homogenous (p = 0.55; I² = 0%) (Fig. 3a).
-
5.
European League of Associations for Rheumatology (EULAR) improvement criteria (week 24): The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for achieving a moderate or a good response on the EULAR improvement criteria (RR = 1.98, 95% CI [1.70, 2.32], p < 0.00001); pooled studies were homogenous (p = 0.77; I² = 0%) (Fig. 3b).
-
6.
Health Assessment Questionnaire-Disability Index (HAQ-DI): The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for improvement on HAQ-DI (RR = 1.75, 95% CI [1.55, 1.97], p < 0.00001); pooled studies were homogenous (p = 0.61; I² = 0%) (Fig. 3c).
-
7.
Sharp/van der Heijde radiologic Score (SHS): The overall effect estimate favored ocrelizumab plus methotrexate group over the placebo plus methotrexate group for response on the Sharp/van der Heijde score (defined as a change in the SHS of 0) (RR = 1.63, 95% CI [1.43, 1.85], p < 0.00001); pooled studies were homogenous (p = 0.12; I² = 58%) (Fig. 3d).
Safety analysis
The frequency of all adverse events (RR = 1.05, 95% CI [1, 1.09], p = 0.05), serious adverse events (RR = 1, 95% CI [0.78, 1.28], p = 0.98), adverse events that led to withdrawal (RR = 1.06, 95% CI [0.59, 1.88], p = 0.85), infections (RR = 1.05, 95% CI [0.97, 1.15], p = 0.22), cellulitis (RR = 0.95, 95% CI [0.17, 5.21], p = 0.95), urinary tract infection (RR = 0.68, 95% CI [0.14, 3.38], p = 0.36), and pneumonia (RR = 1.45, 95% CI [0.53, 3.97], p = 0.47) were not higher in the ocrelizumab plus methotrexate group when compared to the placebo plus methotrexate group; pooled studies were homogenous (Chi-square p > 0.1).
The incidence of IRR was higher in the ocrelizumab containing group (RR = 2.13, 95% CI [1.69, 2.68], p < 0.00001), compared to placebo group. In patients who received two consecutive courses of ocrelizumab (two infusions each); the incidence of IRRs with the first infusion of course 1 (RR = 2.48, 95% CI [1.86, 3.30], p < 0.00001) and course 2 (RR = 2.30, 95% CI [1.35, 3.90], p = 0.002) of ocrelizumab was significantly higher when compared to placebo. However, the frequency of IRRs did not differ between ocrelizumab and placebo containing groups in the second infusion of course 1 (RR = 0.81, 95% CI [0.54, 1.22], p = 0.31) or course 2 (RR = 1.16, 95% CI [0.60, 2.25], p = 0.66). For all IRRs, pooled studies were homogenous (Chi-square p > 0.1). Forest plots of safety outcomes are presented in supplementary file 2.
Subgroup analysis
Subgroup analysis showed that the effect estimate was consistent among both ocrelizumab doses (200 and 500 mg) in all efficacy and safety outcomes, i.e. when the total effect estimates favored ocrelizumab plus methotrexate over placebo plus methotrexate, subgroup analysis showed that both ocrelizumab doses were superior to placebo and vice versa. An exception was the EULAR improvement criteria where the total effect estimate favored ocrelizumab group over placebo (RR = 1.98, 95% CI [1.70, 2.32], p < 0.00001) and subgroup analysis showed that the 500 mg ocrelizumab group achieved similar results to placebo (RR = 2.13, 95% CI [0.69, 6.58], p = 0.19). A summary of the results of subgroup analysis is presented in Table 3.
Discussion
Our meta-analysis pooled data from four clinical trials (2230 patients) that investigated the safety and efficacy of ocrelizumab infusion for treatment of active RA in patients who are resistant or intolerant to methotrexate or TNF inhibitors. Clinical improvements on ACR20, ACR50, ACR70, EULAR criteria, DAS 28-ESR, and HAQ-DI were significantly higher in the ocrelizumab plus methotrexate (intervention) group, compared to methotrexate plus placebo (control) group. Radiologic improvement on Sharp/van der Heijde radiologic Score was also higher in the ocrelizumab group, compared to the placebo group. In terms of safety, no adverse events were significantly higher in the ocrelizumab containing group, except for the incidence of IRRs, especially with the first infusion of course 1 and course 2. Considering the lack of efficacy of methotrexate (the first line anti-rheumatic agent) or TNF blockers in enrolled patients of all included trials, our meta-analysis shows that ocrelizumab can be a safe and a potent anti-rheumatic agent in patients who exhibited resistance to DMARDs (including MTX) or TNF blockers.
Subgroup analysis showed that the effect estimate was consistent across all ocrelizumab doses in all assessed outcomes, i.e. the effect of each independent dose was similar to the combined analysis of all doses. An exception was the EULAR improvement criteria in which the 500 mg dose group showed similar results to the placebo group. We believe this difference can be attributed to the small sample size of 500 mg subgroup in this particular outcome. The optimal ocrelizumab dose would offer the highest therapeutic response, without causing serious adverse events. Comparing different doses to select this dose was not possible using a meta-regression statistical test due to the small number of available studies. However, most of the included studies showed that the 200 mg dose achieved similar therapeutic response to higher doses, with a lower incidence of serious adverse events.
Viewing the current literature, the first study of ocrelizumab on humans was conducted by Genovese et al. in 2008 (ACTION trial) and showed that ocrelizumab at 200 mg or higher is effective and well tolerated in patients with MTX resistant RA [12]. In 2012, two trials by Rigby et al. (STAGE trial), and Tak et al. (SCRIPT trial) showed a potent clinical activity of adding ocrelizumab to methotrexate in resistant RA patients. Rigby et al. reported that the incidence of serious infections was higher in patients receiving 500 mg of ocrelizumab [13]; however, Tak et al. concluded that the rate of serious infections was higher in patients receiving either 200 or 500 mg of ocrelizumab, compared to the placebo group [15]. Another study by Harigai et al. [14] in Japanese population showed that ocrelizumab adds no clinical benefit to methotrexate and they recommended further investigation of more potent anti-B cell drugs [14].
Rituximab is another anti-B cell monoclonal antibody which is approved for treatment of patients with an inadequate therapeutic response to MTX or TNF blockers [8, 9]. A systematic review of three clinical trials has shown that rituximab is a potent anti-rheumatic agent that does not increase the risk of serious adverse events, when compared to placebo. Optimization of its therapeutic response and long-term safety was attempted by molecular modifications of chemical structure, leading to production of ocrelizumab [37]. Compared to the results of the previous systematic review about rituximab, our analysis shows that both ocrelizumab and rituximab can be equally effective in treatment of MTX resistant patients with RA.
All included studies have reported a profound depletion of peripheral B cell count, immediately after ocrelizumab infusions. Genovese et al. suggested that such rapid depletion due to B cell lysis may be responsible for the observed infusion related reactions after ocrelizumab infusions. They also reported that B cell repletion was faster in the lower dose groups. There is a current debate in the literature about whether the level of peripheral B cell depletion, induced by anti-B monoclonal antibodies, is associated with clinical efficacy. Vital et al. concluded that the clinical efficacy of rituximab is determined by the level of B cell depletion rather than the used dose [38]. This finding was reported for ocrelizumab in the ACTION trial by Genovese et al. [12]. On the contrary, Stohl et al. reported that the level of B cell depletion was similar among all infused ocrelizumab doses, which was associated with a variable therapeutic response in treatment-naïve patients [33]. They argued that the subtle changes in peripheral B cell count may be translated into much greater differences in secondary lymphoid and synovial tissues. Of note, the anti-B cell monoclonal activity of ocrelizumab is currently under investigation for primary progressive multiple sclerosis [39, 40], refractory follicular lymphoma [41], and acute proliferative lupus nephritis [42].
Future investigators are recommended to use longer follow up periods to assess the sustainability of therapeutic response and long-term safety of ocrelizumab. Future trials are encouraged to directly compare the safety and efficacy of the two monoclonal antibodies (ocrelizumab versus rituximab). Some patients are severely intolerant to MTX; therefore, the safety and efficacy of ocrelizumab alone should be investigated. Clinicians should consider developing strategies to alleviate the infusion related reactions after ocrelizumab administration to optimize its safety profile.
All included studies were of a low risk of bias, adding to the credibility of our evidence. We performed a subgroup analysis to investigate the therapeutic efficacy of different doses on the prespecified outcomes. We also performed a sensitivity analysis to verify that none of the individual studies affected the results of our analysis and we found that none of the included trials had a much higher weight to shift the effect estimate of our analysis in its direction. Of the 2230 patients included in this analysis, there were 242 (10.8%) withdrawals after randomization and receiving at least one dose of the compared regimens. However, we believe this attrition rate is unlikely to affect the results of our analysis because all included studies performed an intention to treat analysis and specified all reasons for withdrawal.
This meta-analysis has some limitations. The relatively small number of included studies introduces the possibility of publication bias, which cannot be reliably assessed if the number of included studies is small. The variability of clinical inclusion criteria, number of ocrelizumab infusions, and follow up periods among pooled studies could have introduced bias into our meta-analysis. The longest follow up period was 72 weeks in the study by Genovese et al., limiting the available data on the long-term safety and efficacy of ocrelizumab.
Conclusion
Our analysis shows that, in patients who exhibited resistance to methotrexate or a TNF blocker, the combination of ocrelizumab plus methotrexate is superior to methotrexate plus placebo on all clinical and radiographic improvement scales. The incidence of serious adverse events was comparable between both combinations. Future trials should consider assessing the efficacy of ocrelizumab alone and develop strategies to alleviate the infusion related reactions following its administration.
Abbreviations
- ACR:
-
American college of rheumatology
- DAS:
-
Disease activity score
- DMARDs:
-
Disease modifying anti-rheumatic drugs
- EULAR:
-
European League of Associations for Rheumatology
- HAQ-DI:
-
Health Assessment Questionnaire-Disability Index
- MTX:
-
Methotrexate
- OCR:
-
Ocrelizumab
- RA:
-
Rheumatoid Arthritis
- RTX:
-
Rituximab
- SHS:
-
Sharp/van der Heijde Score
- TNF:
-
Tumor necrosis factor
References
Sangha O (2000) Epidemiology of rheumatic diseases. 39(Suppl 2):3–12
Smith JB, Haynes MK (2002) Rheumatoid arthritis—a molecular understanding. Ann Intern Med 136:908–922
Brooks PM (2006) The burden of musculoskeletal disease—a global perspective. Clin Rheumatol 25:778–781
Smolen JS, Landewé R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509. doi:10.1136/annrheumdis-2013-204573
Metlay JP, Puré E, Steinman RM (1989) Control of the immune response at the level of antigen-presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol 47:45–116
Edwards JC, Cambridge G, Abrahams VM (1999) Do self-perpetuating B lymphocytes drive human autoimmune disease? Immunology 97:188–196
Dörner T, Burmester GR (2003) The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol 15:246–252
Emery P, Deodhar A, Rigby WF et al (2010) Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Effi. Ann Rheum Dis 69:1629–1635. doi:10.1136/ard.2009.119933
Emery P, Fleischmann R, Filipowicz-Sosnowska A et al (2006) The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 54:1390–1400. doi:10.1002/art.21778
Kausar F, Mustafa K, Sweis G et al (2009) Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert Opin Biol Ther 9:889–895
Clark EA, Shu G, Ledbetter JA (1985) Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci USA 82:1766–1770
Genovese MC, Kaine JL, Lowenstein MB et al (2008) Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 58:2652–2661. doi:10.1002/art.23732
Rigby W, Tony H-P, Oelke K et al (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight–week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64:350–359
Harigai M, Tanaka Y, Maisawa S et al (2012) Safety and efficacy of various dosages of ocrelizumab in Japanese patients with rheumatoid arthritis with an inadequate response to methotrexate therapy: a placebo-controlled double-blind parallel-group study. J Rheumatol 39:486–495
Tak PP, Mease PJ, Genovese MC et al (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64:360–370
Higgins JP, Green S (2008) Cochrane Handbook for Systematic Reviews of interventions. The Cochrane Collaboration. doi:10.1002/9780470712184
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi:10.1371/journal.pmed.1000097
Felson DT, Anderson JJ, Boers M et al (1995) American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 38:727–735
Van Riel PLCM, van Gestel AM (2000) Clinical outcome measures in rheumatoid arthritis. Ann Rheum Dis 59:i28–i31
Fransen J, Van Riel P (2005) The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 23:S93
Maska L, Anderson J, Michaud K (2011) Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res 63:S4–S13
Van der Heijde D (1999) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 26:743–745
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res 315:629–634. doi:10.1136/bmj.316.7129.469
Boross P, Leusen JHW (2012) Mechanisms of action of CD20 antibodies. Am J Cancer Res 2:676–690
Bossaller L, Rothe A (2013) Monoclonal antibody treatments for rheumatoid arthritis. Expert Opin Biol Ther 13:1257–1272. doi:10.1517/14712598.2013.811230
Bruce SP (2009) Recent developments in the treatment of rheumatoid arthritis. J Pharm Pract 22:65–74. doi:10.1177/0897190008322290
Buch MH, Emery P (2011) New therapies in the management of rheumatoid arthritis. Curr Opin Rheumatol 23:245–251. doi:10.1097/BOR.0b013e3283454124
Edwards JC, Leandro MJ, Cambridge G (2004) Anti-CD20 for rheumatoid arthritis. BMJ PUBLISHING GROUP BRITISH MED ASSOC HOUSE, TAVISTOCK SQUARE, LONDON WC1H 9JR, ENGLAND, pp 25–25
Kappos L, Leppert D, Tinbergen J et al (2012) Risk of infections and malignancies after treatment with anti-CD20 monoclonal antibodies: ocrelizumab and rituximab in rheumatoid arthritis and multiple sclerosis. Mult Scler 18:424
Avivi I, Stroopinsky D, Katz T (2013) Anti-CD20 monoclonal antibodies: beyond B-cells. Blood Rev 27:217–223
Huffstutter JE, Taylor J, Schechtman J et al (2011) Single-versus dual-infusion of B-cell-depleting antibody ocrelizumab in rheumatoid arthritis: results from the phase III FEATURE trial. Int J Clin Rheumatol 6:689–696. doi:10.2217/ijr.11.55
Bokarewa M, Lindholm C, Zendjanchi K et al (2007) Efficacy of anti-CD20 treatment in patients with rheumatoid arthritis resistant to a combination of methotrexate/anti-TNF therapy. Scand J Immunol 66:476–483
Stohl W, Gomez-Reino J, Olech E et al (2012) Safety and efficacy of ocrelizumab in combination with methotrexate in MTX-naive subjects with rheumatoid arthritis: the phase III FILM trial. Ann Rheum Dis 71:1289–1296. doi:10.1136/annrheumdis-2011-200706
Chirinos-Rojas C, Ilivanova E, Boyd P, others (2008) The safety and efficacy of ocrelizumab; a humanized anti CD20 antibody administered as a single infusion regimen to patients with active rheumatoid arthritis [abstract OP-0250]. EULAR
Genovese MC, Kaine JL, Kohen MD et al (2006) Safety and clinical activity of ocrelizumab (a humanized antibody targeting C D20 + B cells) in combination with methotrexate (MTX) in moderate-severe rheumatoid arthritis (RA) patients (pts)(Ph I/II ACTION study). Arthritis Rheum 54:S66–S67
Anandarajah AP (2011) Clinical aspects of rheumatoid arthritis: highlights from the 2010 ACR conference (Part II). Int J 6:393–399
Lee YH, Bae S-C, Song GG (2011) The efficacy and safety of rituximab for the treatment of active rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int 31:1493–1499
Vital EM, Rawstron AC, Dass S et al (2011) Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis Rheum 63:603–608
Kappos L, Li D, Calabresi PA et al (2011) Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. The Lancet 378:1779–1787
Hauser SL, Comi GC, Hartung H-P, et al (2015) Efficacy and safety of ocrelizumab in relapsing multiple sclerosis-results of the interferon-beta-1a-controlled, double-blind, Phase III OPERA I and II studies. In: Multiple Sclerosis Journal. SAGE PUBLICATIONS LTD 1 OLIVERS YARD, 55 CITY ROAD, LONDON EC1Y 1SP, ENGLAND, pp 61–62
Morschhauser F, Marlton P, Vitolo U et al (2010) Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol 21:1870–1876
Mysler EF, Spindler AJ, Guzman R et al (2013) Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, Phase III Study. Arthritis Rheum 65:2368–2379
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abushouk, A.I., Ahmed, H., Ismail, A. et al. Safety and efficacy of ocrelizumab in rheumatoid arthritis patients with an inadequate response to methotrexate or tumor necrosis factor inhibitors: a systematic review and meta-analysis. Rheumatol Int 37, 1053–1064 (2017). https://doi.org/10.1007/s00296-017-3675-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3675-8