Abstract
Reports suggest leptin, which was initially described as a hormone that regulates food intake and energy balance, has an intimate relationship, and interacts with the immune system. Leptin consumption in the synovial cavity in patients with rheumatoid arthritis (RA) reported to have protective effect against erosion. To determine the difference in serum leptin and synovial/serum leptin ratio between RA and control and to assess whether these parameters correlate with systemic inflammation in RA. Also, the hypothesis that synovial/serum leptin ratio could be linked to joint erosion in RA was evaluated. The study subjects consisted of 40 consecutive patients with RA, 30 patients of them had knee effusion, and 30 controls. Ten of these controls had acute knee injury and their synovial fluid was obtained for comparison of synovial/serum leptin ratio with patients with RA. The mean serum leptin in patients with RA was significantly higher than controls. Also, the synovial leptin and synovial/serum leptin ratio in the RA patients with effusion was significantly higher than in the 10 control subjects with traumatic effusion. Serum leptin in the 30 RA patients with effusion was higher than the matched synovial leptin. In RA patients with effusion, synovial/serum leptin ratio was also significantly higher in RA patients with erosion than RA patients without erosion. Serum leptin level and synovial/serum leptin ratio are significantly correlated with the RA duration, DAS28, ESR, CRP, TNF-α, and IL-6. Finally, in regression analysis, only the synovial/serum leptin ratio was positively associated with erosion in patients with RA. In RA, there is a significant increase in circulating leptin levels and synovial/serum leptin ratio compared to non-RA controls. Serum leptin and synovial/serum leptin ratio are significantly in erosive RA than non-erosive RA. Both parameters are correlated with disease duration and parameters of RA activity. In regression analysis, only the synovial/serum leptin ratio was positively associated with erosion in patients with RA. These results indicate that local consumption of leptin in the joint cavity has a protective role against the destructive course of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is the most common form of chronic inflammatory joint disease leading to cartilage and bone destruction. The inflammatory process causes diffuse thickening and hyperplasia of the RA synovium. It is infiltrated with numerous inflammatory cells that produce several proinflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-α [1].
Proinflammatory cytokines increase circulating leptin concentrations, [2] and leptin, in turn, potentiates cytokine release from inflammatory cells [3]. Leptin and its receptors share structural and functional similarities with IL-6 family and their receptors [4]. In addition, leptin stimulates T-cell proliferation and protects T cells from corticosteroid-induced apoptosis [5–7]. Moreover, fasting patients with RA show an evidence of improvement in clinical and biological parameters of disease activity, associated with a decrease in leptin serum concentration [8]. These reports suggest that leptin, which was initially described as a hormone that regulates food intake and energy balance, has an intimate relationship and interacts with the immune system. But leptin also exerts anti-inflammatory activities, which is attributed to increased IL-4 production and release of IL-1 receptor antagonist [6].
Conflicting data on leptin role in RA have been presented by previous studies. In patients with RA, leptin concentrations were found to be similar [9–11], lower [12], or higher [13–15], when compared with those in healthy controls. It was also reported that serum leptin levels were unrelated to disease activity [5, 7, 9, 11, 16]. However, a positive correlation between serum leptin concentration and the value of DAS28, ESR, and the number of tender joints was also reported [17].
Previous studies reported that synovial fluid leptin levels were significantly lower than in matched plasma samples. The association of increased local consumption of leptin in the joint cavity with non-erosive disease emerged the hypothesis that leptin could play a protective role against the destructive course of RA [13]. We suggest that synovial/serum leptin ratio could be more relevant to reflect the extent of local leptin consumption in the joint.
This study was designed to determine the difference in serum leptin and synovial/serum leptin ratio between RA and control and to assess whether these parameters correlate with systemic inflammation in RA. Also, the hypothesis that synovial/serum leptin ratio could be linked to joint erosion in RA was evaluated.
Subjects and methods
This prospective study was conducted on 40 consecutive patients with RA who were attending the outpatient clinic of Rheumatology and Rehabilitation, Mansoura University Hospital, Egypt, between December 2009 and March 2010. All patients fulfilled the American College of Rheumatology criteria for RA diagnosis [18]. There were 5 male and 35 female patients with RA, their age ranged from 19 to 36 years. Disease duration ranged from 1.5 to 20 years. Knee effusion was found in 30 patients. Subjects with signs of a malnutrition syndrome (with body mass index (BMI) <20 kg/m2 and albumin level <3 g/dl), obese patients (with BMI >25 kg/m2), and patients having diabetes mellitus, hypertension, or any systemic disease other than RA were excluded from the study.
Thirty individuals (4 men and 26 women) were chosen to serve as a control group. They were matched for age and sex with the RA group. Among these controls, 10 individuals had recent traumatic knee effusion and their synovial fluid was obtained for comparison of synovial/serum leptin ratio with RA patients. Written consent was obtained from every subject in this study after approval of this study from local ethical committee.
Patient assessment included a standardized interview, physical examination, laboratory tests, and a review of medical records. Besides age and sex, we determined duration of RA and presence or absence of extra-articular manifestations (extra-AM). The RA activity is estimated using disease activity score 28 (DAS28 score) [19]. DAS28, consisting of four items, namely the number of swollen and tender joints, the visual analogue scale of patients’ assessment of their general health, and the erythrocyte sedimentation rate (ESR) in the first hour, gives an absolute number reflecting disease activity. In patients and controls, body weight and body height were measured to obtain the BMI (the result of dividing body weight (in kilograms) by square height (in meters).
At the time of synovial fluid and blood sampling, all the patients were receiving non-steroidal anti-inflammatory drugs with at least one disease-modifying antirheumatic drug (DMARD) as follows: 29 patients (72.5%) used methotrexate (MTX), 31 (77.5%) patients used hydroxychloroquine (HQ), combination of MTX and HQ was used by 27 patients (67.5%), 9 patients (22.5%) used leflunamide (LEF). In 5 patients (12.5%), low-dose prednisone (5–10 mg/day) was used in the treatment.
Collection and preparation of samples
After 12-h fasting, venous blood samples were collected from every subject by sterile venipuncture on the same day of history taking and clinical examination. Two millilitres of blood was delivered into citrated tube for ESR determination. The separated serum was kept frozen at −20° C till the time of estimation of serum C-reactive protein (CRP), IL6, TNF-α, and leptin. Aspiration of the joint fluid was done for the patients with effusion under complete aseptic conditions in the same day of blood sampling. In all cases with effusion, synovial fluid was obtained from knee joints. Synovial fluids were collected into tubes and centrifuged for 20 min, and the supernatant fluid was stored at −20°C until analyzed.
Measurement of serum leptin
Serum and synovial leptin levels were determined by a by a specific ELISA using materials and protocols supplied by the provider. In this assay, The DSL-10-23100 ACTIVE® Human Leptin ELISA (Biosource International Inc., Europe SA) was used (sandwich-type immunoassay); standards, controls, and unknown serum were incubated in the wells that have been coated with anti-human leptin antibody. After incubation and washing, the wells are treated with another anti-human leptin detection antibody labeled with the enzyme horseradish peroxidase. After a second incubation and washing step, the wells are incubated with the substrate tetramethylbenzidine. An acidic stopping solution is then added. The absorbance measured is directly proportional to the concentration of human leptin present and presented as ng/ml. After determining the serum and synovial leptin values, the synovial/serum leptin ratio was calculated in these patients.
IL-6 and TNF-α assays
Solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) kits were used for estimation of serum levels of IL-6 (MEDGENIX IL-6 EASIA kit, Flureus; Belgium) and TNF-α (Biosource International, California, USA).
CRP assay
Quantitative assay of CRP kit was supplied by Turbox® CRP, Orion Diagnostica. Turbox assay for CRP is a liquid-phase immuno-precipitation assay with nephelometric detection. Antiserum to CRP is diluted and added to patient serum. The light scattering caused by antigen–antibody complexes is measured after incubation. The resulting light scattering is directly proportional to the CRP concentration in the sample.
Radiographic examination
Recent radiographs of the hands and feet were obtained for all the patients. The presence of bone erosions, defined as the loss of cortical definition at the joint, was recorded in proximal interphalangeal, metacarpophalangeal, carpus, wrist, and metatarsophalangeal joints. The presence of one erosion was sufficient to fulfill the requirement of an erosive disease.
Statistical analysis
All statistical analyses were performed using SPSS for windows version 10.0 (SPSS, Chicago, IL). Continuous data were expressed as mean ± standard deviation (SD), while categorical data were expressed in number and percentage. The differences among cases and controls were determined by independent samples t test for continuous data or chi square test for categorical data. Paired t test was used to compare the synovial fluid leptin levels with the matched plasma samples. Independent samples t test is also used to further assess if there are differences in serum leptin level and synovial/serum leptin ratio between men and women, in RA patients with and without extra-AM, patients with and without erosion, RF-positive and RF-negative patients, patients using and not using methotrexate (MTX), and patients using or not using steroid. The correlation of serum leptin and synovial/serum leptin ratio with age, duration of RA, DAS28, CRP, IL-6, and TNF-α was calculated using Spearman correlation test. Multivariable regressions were modeled to examine the association between erosion and the serum leptin level, synovial leptin level and the synovial/serum leptin ratio. The 95% confidence intervals (CI) for the difference in means were calculated. Statistical significance was set at P < 0.05.
Results
Patients and controls
Table 1 demonstrates the characteristics of the patients with RA and controls participated in this study. Despite that the patients with RA and controls were similar as regards age, gender, and BMI, the leptin measures are significantly elevated in the patients with RA compared to control group. The mean serum leptin in patients with RA was 32.4 ± 10.1 ng/ml compared to 21.2 ± 7.6 ng/ml in the controls (mean difference, 11.2, 95% CI: 6.88, 15.52, P < 0.001). Also, the synovial leptin in the RA patients with effusion (n = 30) was 23.9 ± 5.5 ng/ml compared to 16.7 ± 5.2 ng/ml in the 10 control subjects with traumatic effusion (mean difference 7.2, 95% CI: 3.31, 11.09, P < 0.001). As regards the synovial/serum leptin ratio, it was also higher in the patients with RA than in controls (0.82 ± 0.13 ng/ml versus 0.61 ± 0.15 ng/ml, respectively). This result was significant (mean difference, 0.21, 95% CI: 0.11, 0.31, P < 0.001).
Figure 1 showed that the serum leptin in the 30 RA patients with effusion was higher than the matched synovial leptin (29.6 ± 6.2 vs. 23.9 ± 5.5 ng/ml, respectively). This difference was significant (P < 0.001).
Association of leptin serum level and synovial/serum leptin ratio with gender and RA-related characters
Table 2 showed that gender significantly affected serum leptin as well as synovial/serum leptin ratio. Among patients with RA, the serum leptin in the women was 33.8 ± 9.9 ng/ml compared with 22.8 ± 5.3 ng/ml in the males (mean difference, 11.00, 95% CI: 2.08, 19.92, P = 0.0206). The synovial/serum leptin ratio in female patients with RA was 0.82 ± 0.12 compared to 0.66 ± 0.14 ng/ml in male patients (mean difference 0.16, 95% CI: 0.03, 0.3, P = 0.021).
From 40 patients with RA, 28 patients had destructive joint disease (erosive RA), and the remaining 12 patients had no changes of the joints as judged by the recent radiologic examination of the hands and feet (non-erosive RA). Table 2 showed also that serum leptin was significantly higher in RA patients with radiologic evidence of erosion (34.7 ± 10.4 ng/ml) than RA patients without erosion (26.8 ± 6.6 ng/ml) (mean difference 7.9, 95% CI: 1.50, 14.30, P = 0.0204). In RA patients with effusion, synovial/serum leptin ratio was also significantly higher in RA patients with radiologic evidence of erosion (0.85 ± 0.09) than RA patients without radiologic evidence of erosion (0.70 ± 0.16) (mean difference 0.15, 95% CI: 0.06, 0.24, P = 0.002).
Table 3 shows that serum leptin level is significantly correlated with the RA duration (r = 0.372, P = 0.018), DAS28 (r = 0.415, P = 0.008), ESR (r = 0.337, P = 0.033), CRP (r = 0.340, P = 0.032), TNF-α (r = 0.382, P = 0.015), and IL-6 (r = 0.343, P = 0.030). This table also shows that synovial/serum leptin ratio is significantly correlated with the RA duration (r = 0.433, P = 0.017), DAS28 (r = 0.494, P = 0.005), ESR (r = 0.400, P = 0.010), CRP (r = 0.410, P = 0.024), TNF-α (r = 0.475, P = 0.008), and IL-6 (r = 0.421, P = 0.020). Serum leptin and synovial/serum leptin ratio did not correlate significantly with age.
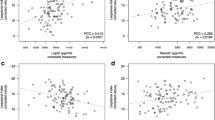
Finally, in regression analysis, only the synovial/serum leptin ratio was positively associated with erosion in patients with RA (Tables 3, 4 and Figs. 2, 3).
Discussion
The main findings of this study are (a) patients with RA have significantly increased serum leptin levels and synovial/serum leptin ratio compared to healthy subjects, (b) synovial fluid leptin levels are significantly lower than the matched plasma samples and (c) presence of radiologic erosion is associated with higher serum leptin and synovial/serum leptin ratio. Serum leptin levels and synovial/serum leptin ratio are significantly higher in women when compared to men. We found a positive significant correlation between serum leptin levels and synovial/serum leptin ratio and RA duration, DAS28, ESR, CRP, serum TNF-α, and serum IL-6.
Many studies reported that serum leptin concentration is not correlated with RA duration [7, 10, 11]. Despite this, according to our results, serum leptin level and synovial/serum leptin ratio is directly correlated with RA duration. Bokarewa et al. [13] found that there is a gradual increase in leptin concentrations with the duration of RA.
In patients with RA, Otero et al. [20] found considerably higher plasma levels of fat-derived hormones (leptin, adiponectin, and visfatin) than in healthy controls. They also observed that CRP levels significantly correlated with leptin. They suggested that adipocytokines play a role in the proinflammatory state in RA.
Parallel to our findings, Targońska Stepniak et al. [17] found a positive correlation between leptin levels and value of DAS28, CRP, and number of tender joints.
On the other hand, no correlations were reported between that serum leptin levels and activity, defined with the value of ESR, CRP [5, 9, 11], DAS [5, 21], number of tender and swollen joints [5], or TNF-α [5, 7].
In the study of Bokarewa et al. [13], it was found that leptin production was significantly increased in patients with RA compared with healthy controls, also, in patients with RA, leptin plasma concentrations were higher than in synovial fluid samples obtained simultaneously and higher than in control plasma samples. Their results showed that decreased leptin level in synovial fluid was associated with non-erosive joint disease.
Our results showed that serum leptin is significantly higher in patients with erosive RA than in patients with non-erosive RA. The significantly higher synovial/serum leptin ratio in erosive than the non-erosive disease indicates that patients with non-erosive RA displayed a more pronounced difference between the levels of leptin in plasma and its matched synovial fluid samples, while in the group of patients with erosive RA, the difference between leptin levels in plasma and synovial fluid was to a lesser extent. This result seems to confirm the hypothesis that local joint consumption of leptin plays a protective role against the destructive course of RA [13].
Interestingly, the most important observation was the association of the synovial/serum ratio only with the presence of erosion in patients with RA in the multivariate regression analyses. This result suggests that the synovial/serum leptin ratio is more appropriate measure that reflects the extent of local consumption of the leptin in the joint than the synovial leptin level.
The positive association between the consumption of leptin in the synovial cavity and the absence of bone destruction may be due to leptin-mediated down regulation of the erosive process in the joints. Leptin induces IL-1 receptor antagonist production [22] Treatment of patients with RA with IL-1 receptor antagonist has been recently proved to stop the joint destructive process [23]. Additionally, chondrocytes and fibroblasts are sensitive to leptin stimulation and responding with increased proliferation [24]. On the other hand, increased levels of proinflammatory cytokines such as TNF-α, locally in the synovial fluid of patients with RA may down regulate local production of leptin inside the joint, aggravating leptin deficiency, a condition that may predisposes to development of erosion [25, 26].
Our data showed that serum leptin levels in patients with RA were higher in women than men. This is consistent with the results of Gunaydin et al. [5]. On the other hand, Bokarewa et al. [13] found that leptin levels were not related to sex. This sex difference was reported not to be related to sex hormones or fat distribution, but possibly to differences in hypothalamic regulation of leptin production or in adipose tissue biologic characteristics [27]. Besides, there are studies reporting that gonadal steroids have an effect on circulating leptin levels. Testosterone inhibited the expression of this hormone, whereas it was increased by ovarian sex steroids [28].
In our study, serum leptin levels did not differ in patients on different DMARD treatment and in patients receiving and not receiving glucocorticoids. Gunaydin et al. [5] found no difference of serum leptin levels in patients receiving MTX versus those not on MTX. In contrast, Bokarewa et al. [13] observed significantly higher leptin levels in patients treated with MTX in comparison with patients receiving other DMARDs. This difference could not be explained by known folic acid’s increasing effect on leptin levels because there was no significant difference in folic acid levels between groups. Folic acid, routinely taken with MTX, could be responsible for the increase in leptin levels in patients treated with MTX. Supplementation of folic acid, used in weight-reducing programmes, correlated positively with an increase in leptin levels [13].
Gunaydin et al. [5] reported higher plasma leptin levels in patients on glucocorticoids, but the difference did not reach statistical significance. Bokarewa et al. [13] found no difference in leptin levels in users and non-users of glucocorticoids. That could be explained by the relatively low doses of glucocorticoids. Glucocorticoids have been shown to increase leptin production in adipose cells [5].
In conclusion, patients with RA had a significant increase in circulating leptin levels and synovial/serum leptin ratio compared to non-RA controls. Serum leptin and synovial/serum leptin ratio are significantly higher in women than in men and in erosive RA than non-erosive RA. Both parameters are correlated with disease duration and parameters of RA activity, suggesting that some important dependence exists between the risk of aggressive course of RA and increased leptin levels. In regression analysis, only the synovial/serum leptin ratio was positively associated with erosion in patients with RA. These results indicate that local consumption of leptin in the joint cavity has a protective role against the destructive course of RA.
Recommendations
Longitudinal studies including patients with early RA are still needed to clarify the potential influence of leptin on disease outcome, and particularly the progression of joint damage. Likewise, it would certainly be worth performing similar studies in other immune-mediated inflammatory rheumatic diseases.
References
Zwerina J, Redlich K, Schett G, Smolen JS (2005) Pathogenesis of rheumatoid arthritis: targeting cytokines. Ann N Y Acad Sci 10:51716–51729
Faggioni R, Feingold KR, Grunfeld C (2001) Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J 15:2565–2571
Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ et al (1998) (Leptin regulates proinflammatory immune responses. FASEB J 12:57–65
Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H et al (1996) The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA 93:8374–8378
Gunaydin R, Kaya T, Atay A, Olmez N, Hur A, Koseoglu M (2006) Serum leptin levels in rheumatoid arthritis and relationship with d activity. South Med J 99:1078–1083
Bernotiene E, Palmer G, Gabay C (2006) The role of lepin in innate and adaptive immune response. Arthritis Res Ther 8:217–226
Wisiowska M, Rok M, Jaszczyk B, Stdpiej K, Cicha M (2007) Serum leptin in rheumatoid arthritis. Rheumatol Int 27:947–954
Fraser DA, Thoen J, Reseland JE, Forre O, Kjeldsen-Kragh J (1999) Decreased CD4 + lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol 18:394–401
Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K (2002) Serum leptin levels in patients with rheumatoid arthritis are correlated with body mass index. Rinsho Byori 50:524–527
Popa C, Netea MG, Radstake TRDS, van Riel PL, Barrera P, van der Meer JWM (2005) Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis 64:1195–1199
Hizmetli S, Kisa M, Gokalp N, Bakici MZ (2007) Are plasma and synovial Xuid leptin levels correlated with disease activity in rheumatoid arthritis? Rheumatol Int 27:335–338
Tokarczyk-Knapik A, Nowicki M, Wyromlak J (2002) The relation between plasma leptin concentration and body fat mass in patients with rheumatoid arthritis. Pol Arch Med Wewn 108:761–767
Bokarewa M, Bokarew D, Hultgren O, Tarkowski A (2003) Leptin consumption in the inXamed joints of patients with rheumatoid arthritis. Ann Rheum Dis 62:952–956
Toussirot E, Nguyen NU, Dumoulin G, Aubin F, Cedoz JP, Wendling D (2005) Relationship between growth hormone-IGF-1–IGFBP-3 axis and serum leptin levels with bone mass and body composition in patients with rheumatoid arthritis. Rheumatology (Oxford) 44:120–125
Salazar-Paramo M, Gonzalez-Ortiz M, Gonzalez-Lopez L, Sanchez-Ortis A, Valera-Gonzalez IC, Martinez-Abundis E, Balcazar-Munoz BR, Garcia-Gonzalez A, Gamez-Nava JI (2001) Serum leptin levels in patients with rheumatoid arthritis. J Clin Rheumatol 7:57–59
Senolt L, Housa D, Vernerová Z, Jirásek T, Svobodová R, Veigl D, Anderlová K, Müller-Ladner U, Pavelka K, Haluzik M (2007) Resistin in rheumatoid arthritis synovial tissue, synovial fluid and serum. Ann Rheum Dis 66:458–463
Targonska-Stepniak B, Majdan M, Dryglewska M (2008) Leptin serum levels in rheumatoid arthritis patients: relation to disease duration and activity. Rheumatol Int 28:585–591
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–48
Otero M, Lago R, Gómez R, Lago F, Dieguez C, Gómez-Reino JJ, Gualillo O (2006) Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis 65:1198–1201
Seven A, Güzel S, Aslan M, Hamuryudan V (2009) Serum and synovial fluid leptin levels and markers of inflammation in rheumatoid arthritis. Rheumatol Int 29(7):743–747 (Epub 2008 Nov 14)
Gabay C, Dreyer M, Pellegrinelli N, Chicheportiche R, Meier CA (2001) Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab 86:783–791
Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW et al (2002) Treatment of rheumatoid arthritis with anakinra a recombinant human interleukin-1 receptor antagonist in combination with methotrexate: results of a twenty-four-week multicenter randomized double-blind placebo-controlled trial. Arthritis Rheum; 46:614–624
Maor G, Rochwerger M, Segev Y, Phillip M (2002) Leptin acts as a growth factor on the chondrocytes of skeletal growth centers. J Bone Miner Res 17:1034–1043
Bruun JM, Pedersen SB, Kristensen K, Richelsen B (2002) Effects of pro-inflammatory cytokines and chemokines on leptin production in human adipose tissue in vitro. Mol Cell Endocrinol 190:91–99
Finck BN, Johnson RW (2002) Anti-inflammatory agents inhibit the induction of leptin by tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol 282:R1429–R1435
Perfetto F, Tarquini R, Simonini G, Bindi G, Mancuso F, Guiducci S, Matucci-Cerinic M, Falcini F (2005) Circulating leptin levels in juvenile idiopathic arthritis: a marker of nutritional status? Ann Rheum Dis 64(1):149–152
Blum WF, Englaro P, Hanitsch S, Juul A, Hertel NT, Müller J, Skakkebaek NE, Heiman ML, Birkett M, Attanasio AM, Kiess W, Rascher W (1997) Plasma Leptin Levels in Healthy Children and Adolescents: Dependence on Body Mass Index, Body Fat Mass, Gender, Pubertal Stage, and Testosterone. Clin Endocrinol Metab 2(9):2904–2910
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olama, S.M., Senna, M.K. & Elarman, M. Synovial/Serum leptin ratio in rheumatoid arthritis: the association with activity and erosion. Rheumatol Int 32, 683–690 (2012). https://doi.org/10.1007/s00296-010-1698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-010-1698-5