Abstract
Leptin is a peptide hormone that has an essential role in the regulation of body weight by inhibiting food intake and stimulating energy expenditure. The role of leptin in the modulation of the immune response and inflammation has been regarded as important. In rheumatoid arthritis (RA) patients it was reported that fasting leads to an improvement of clinical and biological measures of disease activity, which was associated with a marked decrease in serum leptin. These features suggest that leptin may also influence the inflammatory mechanisms of arthritis in humans. In this study we assessed serum leptin levels in RA and osteoarthritis (OA) patients and found a correlation between serum leptin level and other markers as well as bone mass density changes, activity of disease, disease duration and the age of the patients. The blood was collected from 30 RA and 30 OA patients who constituted the control group. Serum leptin level was determined using the DRG Leptin ELISA Kit—a solid phase enzyme—linked immunosorbent assay based on the sandwich principle. The serum level of leptin in RA patients ranged from 1.8 to 81.1 ng/ml and median value was 11.2. There was a positive correlation between body mass index (BMI) of RA patients and serum level of leptin (correlation coefficients Spearman’s r = 0.81). According to correlation coefficients, serum leptin level is independent of age of RA patients, stage of disease, number of painful and swollen joints, duration of morning stiffness, disease duration as well as value of titre of the Waaler–Rose, disease activity score (DAS 28) value and presence of rheumatoid nodules. There was a negative correlation between serum leptin level and glomerular filtration rate (GFR). No correlation between the serum leptin level and T-score was found. An influence of steroid treatment on the serum leptin level was not shown. The median serum leptin level in OA patients was 9.2 ng/ml. There was a positive correlation between body mass index of OA patients and serum level of leptin (correlation coefficients Spearman’s r = 0.57). No correlation was found between serum leptin level and patient’s age, duration of disease and value of laboratory data. There were no correlations between serum leptin level and visual analogue pain scale (VAS) for the lower-limb afflicted patients as well as stage of disease according to Kellgren and Lawrence’s score in OA patients. There was a negative correlation between serum leptin level and T-score value in OA patients (r = −0.58, P < 0.05). No statistically significant differences were found between serum leptin levels for RA and OA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptin is a peptide hormone that has an essential role in the regulation of body weight by inhibiting food intake and stimulating energy expenditure. Leptin also has many other effects, such as the regulation of endocrine function, reproduction, and immunity [1, 2]. Leptin-deficient (ob./ob.) mice and leptin-receptor deficient (db/db) mice are not only severely obese but also display hormonal imbalances, abnormalities in thermoregulation, infertility, and immune and haematopoietic defects. The role of leptin in the modulation of the immune response and inflammation has recently been considered as important. The latest studies have shown that the long isoform of the leptin receptor (OB.-Rb) is expressed in T and B cells and indicated that leptin has dirrect effects on lymphocytes [3–5]. Leptin stimulates the proliferation of T cells in vitro, to promote T helper (Th) 1 responses, and to protect T cells from corticosteroid-induced apoptosis [3–5]. Starvation and malnutrition are connected with changes of the immune response and thymic atrophy, which can be reversed by leptin administration [3–5]. Despite the evidence for the direct effects of leptin on T cells in vitro, the connection between leptin deficiency and immune defects in vivo is more complex. In rodents leptin deficiency after starvation is connected with increased glucocorticoid levels, and decreased levels of thyroid and growth hormone, each of which may mediate immune suppression [6]. In humans, congenital leptin deficiency is associated with a decreased number of circulating CD4 + T cells, and impaired T cell proliferation and cytokine release, all of which can be changed by the administration of recombinant leptin [7]. Leptin-deficient mice are protected from inflammation mediated by T and B cells in different disease models. In experimental animal models, leptin levels are increased by inflammatory and infectious stimuli, such as lipopolysaccharide (LPS), turpentine, and proinflammatory cytokines [8, 9].
In vitro, leptin stimulates both pro- and anti-inflammatory cytokine productions in monocytes and macrophages [10, 11]. Leptin deficient mice show an increased sensitivity to TNF alpha and LPS-induced lethality, indicating that a functional leptin system confers protective anti-inflammatory effects against this systemic proinflammatory stimuli [12].
Rheumatoid arthritis (RA) patients after fasting showed an improvement of different clinical and biological measures of disease activity, which was associated with a marked decrease in serum leptin and a shift towards Th2 cytokine production [13]. These features suggest that leptin may also influence the inflammatory mechanisms of arthritis in humans through the induction of Th1 responses.
The aim of this study was to assess serum leptin levels in RA and OA patients and find a correlation between serum leptin level and other markers as well as bone mass density (BMD) changes, activity of disease, disease duration and the age of the patients.
Materials and methods
Patients
A total of 30 consecutive outpatients aged 38–74 years (average age: 60.2 ± 9.4) with definite or classic RA American Rheumatism Association (ARA) criteria [14] attending the Rheumatology Outpatients Department of the Central Clinical Hospital in Warsaw were included in the study. In the RA group there were 30 women. According to Steinbrocker et al.’s criteria [15] 22 (73%) patients were in stage 3 and 8 (27%) in stage 4 of the disease. According to Larsen and Dale criteria [16] 12 (40%) patients were in stage 2, 13 (43%) in stage 3 and 5 (17%) in stage 4 of the disease. Fifteen patients had a disease activity score (DAS 28) value [17] between 3.31 and 4.8, and 15 patients had a DAS 28 value over 4.8 (maximum 6.21). All of the patients were shown radiologically to have geodes and erosions on the joint surfaces and on bones close to the joints. The number of painful joints ranges from 4 to 12 (average 7.0 ± 1.9), and the number of swollen joints from 2 to 6 (average 3.3 ± 1.4). The presence of subcutaneous nodules was observed in 11 (37%) patients. Morning stiffness duration lasted on average 1.0 ± 0.6 and ranged from 0.5 to 5 h. The duration of disease ranged from 2 to 35 years (mean 14.6 ± 9.4 years).
A Waaler–Rose titre below 8 was confirmed in 18 (60%) patients (seronegatives patients) and over 8 was confirmed in 12 (seropositives). Virtually all of the RA patients were taking nonsteroidal anti-inflammatory drugs (NSAIDs), 11 were taking leflunomide, 9 methotrexate, 3 sulphasalazine, 5 antimalarials and 2 azathioprine. Twenty-six (86.6%) patients were treated with low-dose prednisolone.
The control group 30 patients aged 41–79 years (average age: 62.3 ± 10.0 years); there were 30 women who attended the clinic at the same time with osteoarthrosis of the knee according to the ARA [18] criteria. The duration of disease ranged from 1 to 25 years (mean: 9.4 ± 6.5 years. All the patients had bilateral knee osteoarthritis. The average Western Ontario and McMaster Universities (WOMAC) index pain scale for the lower limbs [19] was 9.5 ± 2.3 ng/ml. According to Kellgren and Lawrance’s score [20], 19 patients were in stages 1 and 2, and 11 were in stages 3 and 4.
Blood was collected from 30 RA and 30 OA patients. Blood samples were collected into tubes without additives. The blood was allowed to clot for 2 h at room temperature and was then centrifuged and stored at −80°C. The study was approved by the local Research Ethics Committee.
Leptin measurements
Serum leptin level was determined using the DRG Leptin ELISA Kit—a solid phase enzyme-linked immunosorbent assay based on the sandwich principle [21]. The microtiter wells are coated with a monoclonal directed towards a unique antigenic site as a leptin molecule.
An aliquot of patient sample containing endogenous leptin is incubated in the coated well with a specyfic rabbit anti-leptin antibody. A sandwich complex is formed. After incubation the unbound material is washed off and a rabbit peroxidase conjugate is added for detection of the bound leptin. Having added the substrate solution, the intensity of colour developed is proportional to the concentration of leptin in the patient sample.
Other analyses
The following data were estimated in all of the patients: the morphology composition of peripheral blood, a level of complete protein and its fractions in serum, ESR, GRF, phosphatase alkaline, electrolytes (Ca, P) and the serum leptin level. In RA patients, serological examinations were made to discover the presence of rheumatoid factor in immunoglobulins (IgM), using Waaler–Rose’s test, with over 8 as a positive level. Overall pain was assessed on a 100 mm visual analogue scale (VAS). The modified 28 join disease activity score (DAS 28)—including 28 tender and swollen join counts, patient’s self report global status on VAS, and erythrocyte sedimentation rate (ESR) according to Westergren—was used to evaluate clinical disease activity.
Radiographic method
All OA patients were examined by standing posteroanterior radiographs of both knee joints in semiflexion with fluoroscopic guidance and with almost all load on the examined leg.
Densitometry examinations
Efficacy measurement included the femur (“total hip” and trochanter). BMD of the total hip T-score less than –2.5 was considered osteoporosis. Dual energy X-ray absorptiometry (DEXA) was the method for measuring BMD using a Lunar Prodigy (GE Medical Systems, Madison, WI, USA).
Statistical calculations
Statistical analyses were done using a statistical software package SPSS/PC+. The following tests were applied [22]:
-
1.
Wilcoxon score and Median tests were used to compare continuous variables whose distributions in the sample were not gaussian.
-
2.
Student’s t-test was used for the comparison of two population means whose distribution was normal.
-
3.
Correlation analysis was performed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient.
The serum levels of leptin were not normally distributed, and were therefore examined with the non-parametric tests and Spearman’s rank correlation coefficient.
All reported P-values are two-sided and a type 1 error level of 0.05 was used [22].
Results
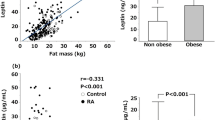
The serum level of leptin in RA patients ranged from 1.8 to 81.1 ng/ml and median value was 11.2 ng/ml. There was a positive correlation between BMI of RA patients and serum level of leptin (correlation coefficients Spearman’s r = 0.81 (Fig. 1). According to correlation coefficients, serum leptin level is independent of age of RA patients (r = 0.15, P = NS), number of painful joints (r = 0.06, P = NS), swollen joints (r = 0.00, P = NS), duration of morning stiffness (r = 0.01, P = NS) and disease duration (r = −0.01, P = NS). There was a lack of difference between median value of serum leptin level and different stages of disease. In patients with stage 3 disease according to Steinbrocker the level of lepin was 11.2 ng/ml (from 1.8 to 41.3), in patients with stage 4 disease it was 11.3 ng/ml (from 4.1 to 81.1). In patients with stage 2 diseases according to Larsen and Dale it was 11.2 ng/ml (from 3.5 to 35.2), in patients with stage 3 disease, it was 8.8 ng/ml (from 1.8 to 39.8) and in those with stage 4 disease, it was 13.8 ng/ml (from 4.1 to 81.1). There was a lack of difference between average median of serum leptin level and titre of the Waaler–Rose test too. The serum leptin level of seropositive patients was 11.3 ng/ml (from 1.8 to 81.1) and of seronegatives 11.2 ng/ml (from 3.5 to 39.8) (Fig. 2). No differences were found between BMI, average ages and stages of disease in observed groups. There was no correlation between DAS 28 value and serum level of leptin (correlation coefficients Spearman’s r = −0.01, P = NS (Fig. 3). There was not a statistically significant difference between median value of leptin level for low and high DAS 28 values. For DAS 28 between 3.3 and 4.8 the serum leptin level was 12.4 ng/ml (ranged from 3.5 to 33.4) and for DAS 28 between 4.84 and 6.21 the serum leptin levels was 8.1 ng/ml (ranged form 1.8 to 81.1).
An influence of rheumatoid nodule presence on the serum leptin level was not shown. The median value of serum leptin level for the patients without rheumatoid nodules was 12.5 ng/ml (ranged from 3.5 to 81.7) and with rheumatoid nodules 8.8 ng/ml (ranged from 1.8 to 41.3) which is not statistically significant (Table 1).
There was a negative correlation between serum leptin level and GRF (Fig. 4). Correlation coefficients of laboratory data with the serum leptin level are presented in Table 2.
Patients with an ESR value below 20 mm/h compared with patients with an ESR value over 60 mm/h, the serum leptin level was observed to be lower (median 12.4 vs. 23.3 ng/ml) but not significantly (Table 3).
The serum leptin levels were examined in different patient subgroups: in the subgroups with normal laboratory results and in the subgroups with abnormal laboratory results (Table 4). Lower leptin levels in sera were observed in patients with decreased haemoglobin values and increased thrombocyte number in comparison with group where these values were normal. However, lower leptin levels can be connected with lower BMI observed in this subgroup. For all patients, some of the examination results did not exceed normal values; therefore, they are not mentioned in Table 4 (Fig. 5).
Table 1 shows the values of laboratory data and BMD in RA patients. No correlation between the serum leptin level and T-score was found.
An influence of steroid treatment on the serum leptin level was not shown. The serum leptin level (median) for patients not treated with steroids was 12.9 ng/ml (ranged from 8.8 to 41.3), which is not statistically significant in comparison with the serum leptin level in patients under steroid treatment, which was 11.2 ng/ml (ranged from 1.8 to 81.1).
The median serum leptin level in OA patients was 9.2 ng/ml and minimum and maximum values were, respectively, 1.5 and 39.2 ng/ml. There was a positive correlation between body mass index (BMI) of OA patients and serum level of leptin (correlation coefficients Spearman’s r = 0.57). No correlation was found between serum leptin level and patients’ age (r = 0.20, P = NS), duration of disease (r = 0.08, P = NS) and value of laboratory data.
There was no correlation between serum leptin level and VAS index pain scale for the lower-limb afflicted patients (r = 0.18, P = NS). No statistically significant differences were found between serum leptin level and stage according to Kellgren and Lawrence’s score in OA patients. For stages 1 and 2, the average leptin level was 8.8 ng/ml and for stages 3 and 4 it was 10.0 ng/ml. There was a negative correlation between serum leptin level and T-score value in OA patients (r = −0.58, P < 0.05).
No statistically significant differences were found between serum leptin levels for RA and OA.
Table 5 shows the serum leptin level depending on duration of disease. There were no differences in the serum leptin level value between RA and OA and duration of disease.
Discussion
It has been confirmed that leptin has influences on myelopoesis, limphopoesis, increased number of macrophages, granulocytes and activation leptin receptors on the macrophage surfaces, which consequently increase the possibility of phagocytosis and proinflammatory cytokines synthesis (TNF, IL-6, IL-12) [23]. On the other hand, proinflammatory cytokines overproduced in chronic inflammatory diseases stimulate ob. gene expression and leptin secretion. It may be one of the mechanisms in which catabolic processes are increases and loss of body weight in RA patients, because cytokines (IL-1, IL-2, IL-6, IL-8, interferon gamma and TNF) are responsible for development of leptin secretion [24].
Roubenoff et al. [25] find, that body mass of RA patients is about 13% lower than body mass of healthy people. It is probably the effect of higher catabolism caused by proinflammatory cytokines (IL-1 and TNF alpha), lower physical activity of these patients and decreased food intake. Interleukine 1 strong proinflammatory interleukine decreased thirst by influence of central nervous regulation of food intake. High concentration of IL-1 beta and TNF alpha during the flare of rheumatoid arthritis accelerates basic metabolism cause hypermetabolism, which lead to decrease of body mass gradual organism deterioration. Saraff et al. [26] proved that in the process of chronic inflammatory diseases proinflammatory cytokines IL-1 and TNF alpha increase ob. gene expression and leptin secretion.
In the present study leptin level in RA patients was 11.2 ng/ml on average and positively correlated with BMI. No essential statistically significant differences were found in serum leptin level between RA and OA patients. Nor was any correlation found between serum leptin level and age of RA patients, stage of disease, number of painful and swollen joints, duration of morning stiffness, disease duration as well as value of titre of the Waaler–Rose, DAS 28 value and presence of rheumatoid nodules. There was a negative correlation between serum leptin level and GRF only. No correlation between the serum leptin level and BMD (T-score) was found. An influence of steroid treatment on the serum leptin level was not shown.
Last year’s Japanese research [26] did not confirm statistically significant difference between leptin concentrations in sera of rheumatoid arthritis patients and healthy individuals. The discoveries did not reveal the dependence between leptin concentration and rheumatoid arthritis activity, either. Anders et al. [27] presented similar results.
Nishiya et al. [28] and Anders et al. [27] showed that serum levels of leptin were not increased in patients with RA as compared with controls. In addition, they did not find any correlation between leptin levels and either clinical or biological signs of disease activity, whereas a positive correlation was seen between leptin and BMI or the percentage of body fat.
Evidence exists, however, for the presence of lower [29] as well as higher [30] leptin levels in RA patients compared to control group of healthy individuals. Another group [29] reported lower plasma leptin levels in patients with RA than in controls. In patients with RA from this cohort, leptin did not correlate with BMI, CRP, total fat mass, or disease activity score.
Bokarewa et al. [30] report increased plasma levels of leptin in 76 patients with RA as compared with healthy controls. The authors also observed that circulating plasma concentrations of leptin were significantly higher than leptin levels in matched synovial fluid samples and that the difference between plasma and synovial fluid was particularly pronounced in non-erosive arthritis. The authors concluded that intra-articular leptin might exert a protective effect against the destructive course of RA. Unfortunately, besides the measurement of C-reactive protein (CRP) levels, the authors did not give any indication about disease activity and body mass index (BMI) of the subject studied. This makes interpretation of the results difficult, because plasma leptin levels are strongly influenced by BMI and fat mass.
In osteoarthritis the influence of leptin on chondrocite proliferation, and anabolism was revealed, too [31].
One of the most important factors influencing bone density is body mass [32]. High mineral bone density accompanies high body mass in obese people [33]. Such people rarely experience osteoporosis [33]. Obese postmenopausal women present slower bone mass loss in comparison to women with normal or small body mass [33]. The correlation was shown between bone mineral density and adipose tissue mass [34]. Therefore the amount of adipose tissue can play an important role in mineral bone density regulation. In our OA patients negative correlation between leptin blood concentration and BMD was observed.
Between 1990 and 2000, examinations showed that leptin stimulates bone marrow stem cells to diferentiate into osteoblasts and inhibits adipocytes’ degeneration [35]. Osteoblasts and adipocytes originate from the same bone marrow cells [36]. It was shown that leptin stimulates the production of alkaline phosphatase, osteokalcin and type I procallagen by osteoblasts [37]. It also increases mineralisation of matrix [37]. Thomas et al. [37] showed that leptin could serve as a regulation factor between adipose tissue and skeletal system.
Ducy et al. [38], however, showed that leptin inhibits bone generation in mice. Mice deficient in leptin or with mutation in leptin receptor characterised with increased bone generation [38]. Leptin infusion into the brain chambers caused the decrease of bone mass [38]. The results of these examinations suggest that leptin can be a factor inhibiting bone growth through it’ influence on central nervous system.
Matkovic et al. [39] showed statistically significant positive correlation between leptynemia and bone mineral density in pubescent women. The authors suggest that leptin has a protective effect in osteoporosis through the increase of bone volume in young people and the increase of peak bone mass [39].
Gulding and Taylor [40] showed statistically significant positive correlation between serum leptin concentration and bone mineral density measured by DEXA in postmenopausal women.
Odabasi et al. [41] also revealed statistically significant positive correlation between leptin concentration in serum and bone mineral density measured by DEXA in postmenopausal women with osteoporosis.
Unlike the other cited works, Rauch et al. [42] did not prove any statistical correlation between serum leptin level and mineral density of forearm bone examined by tomography in postmenopausal women. These authors did not find either any statistical correlation between serum leptin levels and bone marker formation such as osteocalcin, C-terminal propeptide of procolagen type I, bone-specific alkaline phosphatase levels in serum and bone marker resorption such as deoxypyridinoline level in urine [42].
As investigations show, leptin partocipation in osteoporosis pathogenesis although probable, was not, however, definitely proved.
Leptin can exert modulatory effect on different stages of inflammation, yet further researches are necessary to confirm its role in the development and perpetuation of the process.
References
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
Fantuzzi G, Faggioni R (2000) Leptin in the regulation of immunity, inflammation, and, hematopoiesis. J Leukoc Biol 68:437–446
Lord GM, Matarese G, Howard JK et al (1998) Leptin modulates the T-cell immune response and reserves starvation-induced immunosuppression. Nature 394:897–901
Busso N, So A, Chobaz-Peclat V et al (2002) Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol 169:875–872
Howard JK, Lor GM, Matarese G et al (1999) Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob./ob. mice. J Clin Invest 104:1051–1059
Flier JS (1998) Lowered leptin slims immune response. Nat Med 4:1124–1125
Farooqi IS, Matarese G, Lord GM et al (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110:1093–1103
Saraf P, Fantuzzi G, Fuler J et al (1997) Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med 185:171–175
Faggioni R, Fantuzzi G, Fuler J et al (1998) Il-1 beta mediates leptin induction during inflammation. Am J Physiol 274:R 204–208
Loffreda S, Yang SQ, Lin HZ et al (1998) Leptin regulates proinflammatory immune responses. FASEB J 12:577–65
Gabay C, Dreyer M, Pellegrineli N et al (2001) Leptin directly induces the secretion of interleulin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab 86:783–791
Takahashi N, Waelput W, Guisez Y (1999) Leptin is an endogenous protective protein against the toxicity exerted by tumor necrosis factor. J Exp Med 189:207–212
Fraser DA, Thoen J, Reseland JE et al (1999) Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol 18:394–401
Arnett FC (1989) Revised criteria for the classification of rheumatoid arthritis. Bul Rheum Dis 38:1–6
Steinbrocker O, Treger H, Cornelius H (1949) Therapeutic criteria in rheumatoid arthritis. JAMA 140:659–662
Larsen A, Dale K, Eek M (1977) Radiographic eveluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn 18:481–491
Van der Heijde DMFM, van’t Hof MA, van Riel PLCM et al (1993) Development of a disease activity score based on judgement in clinical practice by rheumatologists. J Rheumatol 20:579–581
Altman R (1991) Classification of disease:osteoarthritis. Semin Arthritis Rheum 21(Suppl 2):40–47
Bellamy N, Buchanan WW, Goldsmith CH, et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Kellgren JH, Lawrence SJ (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Conside RV, Sinha MK, Heiman ML (1996) Serum immunoreactive—leptin concentrations in normal weight and obese humans. New Engl J Med 334:292–295
Robert R, Sokal F, Rohlf J (1986) Biometry. Freeman, New York, pp 354–359, 691–714
Gainsford T, Willson TA, Metcalf D et al (1996) Leptin can induce proliferation, differentiation and functional activation of hemopoeitic cells. Proc Natl Acad Sci USA 93:14564–14568
Charles P, Elliott MJ, Davis D (1999) Regulation of cytokines, cytokine inhibitors and acute-phase proteins folowing anti-TNF alpha therapy in rheumatoid arthritis. J Immunol 163:1521–1528
Roubenoff R, Roubenoff RA, Ward LM et al (1994) Rheumatoid cachexia; cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 93:2379–2389
Sarraf P, Frederich RC, Turner EM (1997) Multiple cytokines and acute inflammation raise mouse leptin levels. Potential role in inflammatory anorexia. J Exp Med 185:171–175
Anders HJ, Rihl M, Heufelder A et al (1999) Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48:745–748
Nishiya K, Nishiiyama M, Chang A et al (2002) Serum leptin in patients with rheumatoid arthritis are correlated with body mass index. Abstract Rinshobyori 50:524–527
Tokarczuk-Knapik A, Nowicki M, Wyroslak J (2002) The relation between plasma leptin concentration and body fat mass in patients with rheumatoid arthritis. Pol Arch Med Wewn 108:761–767
Bokarewa M, Bokarew D, Hultgren O et al (2003) Leptin consumption in the inflammed joits of patients with rheumatoid arthritis. Ann Rheum Dis 62:952–956
Dumond H, Presle N, Terlain B (2003) Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 11:3118–3129
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417
Albala C, Yanez M, Devoto E et al (1996) Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes 20:1027–1032
Reid IR, Ames R, Evans M et al (1992) Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab 75:45–51
Bai Y, Zhang S, Kim LS et al (1996) Obese gene expression alters the ability of 30A5 preadipocytes to respond to lipogenic hormones. J Biol Chem 271:13939–13942
Aubin JE, Turksen K, Heershe JNM (1993) Osteoblastic cell linkage (w) Celular and molecular biology of bone, (red) M. Noda, Academic Press, New York, pp 1–45
Thomas T, Gori F, Khosla S et al (1999) Leptin acts on human marrow stromal cels to enhance differentiation to osteoblast and to inhibit differentiation to adipocytes. Endocrinology 140:1630–1638
Ducy P, Amling M, Taskeda S et al (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Matkovic V, Ilich J, Skugor M et al (1997) Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 82:3239–3245
Gulding A, Taylor RW (1998) Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int 63:456–458
Odabasi E, Ozata M, Turan M et al (2000) Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol 142:170–173
Rauch F, Blum WF, Klein K et al (1998) Does leptin have an effect on bone in adult women? Calcif Tissue Int 63:453–455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wisłowska, M., Rok, M., Jaszczyk, B. et al. Serum leptin in rheumatoid arthritis. Rheumatol Int 27, 947–954 (2007). https://doi.org/10.1007/s00296-007-0335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0335-4