Abstract
Leptin is an adypocyte derivated peptide hormone that plays a major role in preventing obesity development by the effects at the hypothalamic level. In our study leptin levels of 41 rheumatoid arthritis (RA) patients and 25 healthy subjects as control group were assessed. Synovial fluid from 21 RA patients were collected to detect leptin levels. Synovial fluid and plasma leptin levels were analysed and correlated with RA duration, ESR, CRP, X ray changes (erosive or non-erosive disease) and negative or positive test for rheumatoid factor. There wasn’t any significant difference at plasma leptin levels between RA patients (3.91 ± 6.15) and control group (4.94 ± 6.44) (p > 0.05). Plasma leptin levels were correlated with body mass index (BMI) in both healthy subjects and RA patients (r = 0.37; p = 0.018). Therefore in RA patients, plasma and synovial fluid leptin levels were not correlated with disease duration, ESR, CRP, negative or positive test for rheumatoid factor and erosive or non-erosive disease (p > 0.05). In conclusion leptin is correlated with BMI both in RA patients and healthy individuals but no considerable relation with disease activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptin is a peptide hormone that plays a major role in, food intake regulation and energy metabolism and preventing obesity development by the effects at the hypothalamic level [1–3]. It has also been shown that leptin has other effects on reproduction, osteogenesis, hematopoiesis, angiogenesis, immunity and regulation of endocrin function [3–8]. Adipose tissue is the predominant release organ of leptin, moreover placenta, pituitary gland and gastric epithelium are the other source organs [9–11].
Leptin is a 16 kD weight, cytokine-like hormone [4]. Plasma leptin level is correlated with BMI and percent body fat. Moreover, hormones, free fatty acids, catecholamines and acute exposure to cold can affect leptin levels [16–21]. Hormonal imbalances, severe obesity, thermoregulation abnormalities immune and hematopoietic defects were detected at leptin deficient (ob/ob) and leptin receptor deficient (db/db) mice [3]. Leptin synthesis at human shows detectable increase as a response to acute inflammation, sepsis, secretion of inflammatory mediators such as IL-1 and TNF-α [9].
Recent studies have suggested the potential role of leptin in modulating the immune response and leptin’s inflammatory or anti-inflammatory effects [3, 4, 12]. Monocyte/macrophage cells are one of the target cells that leptin situmulates to release pro-inflammatory cytokines, IL-6 and TNF-α, on the other hand leptin shows anti-inflammatory effects by inducing IL-1 receptor antagonist secretion [3, 4, 12, 13]. Congenital leptin deficiency in human, is associated with a decreased number of circulating CD4 ( + ) T cells and impaired T cell proliferation and cytokine release, this has been reversed by the administration of recombinant leptin [3].
Leptin levels were found to increase acutely with infectious stimulation and inflammation in experimental animal models [2, 3], however, the effect of chronic inflammatory diseases such as rheumatoid arthritis (RA) on serum leptin levels is still unknown [14].
Our aim in this study was to detect plasma and synovial fluid leptin levels and to evaluate their correlation with disease activation parameters.
Subjects and methods
Patients
RA-diagnosed 41 patients, diagnosed according to ACR criteria [15] attended our Rheumatology Clinic in Cumhuriyet University Medicine Faculty. They were intended for our study and 25 healthy volunteers were chosen as the control group. Hand and feet radiographs of all patients were obtained. The presence of one or more erosion was accepted to be sufficient to say erosive disease.
The medications included methotrexate (20 patients), leflunomide (14 patients ), sulfasalazine (13 patients), cyclosporine (1 patient), low dose steroids (12 patients), non-steroidal anti-inflammatory drugs (19 patients).
Patients with history of diabetes mellitus, infection or unstable weight history were excluded from the study.
Laboratory methods
Venous blood samples for measurement of plasma leptin were collected, between 9 and 10 A.M. from all individuals. Synovial fluid was aspirated from knee joints of 21 patients aseptically. Blood and synovial fluid samples were collected in vacutainer tubes containing K3 EDTA, centrifuged at 3,000 rpm for 15 min, frozen and stored at −20°C until use. Leptin levels were detected using ELISA (Gritols-Tritures) method.
Serum levels of C reactive protein (CRP) were measured by standard nephelometry, with established normal range 0–5 mg/l. The erythrocyte sedimentation rate (ESR) was measured by the Westergren method with an established normal range 0–20 mm/1st h.
Statistical analysis
Results are expressed as means ± standard deviation. Independent samples test, Mann–Whitney U test and Pearson correlation analysis are used for evaluating obtained data. A value of p < 0.05 was considered to be significant.
Results
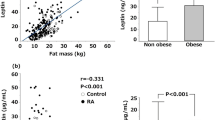
Study was made with 41 RA patients (33 women, 8 men, mean age 49.31 ± 11.76) and 25 healthy volunteers (19 women, 6 men, mean age 51.08 ± 13.39). There wasn’t any statistically significant difference at age, gender, body mass index (BMI) and plasma leptin levels between RA and control group (p > 0.05) (Table 1).
Disease duration of patients with destructive joint disease (erosive RA) was longer then non-erosive group (p = 0.013). Moreover, there was no statistically significant difference at age, ESR, CRP and plasma and synovial fluid leptin levels when erosive group compared with non-erosive group (p > 0.05) (Table 2).
The mean age of patients with positive test for rheumatoid factor (RF) in our study was found to be higher than patients with negative test for RF (p = 0.004), however, no significant difference was detected in BMI, disease duration, ESR, CRP, plasma and synovial fluid leptin levels of these two groups (p > 0.05) (Table 2).
Plasma leptin levels were correlated with BMI in both healthy subjects and RA patients (r = 0.37; p = 0.018). However, in RA patients, plasma and synovial fluid leptin levels were not correlated with disease duration, ESR, CRP, negative or positive test for rheumatoid factor and erosive or non-erosive disease (p > 0.05).
Discussion
In our study RA patients who have a chronical disease with high cytokine levels we didn’t observe any difference at plasma leptin levels between healthy volunteers and RA patients. Although, there was a correlation between BMI and plasma leptin levels in both groups.
We found some previous studies that plasma leptin levels have been researched in RA patients. In Nishiya et al.’s [22] study with 49 RA patients and 20 healthy individuals, there was no significant difference at plasma leptin levels between patients with RA and control group. Although it was noticed in their study that plasma leptin levels were correlated with BMI but it wasn’t correlated with ESR and CRP levels. In another study with similar results, 58 RA patients were evaluated and it has been shown that plasma leptin levels were correlated with percent body fat but not correlated with disease activity [14]. Bokarewa et al. [12] detected RA patients to have higher plasma leptin levels than healthy individuals. The authors observed that circulating plasma concentrations of leptin were significantly higher than matched synovial fluid samples and the difference between plasma and synovial fluid was particularly pronounced in non-erosive arthritis and also noticed that plasma leptin levels were decreased in patients using disease-modifying antirheumatic drugs.
These different results of clinical studies evaluating leptin levels in RA patients suggest us further studies in RA patients with methodological additions including parameters such as BMI, percent body fat, thus smoking [23], catecholamines [21], hormonal factors [16–18] are needed to clarify leptin’s effects in RA.
In conclusion we found plasma leptin levels to be correlated with BMI either in patients or in control group and plasma and synovial fluid leptin level of RA patients were not correlated, with disease duration, ESR, CRP, negative or positive test for rheumatoid factor and having erosive or non-erosive disease.
References
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Considine RV, Sinha MK, Heinman ML, Kriavciunas A, Stepans TW, Nyce MR, et al (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Palmer G, Gabay C (2003) A role for leptin in rheumatic disease. Ann Rheum Dis 62:913–915
Juge-Aubry CE, Meier CA (2002) Immunomodulatory actions of leptin. Mol Cell Endocrinol 194:1–7
Bennett BD, Solar GP, Yuan JO, Thomas GR (1996) A role for leptin and its cognate receptor in hematopoiesis. Curr Biol 6:1170–1180
Chehab FF, Lim ME, Lu R (1996) Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet 12:318–320
Pasco JA, Henry JM, Kotowicz MA, Coller GR, Ball MJ, Ugoni AM, Nicholson GC (2001) Serum leptin levels are associated with bone mass in non-obese women. J Clin Endocrinol Metab 86:1884–1887
Bouloumie A, Drexler HC, Lafontan M, Busse R (1998) Leptin, the product of Ob gene, promotes angiogenesis. Circ Res 83:1059–1066
Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gomez-Reino JJ, Gualillo O (2005) Leptin, from fat to inflammation: old questions and new insights. FEBS Lett 579:295–301
Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG (1997) Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc Natl Acad Sci 94:11073–11078
Morash B, Li A, Murphy PR, Wilkinson M, Ur E (1999) Leptin gene expression in the brain and pituitary gland. Endocrinology 140:5995–5998
Bokarewa M, Bokarew D, Hultgren O, Tarkowski A (2003) Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis 62:952–956
Gabay C, Dreyer M, Pellegrinelli M, Chicheportiche R, Meier CA (2001) Leptin directly induces the secretion of interleukin 1 receptor antagonist in human monocytes. J Clin Endocrinol Metab 86:783–791
Anders HJ, Rihl M, Heufelder A, Oliver L, Schattenkirchner M (1999) Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48:745–748
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Cusin I, Sainsbury A, Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B (1995) The ob gene and insulin. A relationship leading to clues to the understanding of obesity. Diabetes 44:1467–1470
Slieker LJ, Sloop KW, Surface PL, Kriauciunas A, LaQuier F, Manetta J, Bue-Valleskey J, Stephens TW (1996) Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J Biol Chem 271:5301–5304
Gualillo O, Lago F, Garcia M, Menendez C, Senaris R, Cassanueva FF, Dieguez C (1999) Prolactin stimulates leptin secretion by rat white adipose tissue. Endocrinology 140:5149–5153
Rentsch J, Chiesi M (1996) Regulation of ob gene mRNA levels in cultured adipocytes. FEBS Lett 379:55–59
Trayhurn P, Duncan JS, Rayner D (1995) Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J 311:729–733
Scriba D, Aprath-Husmann I, Blum WF, Hauner H (2000) Catecholamines suppress leptin release from in vitro differentiated subcutaneous human adipocytes in primary culture via beta 1- and beta 2-adrenergic receptors. Eur J Endocrinol 143:439–445
Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K (2002) Serum leptin levels in patients with rheumatoid arthritis are correlated with body mass index. Rinsho Byori 50:524–527
Reselan JE, Mundal HH, Hollung K, Haugen F, Zahid N, Anderssen SA, Drevon CA (2005) Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins Leukot Essent Fatty Acids 73:43–49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hizmetli, S., Kisa, M., Gokalp, N. et al. Are plasma and synovial fluid leptin levels correlated with disease activity in rheumatoid arthritis ?. Rheumatol Int 27, 335–338 (2007). https://doi.org/10.1007/s00296-006-0264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-006-0264-7