Abstract
Pulmonary arterial hypertension (PAH) is a severe clinical and pathophysiologic syndrome with no effective treatment at present. Mycophenolate mofetil (MMF), a prodrug of mycophenolic acid (MPA), has been applied widely to the treatment of connective tissue diseases with the effect of immunosuppressant. Its anti-proliferation has been found recently. Thereby, we tried to examine the effects of MMF on rats with PAH which was induced by monocrotaline injection, so as to investigate the mechanisms of treatment on PAH by MMF. The results substantiated that MMF therapy can alleviate thickening of pulmonary arterial walls and inhibit abnormal vascular remodeling, and the MPA concentrations which demonstrated efficacy in this study are within clinical applicable range, suggesting huge potentiality of MMF in the treatment of human PAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary arterial hypertension (PAH) is a severe clinical and pathophysiologic syndrome, which degrades life quality and may even endanger life [1]. The main pathomechanisms of PAH include vasoconstriction, vascular remodeling and thrombosis. Vascular remodeling, led by pulmonary arterial wall thickening and fibrosis, is a key factor to the failure of treatment in PAH, whereas the pulmonary arteriolar wall thickening and fibrosis are significantly influenced by proliferation and dysfunction of vascular smooth muscle cells (VSMCs). At present, therapies such as endothelin receptor blockers, prostacyclin analogs, nitrogen monoxidum, etc. can hardly reverse the progression of PAH [2], mainly because they cannot inhibit pulmonary arterial wall thickening and fibrosis effectively. To improve the prognosis of patients with PAH, it is necessary to look for an effective drug that can actively inhibit the thickening and fibrosis of pulmonary arterial wall. Researches on transplantation and arteriosclerosis revealed that mycophenolic acid (MPA) functioned effectively in the inhibition of proliferation of vascular endothelial cells, VSMCs and fibroblasts, besides lymphocyte [3, 4]. Clinical researches have also noticed that treatment of prednisone combined with mycophenolate mofetil (MMF), a prodrug of MPA, to patients with systemic sclerosis complicated PAH, resulted in obviously descending pulmonary arterial pressure. So MMF is expected to shed light on the creation of a new drug which can inhibit the thickening and fibrosis of pulmonary arterial wall, thus reverse the progress of PAH.
In this study, we observed the effect of treatment with MMF on pulmonary arterial pressure, right ventricle index (RVI) and morphological changes of pulmonary artery of monocrotaline (MCT)-induced PAH in rats; investigated the effects of MPA on the rat pulmonary arterial smooth muscle cells (PASMCs) at cellular level, and cell generation cycle was used to illustrate the inhibitory mechanisms, to confirm the effects of MMF on PAH and provide a pre-clinical account for MMF treatment in PAH.
Materials and methods
Animals, reagents, and equipment
The animals used in this study were male Sprague-Dawley rats (180–220 g body weight, Grade II). MMF was purchased from Roche Bioscience. MPA was obtained from Sigma-Aldrich Biotechnology Inc.
Equipment involved Multichannel physiologic recorder R6000, Pressure transducer P23 ID, Biological signal-amplifier AP-621G, Computer pressure analysis software Acq 3.8.1, Pathological Image analysis system IM50, Flow cytometry Epics-XL II, etc.
MCT-induced PAH rat models
Rats were randomly divided into the following four groups: control group (n = 8), MCT group (n = 8), low-dose MMF group (MMF 20 mg/kg day, n = 8), high-dose MMF group (MMF 40 mg/kg day, n = 8). The control group received an intraperitoneal injection of an equal volume of a solvent mixed by 75% alcohol and saline in a 2:8 volume ratio, and all the other three groups received an intraperitoneal injection of MCT (60 mg/kg). 24 h after the injection, the low-dose and high-dose MMF groups were given MMF 20 mg/kg day and MMF 40 mg/kg day, respectively, through intragastric administration, whereas the control and MCT groups received equal volume of distilled water. This injection was repeated in the following 21 days. Rats had free access to standard chow and water, and were maintained in a controlled temperature (21–22°C)/relative humidity (50–70%) environment on a 12 h light and dark cycle. On the day 22, rats were forbidden to food but water, and then they were killed for evaluation. The PAH rat models’ criteria are systolic pulmonary arterial pressure (SPAP) exceeding 50 mmHg and RVI exceeding 0.33.
Hemodynamic assessment
Rats were anesthetized by intraperitoneal injection of urethane. SPAP was measured by right heart catheterization approach. The catheter inserted into the RV was connected to multichannel physiologic recorder via the pressure transducer. First, calibrating the equipment, fasten the right heart catheter when seeing rising pressure baseline and pulmonary artery waveform. The hemodynamic signals were saved and analyzed with the digital acquisition system, and the average SPAP was calculated. PAH was defined as an elevation of SPAP to more than 50 mmHg [5].
RVI determination
After the rats were executed, collecting their hearts, cutting off vessels and atriums, carefully cutting and keeping the free walls of right ventricle (RV), left ventricles (LV) and interventricular septums remained. The free walls of RV, LV plus interventricular septums were weighed, respectively, and an index of RV hypertrophy was calculated using the following formula: the ratio of the RV (free wall) weight to LV weight plus septum (RV/LV + S). Right ventricular hypertrophy was defined as a RVI over 0.33 [6].
Histology and morphology
Lung tissues were fixed in formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE). Morphometric analysis was performed in pulmonary arterioles with external diameter (ED) less than 200 μm by two persons and the median value is calculated. To rule out the influence of size difference of arterioles, three pulmonary arterioles with ED < 50 μm, five ones with ED 50–100 μm, two ones with ED 101–200 μm were chosen for observation of each rat. In total, ten vessels were counted and the average was calculated.
Image analysis
Transverse sections of arterioles in every pulmonary section of all groups were performed using image analysis system to calculate the following six indexes: (1) total vascular area (inside the adventitia), (2) vascular lumina area (inside the intima), (3) vascular external diameter (mean diameter of adventitia), (4) vascular lumina diameter (mean diameter of intima), (5) vascular wall thickness (the shortest vertical dimension between intima and adventitia), (6) vascular media thickness (the shortest vertical dimension of elastic layer); and to calculate the three ratios below: (1) vascular lumina area/total vascular area (%), named the ratio of vascular lumina area (LA%), (2) vascular wall thickness/vascular external diameter (%), named the ratio of vascular wall thickness (WT%), (3) vascular media thickness/vascular wall thickness (%), named the ratio of vascular media thickness (MT%).
Culture and identification of rat PASMCs
Rat PASMCs of male Sprague-Dawley rats (200–250 g body weight) were grown in culture plates and maintained in complete medium consisting of DMEM supplemented with 10% FCS, referring to Xue’s approach. Cells were identified as smooth muscle cells (SMCs) if they presented typical “hill and valley” growth pattern and positive reaction with antibodies against SMA. SMCs of third to fifth generation in culture were used.
Growth curve
Rat PASMCs were inoculated in 24-well plates at 5,000 cells per well, adhering to the wall of the well, cultured in serum-free medium for 24 h, and then cultured in DMEM supplemented with 10% FCS, adding MPA with final concentration of 0, 10−7, 10−6, 10−5, 10−4 M, respectively, which were named control group, low-dose MPA group, middle dose MPA group, high-dose MPA group and super high-dose MPA group (the same below), respectively, and each group occupied four wells. Cell numbers were measured with a cytometer for everyday, and so were the successive 8 days. The growth curves were drawn based on these data. The same process of drawing the growth curves repeated three times, calculating the average.
Viability of cells
Rat PASMCs were inoculated in 96-well plates at 2,000 cells per well. Serum-free DMEM was added when the cells taking up 60% of the wall, and the plates were placed still for 24 h so that the cell growth was inhibited to G0 phase. DMEM supplemented with 10% FCS was added then. There were four groups: (1) control group, (2) low-dose MPA group (MPA 10−7 M), (3) middle dose MPA group (MPA 10−6 M), (4) high-dose MMF group (MPA 10−5 M), and each group occupied eight wells. MTT tests were used to detect cellular survival value [7]. This experiment had been repeated three times.
Cell cycle and proliferation index
Rat PASMCs were inoculated into six-well plates at 100,000 cells per well, cultured in serum-free medium for 24 h, so that the cell growth was inhibited to G0 phase. Adding MPA into the well with final concentration of 0, 10−7, 10−6, 10−5 M, and maintaining for another 24 h. After fixation in 70% ethanol overnight, incubation in propidium iodide and RNase A for 30 min, DNA contents were measured by a flow cytometer, and the fractions of G1, S, G2M phases in cell cycle as well as the proliferation index were calculated.
Statistical analysis
All statistical data were reported as mean ± SD. Data were analyzed by analysis of variance (ANOVA), paired-sample t test, and multiple linear stepwise regression analysis, using SPSS (version 11.5), with P < 0.05 considered as significant.
Results
MCT-induced PAH rat models
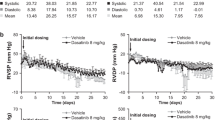
Two of the 32 rats that were given MCT died (one in MCT group, one in low MMF group), on days 15 and 19, respectively, and were excluded from the analysis. In the MCP group, SPAP elevated significantly (61.25 ± 9.60 vs. 27.25 ± 7.82 mmHg, P = 0.000), RVI increased significantly (0.4878 ± 0.0983 vs. 0.2686 ± 0.0464, P = 0.000) compared with the control group. As SPAP in the MCT group exceeding 50 mmHg and RVI exceeding 0.33, we successfully raised the MCT-induced PAH model in rats.
Hemodynamic parameters
In the high-dose and low-dose MMF groups, SPAP was lower than that in the MCT group (42.63 ± 7.63 vs. 61.25 ± 9.60 mmHg, P = 0.000, and 52.00 ± 8.94 vs. 61.25 ± 9.60 mmHg, P = 0.039, respectively). SPAP in the high-dose MMF group was lower than that in the low-dose group (42.63 ± 7.63 vs. 52.00 ± 8.94 mmHg, P = 0.037). All differences showed statistical significance (P < 0.05) (Table 1).
RVI
RVI in the high-dose and low-dose MMF groups was lower than that in the MCT group (0.2984 ± 0.0510 vs. 0.4878 ± 0.0983, P = 0.000, and 0.3855 ± 0.0985 vs. 0.4878 ± 0.0983, P = 0.014, respectively). RVI in the high-dose MMF group was lower than that in the low-dose group (0.2984 ± 0.0510 vs. 0.3855 ± 0.0985, P = 0.032 < 0.05). All differences showed statistical significance (P < 0.05) (Table 1).
Histology and morphology
In the control group, the structures of lung tissue, the inner walls and SMCs of media of pulmonary arterioles were normal (Fig. 1). The infiltration of inflammatory cells had not been observed in lung parenchyma and the surround of vessels (Figs. 2, 3). In the MCT group, the structures of lung tissue were in disorder and lumina areas of pulmonary arterioles decreased significantly with vascular walls thickening markedly and the density of cell nucleus in pulmonary arterioles increased predominantly (Fig. 1). A great deal of inflammatory cells gathered along vascular walls around the pulmonary arterioles in girdle shaped (Fig. 2). Alveolar walls thickened obviously and a great deal of inflammatory cells infiltrated into alveolar walls (Fig. 3).
In the low-dose MMF group, the structures of lung tissue and lumina areas of pulmonary arterioles were generally normal. The walls of pulmonary arterioles thickened in a mild or moderate way (Fig. 1). Inflammatory cells around the pulmonary arterioles infiltrated into the tissue spread in line (Fig. 2). Alveolar wall thickening attenuated obviously. Only a few number of inflammatory cells infiltrated into alveolar walls (Fig. 3). Image analyses of pulmonary arterioles in each group were demonstrated in Table 2.
Culture and identification of PASMCs
On the first day, there was no difference of growing cell numbers among the five groups (P > 0.05). From the second day, cell numbers decreased in middle dose MPA group, high-dose MPA group and super high-dose MPA group except low-dose MPA group compared to the control group, and the difference had statistical significance (P < 0.01). Cell numbers were fewer in super high-dose MPA group and high-dose MPA group compared to middle dose MPA group. In contrast, there was no significant difference between super high-dose MPA group and high-dose MPA group in the number of growing cells (P > 0.05) (Fig. 4).
Effect of MPA on the viability of PASMCs
MTT was adopted to test the viability of PASMCs. The optical density (OD) values of 10% FCS group were higher than that of serum-free control group at a wavelength of 570 nm (0.530 ± 0.038 vs. 0.836 ± 0.036, P = 0.000). OD values in low-dose MPA group decreased compared to 10% FCS group (0.800 ± 0.042 vs. 0.836 ± 0.036, P = 0.140). OD values in middle dose MPA group and high-dose MPA group represented to be lower than in 10% FCS group (0.785 ± 0.020 vs. 0.836 ± 0.036, P = 0.017; 0.718 ± 0.054 vs. 0.836 ± 0.036, P = 0.002, respectively). Effect of MPA on the viability of PASMCs was dose dependent (Fig. 5).
Effect of MPA on cell cycle of PASMCs
Tables 3 and 4 and Fig. 6 show the effects of MPA on cell cycle and proliferation index of PASMCs. Compared to the control group, the proliferation index in low-dose MPA group decreased (29.25 ± 2.82 vs. 26.19 ± 3.37, P = 0.087), and kept falling in middle dose MPA group (29.25 ± 2.82 vs. 22.80 ± 0.91, P = 0.001), decreased further in high-dose MPA group (29.25 ± 2.82 vs. 13.91 ± 1.87, P = 0.000), with dose dependency.
Interrelationship between morphological index and SPAP
SPAP positively correlated with RVI (r = 0.862, P = 0.000), WT% (r = 0.912, P = 0.000), MT% (r = 0.880, P = 0.000), and negatively correlated with LA% (r = 0.893, P = 0.000). Interrelationships among WT%, MT% and LA% were analyzed by multiple linear stepwise regression, the regression equation was \( \hat{Y} = - 19.092 - 0.562X_{1} + 1.472X_{2} + 0.590X_{3} \) (r = 0.965, P = 0.000). The absolute standard coefficient was 5.533, 3.134, and 2.624 for WT% (X 2), LA% (X 1), and MT% (X 3), respectively, suggesting that the strength of the three parameters correlated with RVI were in the following sequence: WT%, LA%, and MT%.
Discussion
Pulmonary arterial hypertension is a severe issue in medical treatment and health care at present, for its high rate in morbidity, disability and mortality. The development of PAH can lead to intractable right ventricular failure, degrading life quality and even death [1, 2]; however, no effective treatment for PAH has been established. Connective tissue disease (CTD)-associated PAH was an important ingredient of PAH, the PAH which may occur at any stage of CTD will worsen the prognosis [8]. For many years, PAH researches attached more importance on the vasodilatation and regulation of vascular endothelial cells. Although great progress has been made in this field, they did not completely solve the persistent progressing of PAH. The typical pathological change of PAH is the thickening of pulmonary arteriolar wall in which proliferation, dysfunction of SMCs and muscularization of non-muscular layer arteries (inside the alveolar) take important position. Therefore, pulmonary vascular remodeling is considered as an important factor of the persistency and irreversibility properties of PAH [9]. In recent years, physiopathology of PAH has been developed from a simple vasoconstrictor mode to a neurohumoral proliferative mode [10]. Researches have also turned to reverse the proliferation and fibrosis of VSMCs.
As an immunosuppressant, MMF, which is active component of MPA, is widely used in the transplantation and treatment of autoimmune diseases [11–13]. Researches about transplantation have disclosed that in addition to lymphocyte, MPA is able to inhibit proliferation of non-immunological cells such as vascular endothelial cells, SMCs, fibroblasts [14, 15]. Shimizu found that MPA could prevent transplanted vessels from sclerosis by the inhibition of VSMC proliferation. Findings of Voisard et al. [4] demonstrated that MPA inhibited proliferation of human coronary SMCs in a dose-dependent manner and the effective concentration (0.5 μg/ml) in vitro was below clinically applicable plasma level.
Inspired by researches on transplantation, we hypothesize that MPA has beneficial effect on PAH through inhibiting proliferation of vascular endothelial cells, SMCs and fibroblasts in pulmonary, as well as by its strong anti-inflammatory action. Clinical researches also verified the potent effects of MMF on PAH. Our previous investigations showed a combined therapy of glucocorticoid and MMF can decrease SPAP significantly in patients with SLE associated with PAH. The most favorable clinical outcome up to now is a decrease of 76 mmH2O of SPAP in a SLE-associated PAH patients. The longest course of this treatment has lasted more than 2 years and the patient keeping a stable SPAP (published in another article).
In this study, rats were injected intraperitoneally with MCT to induce PAH. Various doses of MMF were injected at the same time and finally resulting in reduced SPAP and RVI. It suggests that MMF can prevent the development of MCT-induced PAH in rats and attenuate right ventricle hypertrophy.
Moreover, our data also revealed reduced pulmonary lumina areas, controlled the vascular wall thickening, marked mitigated inflammation around the pulmonary arterioles, and lightened structural damage and inflammation of lung tissue. All these results indicated that MMF, in pulmonary arterioles of PAH rat model, can effectively inhibit proliferation of vascular walls, reduce vascular wall thickening, improve stenosis of lumina, inhibit vascular remodeling and the development of alveolitis and pulmonary vasculitis as well. Consequently, the PAH was relieved. In addition, the therapeutic effects of the high-dose MMF group were superior to the low-dose group. Combined with the results of cell experiments, the effect of MMF on SPAP in rat PAH model was confirmed in dose dependency. Moreover, our date demonstrated that the thickening tendencies in vascular wall were effectively controlled by MMF. Yet, further studies are still required. Hypothesis that increased dose of MMF and prolonged course of treatment may be good to the PAH patients needs to be confirmed.
The study showed that there was a favorable linear relationship between SPAP and lumina area ratio, vascular wall thickness ratio, tunica media thickness ratio in PAH rats. The effects of the three parameters on SPAP were in the following sequence: WT%, LA%, and MT%, suggesting that the elevated SPAP, which was induced by pulmonary arterial wall thickening, was relevant to tunica media thickening (mainly posed by vascular smooth muscles), as well as changes to other components in the pulmonary arteriolar wall, such as proliferation of endotheliocytes and fibroblasts, accumulation of extracellular matrix. MMF is not only limited to the inhibition of the proliferation of vascular smooth muscle, but also effects other components in pulmonary arteriolar wall. Researches on this issue are undertaking now.
Several methods were used to investigate the effects of MPA on PASMCs of rats in this study. The study preliminarily confirms that MPA had experimental groundwork in the treatment of PAH. The results showed MPA can inhibit PASMC proliferation in rats in a dose-dependent manner. The 10% FBS-induced PASMC proliferation in the MPA 10−4 M and 10−5 M groups was more than that in the MPA 10−6 M group. The minimum effective drug concentration of MPA was 10−6 M. The direct inhibition of MPA on PASMC suggested that MPA can inhibit vascular wall remodeling of pulmonary arterioles. Researches by Suzuki [16] has also indicated that MPA can inhibit serum-stimulated human PASMC proliferation in a dose-dependent manner (10−8–10−6 M), and our results tallied with it.
Raisanen reported that the ratio between MMF dose with significant inhibitory effect on inflammation and SMC proliferation in vitro and the maximal systemic plasma level of MMF in vivo was 0.58 in artery transplantation rat model, which was far higher than the ratio 0.014 (≥0.5 μg/ml) that between a significant anti-proliferation effect in vitro and the maximal systemic plasma level in vivo, confirming that MMF dose with significant inhibitory effect on SMCs proliferation had clinical applicability [17]. Later, Moon et al. [18] demonstrated that the MPA dose with significant anti-proliferation effect on SMCs of rat aorta was within the clinical applicable scope. It is also reported that clinically applied dose (10−6 M) of MPA could inhibit the proliferation of cultured human arteriolar SMCs [19]. This study found the effective MMF dose with a significant anti-proliferation on serum-stimulated PASMCs was 10−7–10−5 M, which was lower than above-mentioned effective doses, further confirming that the inhibitory effect of MMF on PASMCs has clinical applicability.
This study revealed the effect of MPA on cell cycle of PASMCs as following: an increased percentage of G1 phase and decreased percentage of S, G2M, dividing phases, suggesting the anti-proliferation role of MPA was mainly performed in S, G2M and dividing phase of PASMCs, especially the S phase.
This study substantiated that MPA can inhibit the development of MCT-induced rat PAH effectively. MMF therapy can alleviate thickening of pulmonary arteriolar wall, inhibit abnormal vascular remodeling. MPA inhibits PASMCs proliferation in a dose-dependent manner and thus there are experimental bases of treatment in PAH. The effective concentration of MPA is within clinical applicable scope.
MPA significantly inhibits PASMC proliferation by inhibition of DNA synthesis in cell cycle. These results suggested MPA has a huge potentiality in the treatment of human PAH. The application of MMF in PAH is a pending issue. Therefore, we will do further researches about the effects of MPA on function (proliferation, immigration, contraction, and so on) and phonotype transformation of PASMCs, the effect of MPA on the action mode in animal PAH model and the prospect of clinical application, and try to provide a potential drug against PAH.
References
Farber HW, Loscalzo J (2004) Pulmonary arterial hypertension. N Engl J Med 351:1655–1665. doi:10.1056/NEJMra035488
Puri A, McGoon MD, Kushwaha SS (2007) Pulmonary arterial hypertension: current therapeutic strategies. Nat Clin Pract Cardiovasc Med 4:319–329. doi:10.1038/ncpcardio0890
Shimizu H, Takahashi M, Takeda S et al (2004) Mycophenolate mofetil prevents transplant arteriosclerosis by direct inhibition of vascular smooth muscle cell proliferation. Transplantation 77:1661–1667. doi:10.1097/01.TP.0000127592.13707.B6
Voisard R, Gecquner L, Baur R et al (2005) Antiproliferative profile of sirolimus and mycophenolate mofetil: impact of the SI/MPL ratio. Int J Cardiol 102:435–442. doi:10.1016/j.ijcard.2004.05.039
Lipke DW, Arcot SS, Gillespie MN et al (1993) Temporal alterations in specific basement components in lungs from monocrotaline-treated rats. Am J Respir Cell Mol Biol 9:418–428
Hessel MH, Steendijk P, den Adel B et al (2006) Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol 291:H2424–H2430. doi:10.1152/ajpheart.00369.2006
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi:10.1016/0022-1759(83)90303-4
Coghlan JG, Handler C (2006) Connective tissue associated pulmonary arterial hypertension. Lupus 15:138–142. doi:10.1191/0961203306lu2280rr
Mandegar M, Fung YC, Huang W et al (2004) Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res 68:75–103. doi:10.1016/j.mvr.2004.06.001
Simonneau G, Galiè N, Rubin LJ et al (2004) Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43:5S–12S. doi:10.1016/j.jacc.2004.02.037
Kasitanon N, Petri M, Haas M et al (2008) Mycophenolate mofetil as the primary treatment of membranous lupus nephritis with and without concurrent proliferative disease: a retrospective study of 29 cases. Lupus 17:40–45. doi:10.1177/0961203307085114
Kahu J, Lõhmus A, Ilmoja M et al (2007) Successful rescue therapy with mycophenolate mofetil in kidney transplantation improves the long-term graft survival. Medicina (Kaunas) 43:953–958
Mele TS, Halloran PF (2000) The use of mycophenolate mofetil in transplant recipients. Immunopharmacology 47:215–245. doi:10.1016/S0162-3109(00)00190-9
Huang Y, Liu Z, Huang H et al (2005) Effects of mycophenolic acid on endothelial cells. Int Immunopharmacol 5:1029–1039. doi:10.1016/j.intimp.2005.01.015
Morath C, Zeier M (2003) Review of the antiproliferative properties of mycophenolate mofetil in non-immune cells. Int J Clin Pharmacol Ther 41:465–469
Suzuki C, Takahashi M, Morimoto H et al (2006) Mycophenolate mofetil attenuates pulmonary arterial hypertension in rats. Biochem Biophys Res Commun 349:781–788. doi:10.1016/j.bbrc.2006.08.109
Räisänen-Sokolowski A, Vuoristo P, Myllärniemi M et al (1995) Mycophenolate mofetil (MMF, RS-61443) inhibits inflammation and smooth muscle cell proliferation in rat aortic allografts. Transpl Immunol 3:342–351. doi:10.1016/0966-3274(95)80021-2
Moon JI, Kim YS, Kim MS et al (2000) Effect of cyclosporine, mycophenolic acid, and rapamycin on the proliferation of rat aortic vascular smooth muscle cells: in vitro study. Transplant Proc 32:2026–2027. doi:10.1016/S0041-1345(00)01542-6
Dubus I, Sena S, Labouyrie JP et al (2005) In vitro prevention of cyclosporin-induced cell contraction by mycophenolic acid. Life Sci 77:3366–3374. doi:10.1016/j.lfs.2005.05.050
Acknowledgments
This study was sponsored by National Natural Science Foundation of China and Wu Jieping Medical Foundation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, Y., Li, M., Zhang, Y. et al. The effects and mechanisms of mycophenolate mofetil on pulmonary arterial hypertension in rats. Rheumatol Int 30, 341–348 (2010). https://doi.org/10.1007/s00296-009-0966-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-009-0966-8