Abstract
In a randomized multicenter, double-blind, double-dummy, parallel group study a comparison of the efficacy and safety of 1 μg alfacalcidol to 880 IU vitamin D plus calcium carbonate (1 g calcium) once daily per os was performed on 148 postmenopausal osteoporotic Caucasian patients with normal vitamin D serum levels for 18 months. Bone mineral density (BMD) was measured at baseline, 12 and 18 months. Safety parameters were followed during the entire study period. Sixty-nine (90.8%) in the alfacalcidol group and 67 (93.1%) in the vitamin D group were included in the ITT analysis. Lumbar BMD in the alfacalcidol group increased by 0.017 g/cm2 (2.33%) and 0.021 g/cm2 (2.87%) from baseline (P<0.001) at 12 and 18 months, respectively, whereas in the vitamin D plus calcium group the increase was 0.005 g/cm2 (0.70%) from baseline (N.S.) at both 12 and 18 months. The higher changes from baseline in the alfacalcidol group, as compared to the changes in the vitamin D plus calcium group at both 12 and 18 months, were found to be statistically significant (P=0.018, 0.005). A small increase of mean femoral BMD was achieved in both groups (N.S.). Adverse events were similar in both groups. No significant differences were noted between the groups in serum calcium. In conclusion, alfacalcidol was found to be superior in significantly increasing lumbar BMD as compared to vitamin D plus calcium while safety characteristics were found to be similar in both treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis (PMO) is a disease characterized by increased skeletal fragility and susceptibility to fractures. It is a significant cause of morbidity and even mortality. Estrogen deficiency and changes in vitamin D metabolism are important contributors to the development of PMO. The decrease in calcium absorption can, in part, be attributed to a decrease in serum 1,25-dihydroxyvitamin D (calcitriol; D-hormone). There is, however, also evidence of an age-related impairment in the sensitivity or response of the intestines and other target organs to circulating endogenous D-hormone levels due to the reduction of vitamin D receptors (VDRs) [1]. D-hormone is clearly involved in the bone modeling and remodeling systems, and can, therefore, contribute markedly to bone strength.
D-hormone analogs (alfacalcidol, calcitriol) have been proven to be active in increasing bone mineral density (BMD), and in reducing vertebral and nonvertebral fractures in several prospective, randomized, mainly placebo-controlled studies [2–8]. A recently published meta-analysis conducted by two independent research groups from the USA (The Osteoporosis Methodology Group) and Canada (The Osteoporosis Research Advisory Group) clearly showed the advantageous efficacy of hydroxylated vitamin D (alfacalcidol, calcitriol) versus plain vitamin D [9].
While plain vitamin D does have an important role as a supplementary drug in osteoporosis therapy with agents, such as bisphosphonates or raloxifene, clinical studies with plain vitamin D as a monotherapy have yielded unsatisfactory results in PMO [9, 10]. Gallagher et al. [11] compared the efficacy of D-hormone with a low dose of vitamin D in a randomized, double-blind controlled pilot trial over 2 years in 50 patients with PMO and vertebral fractures: they found a statistically significant difference in vertebral BMD in favor of D-hormones after 18 and 24 months. In a randomized, single-masked, multicenter study, the effects of alfacalcidol treatment and plain vitamin D supplementation on calcium absorption were evaluated in women with radiological evidence of vertebral fractures [12]: fractional calcium absorption increased significantly after 3 months of treatment with alfacalcidol, but remained unchanged after taking plain vitamin D. Other “head to head” studies performed in secondary osteoporosis confirmed the superiority of alfacalcidol as compared to plain vitamin D [13, 14].
Vitamin D + calcium supplementation demonstrated an improvement in digestive calcium absorption, in the suppression of parathyroid hormone synthesis, decreased bone loss and reduced nonvertebral fracture risks in elderly [15, 16]. Such treatment proved to be ineffective in patients with PMO [17]. As for the safety of D-hormone analogs, both alfacalcidol and calcitriol may induce an increase of serum calcium levels and urinary calcium excretion. However, a large Post-Marketing Surveillance Study on osteoporotic patients in Japan proved that there was a very low risk of hypercalcemia, and no kidney stones were reported [18]. In any case, there is still a certain unfounded concern as to whether the Caucasian patients react like the Japanese, especially in countries with high calcium intake. On the other hand, it was reported that by administering similar doses per os, the levels of D-hormone in bone were higher after taking alfacalcidol than those levels observed after taking calcitriol [19].
The aim of the present study was to compare the efficacy and adverse events of alfacalcidol with plain vitamin D in combination with calcium in internationally accepted dosages in patients with established PMO, with or without vertebral fractures.
Material and methods
We recruited 170 osteoporotic postmenopausal women in 11 clinical centers all over Italy for this double-blind, double-dummy, randomized, multicenter parallel group study. Women were eligible for the study if they were between the ages of 55 and 75, had been postmenopausal for at least 5 years, and had a history of at least one prior vertebral fracture confirmed by spinal radiography and/or had a lumbar or femoral BMD T-score <−2.5.
Patients with secondary osteoporosis, other bone diseases, significant concomitant diseases, hypercalcemia, hypercalcuria, treated with drugs that influence bone metabolism (estrogens, progesterone), SERMS, calcitonins, vitamin D and calcium supplements taken for more than 1 month in the previous 3 months; bisphosphonates, fluoride, ipriflavone, glucocorticoids, immunosuppressant agents, anticonvulsants, lithium taken for more than 1 month in the previous 6 months, or with an abnormal vitamin D status (25(OH)D3 in a serum less than 30 nmol/l measured by HPLC) were excluded from the study. All patients gave their written informed consent to participate in the study before enrollment. The protocol was approved by the Ethics Committees of all 11 participating centers.
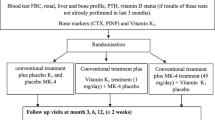
At the screening visit the following evaluations and measurements were taken: medical history, physical examination, complete blood analysis and vital signs. After confirmation of inclusion/exclusion criteria patients were allocated randomly to receive either alfacalcidol 1 μg (capsules, Diseon®/AlphaD ®3 , Teva Pharmaceutical Industries Ltd.) or Placebo of vitamin D3 880 IU + calcium carbonate 2,500 mg or Placebo of alfacalcidol 1 μg and vitamin D3 880 IU + calcium carbonate 2,500 mg (1,000 mg calcium) (sachets, Cacit D3®, Procter & Gamble) in a ratio 1:1. All the drugs were administered once a day. Patients were instructed to take a sachet after lunch and a capsule in the evening.
Patients were examined after 3, 6, 12 and 18 months of treatment. At each visit treatment compliance was checked by counting the number of capsules and sachets, and all adverse events found by the investigator were recorded. Serum calcium, phosphorus and creatinine were measured at each visit.
At the time of screening, BMD was measured at lumbar spine or femoral site by dual-energy X-ray absorptiometry and again after 12 and 18 months of treatment. All the scans were analyzed centrally at the coordinator site.
A vertebral column X-ray was taken at screening (if such an X-ray had not been taken during the 3 months prior to the screening) and again at the end of the study. A vertebral fracture was defined by a reduction of at least 4 mm or 15% of the anterior height, as compared to the posterior height (cuneus fracture), or a reduction of at least 4 mm or 15% of the central height, as compared to the posterior height (biconcave fracture), or by a reduction of all the heights by at least 4 mm or 15%, as compared to the average of the corresponding heights of the upper and lower adjacent vertebrae (compression fracture).
The main efficacy analysis was performed on an intention-to-treat basis, and included all patients who were randomized, who took double-blind study medication, and for whom any assessment of efficacy or serum calcium value was available while taking double-blind study medication.
All demographic and baseline variables were described by statistical characteristics in the randomized and intention-to-treat population. The two-sided Student t test was used to compare mean values of continuous data in the two groups at baseline, while the chi-square test was used for categorical data.
Statistical analysis on BMD values was performed using a linear model on the difference at each visit to the baseline, including baseline values as covariate (ANCOVA model). Missing post-treatment values have been replaced using the last observation after randomization carried forward method (LOCF method).
For serum calcium phosphorus and creatinine, a noninferiority analysis was performed. The values obtained for LOCF were analyzed using a linear model on log-transformed values, including treatment and center as factors, and log-transformed baseline values as covariates.
The two-sided 95% confidence interval (CI) of the difference between the log-transformed adjusted means obtained from the two treatment groups was calculated, and the values obtained for the point estimate and the bounds were transformed back into the original unit, leading to an estimation of the 95% CI of the ratio of the geometric means. Noninferiority is assessed referring to the commonly used 80–125% equivalence range.
A C.R.O., Phidea S.p.a. was in charge of monitoring, data collection and statistical analysis. The Data, Safety and Monitoring Board established by Phidea S.p.a. (Via C. Colombo 1, 20094 Corsico, Milan, Italy) reviewed the conduction of the Study, and also performed the statistical analysis.
Results
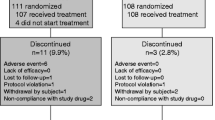
Out of 170 recruited women, 22 did not meet the required criteria. One hundred and forty-eight Caucasian patients entered the double-blind phase of the Study, 76 were randomized to receive alfacalcidol and 72 vitamin D + calcium; 136 of them (91.9%), 69 for the alfacalcidol group and 67 for the vitamin D + calcium group, made up the population for the intention-to-treat analysis (Fig. 1). One patient in the alfacalcidol group and two patients in the vitamin D + calcium group had T-scores of >−2.5, with at least one prior vertebral fracture.
The baseline characteristics of the two groups were similar both in the intention-to-treat population (Table 1), and in the randomized population. A significant difference between the groups was found only in the measurement of the mean lumbar BMD. A remarkable difference was found between the number of patients who did have previous vertebral fractures (N.S., P=0.208).
A total of 102 patients (75% of the intention-to-treat population), 52 patients from the alfacalcidol group, and 50 patients from the vitamin D + calcium group completed the study at the end of 18 months of treatment.
A total of 97 patients, 50 in the alfacalcidol group and 47 in the vitamin D + calcium group, were included in the lumbar BMD analysis. No significant difference was found between the groups at baseline (Table 2). In the alfacalcidol group, mean BMD increased by 0.017 g/cm2 (2.33%) after 12 months treatment versus baseline, and an increment of 0.021 g/cm2 (2.87%) after 18 months treatment versus baseline was found. Both increases were found to be statistically significant (P<0.001 at month 12 vs. baseline and at month 18 vs. baseline).
In the vitamin D + calcium group an increase of 0.005 g/cm2 (0.70%) was found versus baseline after both 12 and 18 months’ treatment, respectively. These increases were not statistically significant. The differences between the mean BMD increase after 12 and 18 months achieved with alfacalcidol versus that achieved with vitamin D + calcium were significant both after 12 and 18 months treatment (P=0.018 at month 12 vs. baseline; P=0.005 at month 18 vs. baseline) (Fig. 2).
The femoral BMD analysis was performed on 92 patients: 44 in the alfacalcidol group and 48 in the vitamin D + calcium group. No significant difference was found between the groups at baseline. A small increase (not statistically significant) of femoral BMD was achieved in both groups (Table 2).
Vertebral fracture evaluations were performed only on patients who had a basal and a final X-ray (42 patients for each treatment group). The number of patients with vertebral fractures at the screening visit was higher in the alfacalcidol group: 11 patients (26.2%) with a total number of 18 fractures in the alfacalcidol group, and 5 patients (11.9%) with a total number of 7 fractures in the vitamin D + calcium group (P=0.208). At the end of the double-blind treatment period the number of patients with vertebral fractures in the alfacalcidol group was 14, with a total of 24 vertebral fractures, while in the vitamin D + calcium group there were 10 patients with vertebral fractures, with a total number of 14 vertebral fractures. Three patients (7.1%) in the alfacalcidol group versus five patients (11.9%) in the vitamin D + calcium group had no fractures at screening, but had at least one vertebral fracture during the Study. In the alfacalcidol group, one patient with a vertebral fracture at baseline had a new fracture. There was a total of 6 new fractures that occurred in the alfacalcidol group versus 7 in the vitamin D + calcium group (Table 3). The pattern of BMD changes in this subgroup of patients was similar to the one reported in all the population studies.
Mean values for serum calcium, phosphorus and creatinine at baseline and at the end of the treatment period are shown in Table 4. Serum calcium concentrations at baseline were similar in the two groups: 9.35±0.70 in the alfacalcidol group, and 9.39±0.48 in the vitamin D + calcium group. At the end of the Study, serum calcium concentration was slightly higher in the alfacalcidol group, as compared to the vitamin D + calcium group (9.56±0.48 vs. 9.45±0.49). Serum phosphate and creatinine levels did not change significantly over the 18 month period. The statistical analysis performed on serum calcium, phosphorus and creatinine values demonstrated a noninferiority between the groups at 2.5% level (95% CI ratio geometric means: serum calcium 1.00–1.03; phosphorus 0.93–1.00; creatinine 0.96–1.05).
In the alfacalcidol group 22 (30.1%) of the patients had one or more adverse events related to their treatment. In the vitamin D + calcium group there were 19 (27.1%) patients who had one or more adverse events related to their treatment. The most common side effect was gastrointestinal (10 patients in the alfacalcidol-treated group, and 9 patients in the vitamin D + calcium-treated group). No case of renal calculus was reported.
Discussion
The growing awareness concerning the role of vitamin D in the pathogenesis of postmenopausal bone loss has stimulated interest in the use of vitamin D and D-hormone analogs in the management of PMO.
The crucial importance of postmenopausal estrogen deficiency in the development of osteoporosis is supported by the increased number and activity of osteoclasts, and by the intestinal calcium malabsorption related to impaired vitamin D metabolism that occur after menopause [1, 20]. The osteoclastogenic effect of ovariectomy was recently confirmed in vitro by a specific dendritic cell activation in the bone marrow that leads to increased activation of antigen-producing T cells and T cell TNF-α production [21]. Moreover, in elderly osteoporotic patients a deficiency in VDRs related to lack of estrogen may contribute to the PMO pathogenesis [22].
Our data demonstrate that treatment with 1 μg alfacalcidol daily without additional calcium supplementation induces a statistically significant increase in lumbar BMD after 12 and 18 months, in comparison to 880 IU vitamin D plus 1 g calcium daily in osteoporotic women characterized by normal vitamin D status (Fig. 2). The small increase in femoral BMD may be accounted for by the lower and slower effect that a bone active agent usually has on cortical bone. In addition, alfacalcidol treatment has shown a clinically relevant, though not statistically significant reduction in the number of patients having new vertebral fractures (Table 3). Considering that previous fractures represent a remarkable risk factor for future vertebral fractures [10], it is important to mention that there were more patients with one or more prevalent fractures in the alfacalcidol group than in the vitamin D + calcium group (Table 1). The fact that alfacalcidol is superior, despite this bias, is remarkable.
It has been speculated that VDR gene polymorphisms could be responsible for the greater therapeuthic response to vitamin D and its metabolites observed in Asian populations with respect to Caucasians. However, our study confirms that alfacalcidol is also effective in Caucasian patients. Moreover, recent preliminary investigations have not evidenced any differences in calcitriol response to treatment according to different VDR genotypes [23].
There are often controversial discussions as to whether D-hormone analogs are really more effective than plain vitamin D in osteoporotic patients. The efficacy of a vitamin D and calcium combination has been clearly demonstrated in patients with vitamin D deficiency. In vitamin D-repleted women with PMO, no significant effects can be expected. Supplementation with plain vitamin D is not a pharmacological therapy, but a dietary substitute. As a result of the negative feedback regulating the final activation step of 25(OH)D into the active 1.25-dihydroxyvitamin D hormone by the kidneys, the oral supplements of plain vitamin D will never lead to an increase of the D-hormone levels [24, 25]. This means that in vitamin D-repleted patients therapeutic effects on bone, muscle, or other target organs can only be achieved with the use of D-hormone analogs [26]. Similarly, D-hormone deficient patients due to impaired 1-alpha-hydroxylase activity in the kidney (e.g., patients with PMO) seemed to be more or less resistant to plain vitamin D. A condition of vitamin D resistance was also reported in patients with reduced VDR affinity [22, 24, 25]. Alfacalcidol is activated in the liver and in other target organs, like bones, and is a pro-drug of the D-hormone. Thus, the D-hormone deficiency can be treated by bypassing the body’s natural regulation in the kidneys [24–26]. Vitamin D resistance, based on VDR deficits, can also be treated with D-hormone analogs through their influence on the expression, activation and reduction in the quality of VDRs [27].
Alfacalcidol prevents rapid postmenopausal bone loss and improves bone quality by improving calcium malabsorption in the gut [12] through normalization of the increased bone remodeling that results from a direct reduction of osteoclast precursors in vivo [28] and by a subtle regulation of osteoblast differentiation and metabolism [29]. It may be that the known, very specific, T cell immunoregulating properties are, in part, also responsible for the efficacy of alfacalcidol by producing more tolerogenic antigen-presentating cells, decreasing T-helper cells, increasing suppressor cells and inducing cytokine homeostasis [30, 31].
In the ovariectomized rat model of osteoporosis, there is histomorphometric and biochemical evidence that oral administration of alfacalcidol causes dose-dependent suppression of osteoclastic bone resorption, as opposed to well-known in vitro “stimulation”. The explanation for the direct inhibition of bone resorption by D-hormone analogs is based on the new findings that alfacalcidol inhibits osteoclastogenesis in vivo by decreasing the pool of osteoclast precursors within the bone marrow, which is one of the pathogenetic key factors of postmenopausal bone loss [28]. Unlike typical inhibitors of bone resorption such as estrogens and bisphosphonates, alfacalcidol does not suppress, but rather stimulates bone formation [32]. This results in a significant improvement in the bone quality, as well as the quantity of cortical and cancellous bone. Alfacalcidol increases BMD and bone strength more effectively than plain vitamin D in osteoporotic patients [33]. These potential advantages of alfacalcidol over plain vitamin D on bone microstructure have been confirmed by micro-CT scanning [34]. It has also been proven that alfacalcidol exerts a direct anabolic effect on bone mass and strength, independent of calcium absorption and PTH suppression [33] in parathyroidectomized rats under constant PTH infusion.
There is clear evidence of the direct effect of D-hormone analogs on osteoblast function (proliferation, apoptosis, expression of specific bone proteins and growth factors) and mineralization [20, 29, 35]. Recently, bone anabolic effects of D-hormone have been detected in OVX rats alone, and, especially, in combination with a powerful antiresorptive agent [36]. This anabolic bone efficacy occurred despite significant decreases in osteoclast activity, suggesting an independent effect of D-hormone on osteoblast function and activity.
Dendritic cells (DC) play a previously unrecognized role in the mechanism of postmenopausal bone loss [21]. Several investigators have shown that DC are inhibited by VDR in vitro and in vivo in their ability for antigen presentation that stimulates T cell activation and proliferation and bone resorbing cytokine production following exposure to D-hormone analogs [31, 37]. The data demonstrate that DC may represent a novel D-hormone target to reduce bone loss in PMO.
The clinical counterpart of OVX animals is induced menopause in humans. Bone loss is known to be significantly increased after surgery. A study by DEXA involving women who had undergone bilateral ovariectomy evaluated the effect of alfacalcidol treatment [38]. Premenopausally ovariectomised women (>6 months after surgery) with bone loss above the standard deviation (−1 SD T-score) were divided into three groups (control; 0.25 μg alfacalcidol daily; 0.5–0.75 μg alfacalcidol daily). All patients were on a diet containing approximately 800 mg of calcium daily. After 1 year, lumbar BMD had decreased by 3.6% in the placebo group. While 0.25 μg alfacalcidol showed a small effect (−3.2%), 0.5 to 0.75 μg alfacalcidol taken daily appeared to be sufficient in order to achieve a statistically significant decrease in bone loss (−0.8%) [38]. This study demonstrated a clear dose–effect ratio for alfacalcidol.
A meta-analysis of clinical studies in PMO clearly showed the advantageous efficacy of D-hormone analogs (alfacalcidol, calcitriol) versus plain vitamin D [9]. D-hormone analogs had a consistently greater impact on BMD than plain vitamin D. After 12 months the difference between the groups was statistically significant for total body (P<0.03) and for both forearms (P<0.01). Treatment with D-hormone analogs significantly reduced the risk of vertebral fractures (Relative Risk, RR=0.64; 95% CI 0.44–0.92), while plain vitamin D therapy failed to achieve statistical significance [9]. The vertebral antifracture efficacy of D-hormone analogs is in the range of that observed with bisphosphonates or raloxifene. The number needed to treat (NNT), i.e., the number of patients who have to be treated for 2 years in order to prevent one vertebral fracture does not differ when using D-hormone analogs (NNT=94) in comparison to other antiosteoporotic agents, e.g., risedronate (NNT=96) or raloxifene (NNT=99), while only for alendronate (NNT=72) it was slightly inferior as shown in a summary of meta-analyses [39]. A second meta-analysis confirmed the positive effects of D-hormone analogs on bone mass, and, very importantly, on vertebral fracture risk (RR=0.53; 95% CI 0.47–0.60) [40]. In this meta-analysis a reduction of nonvertebral fractures (RR=0.34; 95% CI 0.16–0.71) was proven. The fact that two independent meta-analyses show corresponding results is a very strong proof of the efficacy of D-hormone analogs in the reduction of vertebral fractures. In a comparative meta-analysis, Richy et al. [41] confirmed that D-hormone analogs exhibit better efficacy in increasing vertebral bone loss and in preventing vertebral and nonvertebral fractures, as compared to plain vitamin D in PMO.
All the above data demonstrated the efficacy of D-hormones on bone loss and fracture prevention in PMO. In future, a clear differentiation must be made between calcium and plain vitamin D supplementation in vitamin D-deficient old women and men (>75 years), and the pharmacological treatment of patients with established osteoporosis using D-hormone analogs, independent of the patients’ vitamin D status [26].
Adverse drug reactions in our study were similar in both treatment groups. The high rate of gastrointestinal side effects in the vitamin D plus calcium group is a known phenomenon and is due to the presence of calcium carbonate. In the alfacalcidol-treated group a placebo containing various carbonate salts (calcium free) was used, and, therefore, it was not surprising that a similar number of gastrointestinal side effects were recorded in both groups. No significant differences in serum calcium were noted between the groups (Table 4). During the study there was no report on hypercalcemia in the group treated with alfacalcidol. Importantly, no cases of renal stone were recorded. Our findings have been confirmed by a large Post-Marketing Surveillance Study [18]. Patients (13,550) with PMO, over 60 years of age, and receiving alfacalcidol therapy were followed for over a 6-year period. The dosage was between 0.5 and 1 μg daily. Adverse effects were found in 1.1% of the patients. Hypercalcemia (Ca serum>11 mg/dl) was found in 0.22% of the patients, but there was not a single renal stone formation recorded [18]. The risk of getting affected by hypercalcemia as a result of taking calcitriol may be higher. Calcitriol, upon ingestion, acts immediately and directly on the VDR in the intestinal mucosal cells to promote intestinal calcium absorption, leading to a rapid increase in serum calcium [25]. No significant changes in serum creatinine occurred in either treatment group during the study period.
Bisphosphonates and selective estrogen receptor modulators (SERMs) are very effective in preserving BMD and preventing fractures in PMO. However, oral bisphosphonates may have gastrointestinal side effects and may induce musculoskeletal pain with weekly treatment [42]. SERMs may induce a postmenopausal syndrome and may increase the risk of thrombosis. In addition, these treatments are much more expensive than treatment with alfacalcidol. Hormone replacement therapy (HRT) was once regarded as the gold standard, but, based on its potential cardiovascular and neoplastic side effects, HRT is now not fully accepted for the treatment of PMO [43].
On the other hand, it has been proven that switching from HRT to alfacalcidol is possible [44]. D-hormone analogs seem to be interesting candidates for combination with bisphosphonates in severe PMO. The independent bone anabolic effects obviously occur more intensively, and bone quality has been shown to be significantly improved in animal studies [34, 36, 45].
This study had several limitations. The patients had normal serum levels of vitamin D (25(OH)D>12 ng/ml), therefore, the effect of vitamin D in vitamin D-deficient patients could not be determined. The study had limited power to evaluate the efficacy on femoral BMD due to its short duration, or to find differences in fracture rates due to the relatively low number of patients available for evaluation.
Our study shows alfacalcidol to be an efficient agent in the therapy of PMO. Alfacalcidol was significantly superior to vitamin D in terms of bone mass gain, and, possibly, in the reduction of vertebral fractures. The results serve as a response to the controversy concerning treatment with alfacalcidol (i.e., higher costs and increased risk of side effects): plain vitamin D is insufficient for the management of PMO, while safety characteristics were found to be similar in both treatments. Due to the above-described efficacy and excellent tolerability, the long-term safety and simple mode of administration (which all promote long-term patient compliance), together with its moderate daily cost, it can be concluded that the alfacalcidol therapy is an important treatment option for patients with PMO.
References
DeLuca HF (1997) 1,25-dihydroxyvitamin D3 in the pathogenesis and treatment of osteoporosis. Osteoporos Int 7(Suppl 3):S24–S29
Hayashi Y, Fujita T, Inoue T (1992) Decrease of vertebral fracture in osteoporotics by administration of 1α-hydroxyvitamin D3. J Bone Miner Metabol 10(2):184–188
Tilyard MW, Spears GF, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. New Engl J Med 326(6):357–362
Menczel J, Foldes J, Steinberg R, Leichter I, Shalita B, Bdolah-Abram T, Kadosh S, Mazor Z, Ladkani D (1994) Alfacalcidol (Alpha D3) and calcium in osteoporosis. Clin Orthop 300:241–247
Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Miyazaki S, Kurosawa H, Nakamura T, Ogawa N (1994) Effects of 1α-hydroxyvitamin D3 on lumbar bone mineral density, vertebral fractures in patients with postmenopausal osteoporosis. Calcif Tissue Int 54:370–376
Shiraki M, Kushida K, Yamazaki K, Nagai T, Inoue T, Orimo H (1996) Effects of 2 years treatment of osteoporosis with 1α-hydroxy vitamin D3 on bone mineral density and incidence of fracture: a placebo-controlled, double-blind prospective study. Endocr J 43(2):211–220
Nuti R, Martini G, Valenti R, Giovanni S (1996) Controlled study on the metabolic and absorptiometric effects of calcitriol in involutional osteoporosis. Clin Drug Invest 11(5):270–277
Gallagher JC, Fowler SE, Detter JR, Sherman SS (2001) Combination treatment with estrogen and calcitriol in prevention of age-related bone loss. J Clin Endocrinol Metab 86:3618–3628
Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, Greenwood C, Guyatt G (2002) The osteoporosis methodology group, and the osteoporosis research advisory group. Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569
Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E (2001) Risk of new vertebral fracture in the year following a fracture. JAMA 285(3):320–323
Gallagher JC, Goldgar D (1990) Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med 113(9):649–655
Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, Cowan RA, Downes N (1996) A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos Int 6:284–290
Ringe JD, Dorst A, Faber H, Schacht E, Rahlfs VW (2004) Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid-induced osteoporosis. Rheumatol Int 24:63–70
Scharla SH, Schacht E, Bawey S, Kamilli I, Holle D, Lempert UG (2003) Pleiotropic effects of alfacalcidol in elderly patients with rheumatoid arthritis. Arthritis Rheuma 23(5):268–274
Chapuy MC, Arlot ME, Duboeuf F et al. (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642
Chapuy MC, Pamphile R, Paris E et al. (2002) Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int 13:257–264
Cooper L, Clifton-Bligh PB, Nery ML et al. (2003) Vitamin D supplementation and bone mineral density in early postmenopausal women. Am J Clin Nutr 77:1324–1329
Orimo H (1994) Clinical application of 1α(OH)D3 in Japan. Akt Rheumatol 19(Suppl 1):27–30
Nishii Y, Sato K, Kobayashi (1993) The development of vitamin D3 analogues for the treatment of Osteoporosis. Osteoporosis Int 3(Suppl 1):S190–S193
Schacht E (1999) Rationale for treatment of involutional osteoporosis in women and for prevention and treatment of corticosteroid-induced osteoporosis with alfacalcidol. Calcif Tissue Int 65:317–327
Grassi F, Leavey JK, Dark K, Qian W, Weitzmann MN, Pacifici R (2004) Bone marrow restricted antigen presentation by dendritic cells induces T cell activation and T CELL TNF production in ovariectomized mice. J Bone Miner Res 19(Suppl 1):S16
Horst RL, Goff JP, Reinhardt TA (1990) Advancing age results in reduction of intestinal and bone 1,25-dihydroxy-vitamin D receptors. Endocrinology 126(2):1053–1057
Rapuri PB, Gallagher JC, Haynatzka V (2004) Interaction between vitamin D receptor and estrogen receptor genotypes does not influence BMD, rate of bone loss and the response to treatment with calcitriol or estrogen in elderly women. J Bone Miner Res 19(Suppl 1):S246
Nordin BEC, Need AG, Morris HA, Horowitz M (1999) The special role of “Hormonal” forms of Vitamin D in the treatment of osteoporosis. Calcif Tissue Int 65:307–310
Lau KHW, Baylink DJ (1999) Vitamin D therapy of osteoporosis: plain vitamin D therapy versus active vitamin D analog (D-hormone) therapy. Calcif Tissue Int 65:295–306
Ringe JD, Schacht E (2004) Prevention and therapy of osteoporosis: the respective roles of plain vitamin D and alfacalcidol. Rheumatol Int 24:189–197
Li XY, Boudjelal M, Xiao JH, Peng ZH, Asuru A, Kang S, Fisher GJ, Voorhees JJ (1999) 1,25-dihydroxyvitamin D3 increases nuclear vitamin D3 receptors by blocking Ubiquitin/Proteasome-mediated degradation in human skin. Mol Endocrinol 13(10):1686–1694
Shibata T, Shira-Ishi A, Sato T, Masaki T, Sasaki A, Masuda Y, Hishiya A, Ishikura N, Higashi S, Uchida Y, Saito M, Ito M, Ogata E, Watanabe K, Ikeda K (2002) Vitamin D hormone inhibits osteoclastogenesis in vivo by decreasing the pool of osteoclast precursors in bone marrow. J Bone Miner Res 17:622–629
van Driel M, Pols HAP, van Leeuwen JPTM (2004) Osteoblast differentiation and control by vitamin D and vitamin D metabolites. Curr Pharm Des 10:2535–2555
DeLuca HF, Cantorna MT (2001) Vitamin D. Its role and uses in immunology. FASEB J 15:2579–2585
Mathieu C, Adorini L (2002) The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol Med 8:174–179
Shiraishi A, Takeda S, Masaki T, Higuchi Y, Uchiama Y, Kubodera N, Sato K, Ikeda K, Nakamura T, Matsumoto T, Ogata E (2000) Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: distinct action from estrogen. J Bone Mineral Res 15:770–779
Shiraishi A, Higashi S, Ohkawa H, Kubodera N, Hirasawa T, Ezawa I, Ikeda K, Ogata E (1999) The advantage of alfacalcidol over vitamin D in the treatment of osteoporosis. Calcif Tissue Int 65:311–316
Ito M, Azuma Y, Takagi H, Komoriya K, Ohta T, Kawaguchi H (2002) Curative effect of combined treatment with alendronate and 1α-hydroxyvitamin D3 on bone loss by ovariectomy in aged rats. Jpn. J. Pharmacol 89:255–266
Duque G, El Abdaimi K, Henderson JE, Lomri A, Kremer R (2004) Vitamin D inhibits Fas ligand-induced apoptosis in human osteoblasts by regulating components of both the mitochondrial and Fas-related pathways. Bone 35:57–64
Reszka AA, Pun S, Rodan GA, Freedman LP, Kimmel DB (2004) Bone anabolic effects of 1,25(OH)2 Vitamin D3 are detected only in the presence of a powerful antiresorptive. J Bone Min Res 19(Suppl 1):S483
Griffin MD, Lutz WH, Phan VA, Bachmann LA, McKean DJ, Kumar R (2001) Dendritic cell modulation by 1α, 25-dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U.S.A. 98(12):6800–6805
Kato T, Chen JT, Katase K, Hirai Y, Hasumi K, Ogata E, Shiraki Y, Shiraki M (1997) Effect of 1α-hydroxyvitamin D3 on loss of bone mineral density immediately after artificial menopause. Endocr J 44(2):299–304
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C (2002) The osteoporosis methodology group, and the osteoporosis research advisory group. Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23:570–578
Richy F, Ethgen O, Bruyere O, Reginster J-Y (2004) Efficacy of alphacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int 15:301–310
Richy F, Schacht E, Bruyère O, Ethgen O, Gourlay M, Reginster JY (2005) Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif Tissue Int 76(3):176–186
Bock O, Boerst H, Degner C, Stephan-Oelkers M, Felsenberg D (2004) Underestimated musculoskeletal adverse effects of oral treatment with once weekly alendronate 70 mg and risedronate 35 mg: including possibilities for their prevention. Osteoporos Int 15(Suppl 1):S106
Compston JE (2004) The risks and benefits of HRT. J Musculoskel Neuron Interact 4(2):187–190
Ushiroyama T, Ikeda A, Sakai M, Higashiyama T, Ueki M (2003) Prevention of postmenopausal bone loss with exchange for short-term HRT for 1α-hydroxycholecalciferol. Maturitas 45:119–127
Shiraishi A, Ito M, Hayakawa N, Kubota N, Imai N (2004) Combination therapy with alfacalcidol and risedronate at their subtherapeutic doses can additively improve bone dynamics in ovariectomized rat model of osteoporosis. J Bone Miner Res 19(Suppl 1):S180
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuti, R., Bianchi, G., Brandi, M. et al. Superiority of alfacalcidol compared to vitamin D plus calcium in lumbar bone mineral density in postmenopausal osteoporosis. Rheumatol Int 26, 445–453 (2006). https://doi.org/10.1007/s00296-005-0073-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-005-0073-4