Abstract
Supplementation therapy with plain vitamin D plus calcium is in general regarded as effective prevention or first-step treatment of glucocorticoid-induced osteoporosis (GIOP). The aim of our study was to compare the therapeutic efficacy of the D-hormone analog alfacalcidol with plain vitamin D in patients with established GIOP with or without vertebral fractures. Patients on long-term glucocorticoid (GC) therapy were included as matched pairs to receive randomly either 1 μg alfacalcidol plus 500 mg calcium per day (group A, n=103) or 1,000 IU vitamin D3 plus 500 mg calcium (group B, n=101). The two groups were well matched in terms of mean age, sex ratio, mean height and weight, daily dosage, and duration of GC therapy, and the percentages of the three underlying diseases included chronic obstructive pulmonary disease, rheumatoid arthritis, and polymyalgia rheumatica. The baseline mean bone mineral density (BMD) values at the lumbar spine for the two groups were −3.26 (alfacalcidol) and −3.25 (vitamin D3) and, at the femoral neck, −2.81 and −2.84, respectively (T scores). Rates of prevalent vertebral and nonvertebral fractures did not differ between groups. During the 3-year study, we observed a median percentage increase of BMD at the lumbar spine of 2.4% in group A and a loss of 0.8% in group B (P<0.0001). There also was a larger median increase at the femoral neck in group A (1.2%) than in group B (0.8%) (P<0.006). The 3-year rates of patients with at least one new vertebral fracture were 9.7% among those assigned to the alfacalcidol group and 24.8% in the vitamin D group (risk reduction 0.61, 95% CI 0.24–0.81, P=0.005). The 3-year rates of patients with at least one new nonvertebral fracture were 15% in the alfacalcidol group and 25% in the vitamin D group (risk reduction 0.41, 95% CI 0.06–0.68, P=0.081). The 3-year rates of patients with at least one new fracture of any kind were 19.4% among those treated with alfacalcidol and 40.65% with vitamin D (risk reduction 0.52, 95% CI 0.25–0.71, P=0.001). In accordance with the observed fracture rates, the alfacalcidol group showed a substantially larger decrease in back pain than the plain vitamin D group (P<0.0001). Generally, side effects in both groups were mild, and only three patients in the alfacalcidol group and two in the vitamin D group had moderate hypercalcemia. We conclude that alfacalcidol plus calcium is highly superior to plain vitamin D3 plus calcium in the treatment of established GIOP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GCs) are widely used in clinical practice and play a major role in treating a variety of chronic diseases. Despite their indisputable therapeutic advantages, long-term GC treatment is often overshadowed by severe side effects that sometimes produce morbidity comparable to that of the original illness. One of these is the development of osteoporosis, which is known to arise as a consequence not only of chronic oral GC administration but also of deleterious effects on bone metabolism by the underlying disease. Bone loss is highest in the initial months of therapy, and the fracture incidence of GC-induced osteoporosis (GIOP) is estimated at 30–50% among patients receiving this type of treatment over long periods. This high rate cannot be explained alone by the loss of BMD. It is suggested, however, that early negative influences on bone quality contribute to the rapid increase in bone fragility. Therefore early, efficient, and cost-effective treatment is mandatory for this condition.
It is well established that GCs affect bone through multiple mechanisms. Their pathophysiological effects on bone and calcium homeostasis include decreased intestinal calcium uptake, enhanced renal excretion of calcium, impairment of osteoblast function, increased osteoclastic bone resorption, promotion of osteocyte apoptosis, and myopathy. All these deleterious pharmacological effects on bone or muscle can be counteracted directly by the biologically active form of vitamin D, calcitriol (D-hormone) [1]. Moreover, D-hormone was recently shown to be a potent immunomodulating agent. There is strong experimental evidence for a disease-modifying influence of calcitriol in murine models of human rheumatoid arthritis [2] and other chronic inflammatory autoimmune diseases [3], and there have been preliminary clinical investigations with alfacalcidol, a prodrug of the D-hormone, to corroborate this finding [4, 5, 6]. Therefore, these D-hormone analogs have attracted considerable attention as therapeutic options in the treatment of GIOP. Although both plain vitamin D and D-hormone analogs act through a common, biologically active metabolite (the D-hormone calcitriol), there is evidence that the latter has a higher therapeutic potential, particularly in patients with higher GC dosage (>2.5 mg prednisolone daily) and insufficiently controlled inflammatory diseases.
Several clinical studies have shown the efficacy of D-hormone analogs in GIOP such as alfacalcidol [7, 8, 9] and calcitriol [10, 11]. In contrast, plain vitamin D has an important role as a supplementary drug in osteoporosis therapy with agents such as bisphosphonates. Studies with plain vitamin D as a monotherapy have yielded unsatisfactory results [12, 13] in cases with higher GC dosage. On the other hand, alfacalcidol is more expensive than plain vitamin D and might be associated with a higher incidence of side effects such as hypercalcuria and hypercalcemia. Therefore, we designed this study to compare the efficacy and adverse events of alfacalcidol (1α-hydroxy-vitamin D) vs plain vitamin D3 in patients with established GIOP with or without vertebral fractures.
Materials and methods
Study design and subjects
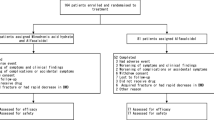
Included were patients on long-term GC therapy with established osteoporosis, i.e., bone mineral density (BMD) levels at the lumbar spine below −2.5 SD of the mean peak value in young adults (T score). The mean daily dose of GC was 9.0 mg prednisolone equivalents in the alfacalcidol group and 8.7 mg in the vitamin D3 group. The mean duration of GC treatment was 4.4 years in both groups. Patients with three underlying diseases were enrolled: chronic obstructive pulmonary disease, rheumatoid arthritis, and polymyalgia rheumatica. They were grouped in matched pairs as judged by baseline variables and assigned randomly to receive either 1 μg of alfacalcidolFootnote 1 plus 500 mg of calcium (n=103) or 1000 IU of vitamin D3 plus 500 mg of calcium (n=101) for 3 years.
The BMD was measured at onset and 12, 24, and 36 months by a technician blind to the therapy with dual X-ray absorptiometry (DXA) (Lunar, Madison, Wis., USA) at the lumbar spine (L2–4) and femoral neck. Lateral X-ray studies of the thoracic and lumbar spine were performed at onset and each year thereafter. Prevalent or new vertebral fracture was diagnosed by an independent radiologist (blind to the therapy) at vertebrae with 20% or more loss of anterior, median, or posterior height. Routine laboratory examinations were carried out at 6-month intervals, and patients were interviewed for adverse events and back pain.
Statistical analysis
Efficacy criteria
Efficacy criteria were BMD (lumbar spine and neck of femur), 3-year rate of patients with at least one new fracture (vertebral, nonvertebral, and both combined), and back pain (rating scale: 0=none, 1=mild, 2=moderate, and 3=severe). Other criteria were evaluated but are not the subject of this report.
Operationalization of efficacy criteria
The criterion BMD of lumbar spine and of neck of femur was evaluated as percentage of change from baseline. As the criterion back pain rating includes "0" values, change from baseline was used in this case. Missing values were substituted by the Last Value Carried Forward technique.
For data evaluation of fractures, the 3-year rate of new fractures was used. For patients who discontinued the study, the rate of fractures over 3 years was extrapolated, based on the rate while still participating in the study (so that a constant fracture risk in the course of time was assumed).
Methods of analysis
For comparison of treatments at baseline and different time points, a generalized Wilcoxon-Mann-Whitney procedure according to Wei and Lachin was used [33, 34, 35]. This procedure may be performed either as an Omnibus test without direction (used for baseline data) or as a directional test, with specification of a common direction of interest (improvement). The difference between groups can be quantified using the Mann-Whitney estimator and its confidence interval (CI) [36]. The Mann-Whitney (MW) statistic is interpreted as: 0.50̇ no difference, 0.44–0.56 small difference, 0.36–0.64 medium difference, and 0.29–0.71 large difference.
Concerning the 3-year rates of patients with at least one new fracture (vertebral and nonvertebral fractures or both combined), cross-tabulations with analysis for 2×2 tables were performed (relative risk, risk reduction). For underlying disease, additional analysis was performed to adjust results for confounding potentials with the Cox-Mantel test. All analyses were performed with the Testimate version 6.0.19 and SmarTest version 1.2 (idv, Gauting, Germany) validated software packages.
Results
Subject characteristics
Initially, 103 patients were assigned to the alfacalcidol group and 101 to the vitamin D group. As shown in Table 1, treatment groups were well matched with regard to mean age, sex, mean height and weight, mean daily dose, duration of GC therapy, and percentages of the three underlying diseases. The mean initial BMD T-score values at the lumbar spine for the two groups were −3.26 (alfacalcidol) and −3.25 (vitamin D3) and, at the femoral neck, −2.81 and −2.84 SD, respectively (Table 1). Baseline characteristics for fracture status and bone pain did not differ substantially between the treatment groups. The baseline test for homogeneity resulted in Mann-Whitney estimators between 0.4864 (height) and 0.5299 (lumbar spine BMD), so that only trivial group differences were found.
A total of 89 patients in the alfacalcidol group (86.4%) and 88 in the vitamin D group (87.1%) completed treatment normally after 36 months. There were only trivial differences between both groups with respect to reasons for discontinuing treatment (Table 2).
Bone mineral density
At the lumbar spine, gains in BMD were observed in both treatment groups after 12 months (Fig. 1). However, while median percentage gain in BMD continued to increase moderately in the alfacalcidol group during the course of the study (+2.4% at the end), the initial increase in the vitamin D group was neutralized by subsequent loss at months 12 and 24, which remained almost steady until the end of the study (−0.8% at 36 months). The Wilcoxon-Mann-Whitney directional test for the criterion "percent change from baseline" showed a noticeable superiority of alfacalcidol at month 12 and a great superiority at months 24 and 36 (month 36: MW score 0.8675, 95% CI 0.7806–0.9543, P<0.0001). Concerning BMD at the femur neck, no noteworthy median change was observed in the vitamin D group (+0.8% at the end of the study). There was a steady gain in BMD until month 12 and between months 24 and 36 (+1.2% at the end of the study) (Fig. 2), denoting a moderate superiority of alfacalcidol at the end of the study (MW score 0.6394, 95% CI 0.5596–0.7193, P<0.006). Stratified analysis of the BMD measurements with respect to the underlying diseases indicated the same treatment effect as the unstratified analysis.
Fracture rate
A total of 35 out of 204 patients had one or more vertebral fractures. At the end of the study, the numbers of new vertebral fractures were 16 in ten patients in the alfacalcidol group and 35 for 25 patients in the vitamin D group. The 3-year rates of patients with at least one new vertebral fracture were 9.7% for the alfacalcidol group and 24.8% for the vitamin D group (risk reduction 0.39, 95% CI 0.20–0.76, and P=0.005 vs risk reduction 0.61, 95% CI 0.24–0.81, and P=0.005, respectively) (Fig. 3).
The 3-year rates of patients with at least one new nonvertebral fracture were 14.6% in the alfacalcidol group and 24.8% in the vitamin D group (risk reduction 0.59 and 95% CI 0.32–1.06 vs risk reduction 0.41 and 95% CI 0.06–0.68, respectively) (P=0.081). The difference was not statistically significant, even in a descriptive sense, which might have been caused by the small sample size.
At the end of the study, the numbers of new fractures of any kind were 32 in 20 patients in the alfacalcidol group and 68 in 41 patients in the vitamin D group. The 3-year rates of patients with at least one new fracture were 19.4% for the alfacalcidol group and 40.6% for those treated with plain vitamin D and calcium (risk reduction 0.48, 95% CI 0.20–0.75, and P=0.001 vs risk reduction 0.52, 95% CI 0.25–0.71, and P=0.001, respectively) (Fig. 4).
Back pain
At 36 months, back pain showed mean decreases of 1.7 points in the alfacalcidol group and 0.8 points in the vitamin D group (Fig. 5). This effect was not significant for the vitamin D group. Concerning the percentage change from baseline, a large superiority of alfacalcidol was demonstrated with the Mann-Whitney test after both 2 and 3 years (month 24: MW score 0.7244, 95% CI 0.6523–0.7965, and P<0.001 vs month 36: MW 0.7395, 95% CI 0.6683–0.8108, and P<0.001, respectively).
Side effects
There were no relevant differences in frequency, type, or severity of side effects between treatment groups (Table 3). Generally, all side effects were mild to moderate. Epigastric discomfort was the most frequent adverse effect, which was supposed to be related mainly to the calcium supplementation. Three patients in the alfacalcidol group and two in the vitamin D group had moderate hypercalcemia. One alfacalcidol patient dropped out due to hypercalcemia, i.e., a treatment-related adverse event. Four patients died during the course of the study (two in each group) based on intercurrent diseases most likely unrelated to the study drugs (two of stroke, one of cor pulmonale, and one of status asthmaticus).
Discussion
Plain vitamin D is active only in patients with vitamin D deficiency. This could explain the effect on vertebral BMD in the first 12 months. Alfacalcidol, a prodrug of the D-hormone calcitriol, is a pharmacologically active antiosteoporotic drug which works independently of vitamin D status [14]. D-hormone analogs such as alfacalcidol and calcitriol have been demonstrated to be useful in the therapy of GIOP. All clinical studies with these agents in GIOP have shown an increase or stabilization of BMD in comparison to control groups.
Some studies looking at prevention of GIOP by alfacalcidol in patients with different underlying diseases demonstrated an inhibition of bone loss, even for very high doses of GC [8, 9]. Van Cleemput et al. investigated the relative efficacies of alfacalcidol and etidronate in patients after cardiac transplantation under therapy with GC and cyclosporine A. They showed better efficacy on BMD in the alfacalcidol group at both the lumbar spine and the femoral neck than in the etidronate group. More fractures were found in the etidronate group [7]. This result has to be interpreted with caution, as only very few fractures had occurred. Similar results were obtained in a more recent study of patients with cardiac or lung transplants [11], and calcitriol reduced significantly the number of vertebral fractures.
The prevention of bone loss after cardiac transplantation was recently shown in a 1-year, prospective, randomized, double-masked, clinical trial comparing 0.5 μg calcitriol and 10 mg alendronate daily with a nonrandomized untreated control group. Bone loss was minimal and did not differ between the calcitriol and alendronate groups, but both sustained significantly less than the control group [15].
Taken together, there is very good evidence that treatment with D-hormone analogs is able to maintain bone mass also in patients with very high dosages of GC. This fact was taken into consideration in the guidelines of the American College of Rheumatology [16] and confirmed by a new meta-analysis [17].
While there is ample evidence that alfalcalcidol and calcitriol are effective in reducing vertebral and nonvertebral fracture incidence in age-related and postmenopausal osteoporosis [17, 18, 19, 20, 21], fracture data for GIOP have largely been uninformative and discrepant due to the relatively small sample sizes of the respective studies.
Thus, the present study is the first one large enough to evaluate statistically the effect of alfacalcidol not only on BMD but also on fracture risk in patients with established GIOP. The results corroborate previous findings that alfacalcidol is capable of significantly increasing the BMD in both lumbar spine and femoral neck in comparison to control groups. These results are of special interest, because our control group received a standard basic supplementation therapy with vitamin D and calcium. Alfacalcidol treatment led to significant reductions of 61% in vertebral fractures and 52% in all fractures, vertebral and nonvertebral combined, in comparison to the vitamin D group. The study patients also had a 41% risk reduction for new nonvertebral fractures, which is clinically relevant but statistically not significant.
The superiority of alfacalcidol with respect to the occurrence of vertebral fractures was reflected by a significantly higher efficacy of alfacalcidol in back pain reduction than with vitamin D. It is not clear whether the advantageous symptomatic efficacy is only related to the lower vertebral fracture rate or if special effects on muscle metabolism [22] and/or the immune system [3] are involved. Although the vitamin D dose of 1000 IU used in this study is a well-established standard supplementation in osteoporosis management, it might be argued that this amount was too low for the agent to exert fully its antiosteoporotic potential. However, another study on GIOP has shown no therapeutic efficacy with vitamin D doses as high as 7,000 IU per day [12]. An interesting question is why the two agents differ so strongly in their antiosteoporotic potency although they share the same active metabolite, the D-hormone.
It has to be considered, though, that the bioactivation of native vitamin D requires renal 1a-hydroxylation. In contrast to the preceding 25-hydroxylation in the liver, this enzymatic metabolization is a tightly regulated process similar to that associated with the metabolism of other steroidal hormones. While native vitamin D has to undergo this enzymatic activation step, the preactivated metabolites alfacalcidol and calcitriol are already hydroxylated at the 1α-position. Moreover, recent research has shown that GCs reduce the amount of D-hormone receptors on effector cells, thereby decreasing de facto the activity of this hormone. Interestingly, this effect might still be aggravated in chronic inflammatory diseases, where high levels of circulating proinflammatory cytokines such as tumor necrosis factor (TNF)-α or interleukins (IL)-1, -6, and -12 prevail in target tissues or serum. Experimental data suggest that TNF-α inhibits renal 1-alpha-hydroxylase [23]. This is in accordance with clinical findings that inflammatory diseases are associated with low serum calcitriol levels [24, 25], depending on disease activity [24].
There is general consensus that the above cytokines induce bone resorption and also interfere with bone formation. Tumor necrosis factor α promotes apoptosis of osteoblasts, and D-hormone is able to protect against TNF-α-induced cell death. This mode of action has been demonstrated in vivo in an inflammation-mediated animal osteopenia model that simulated bone loss in rheumatoid arthritis [26]. D-hormone analogs have immunoregulating properties, inducing cytokine homeostasis, decreasing T helper cells, and increasing suppressor cells [1, 3]. In clinical pilot studies, it has been shown that alfacalcidol decreases TNF-α and IL-6 serum levels and increases IL-4 serum levels [5, 6]. In a study comparing the efficacy of alfacalcidol and plain vitamin D on bone metabolism, muscle function, joint pain, and cytokine levels, it has been clearly shown that the former significantly improves muscle strength in comparison to plain vitamin D [6].
In GC/inflammation-induced osteoporosis, the fracture risk is higher than expected by the loss of BMD, because early destruction of the trabecular architecture occurs, e.g., a decrease in bone quality and bone strength. In animal trials, it has been shown that alfacalcidol increases bone strength more effectively than plain vitamin D [27]. In conclusion, there are several potential reasons for the superiority of alfacalcidol over vitamin D in GIOP therapy, and the most important may be the greater availability of D-hormone in target tissues.
Given the relatively high increase in BMD from antiosteoporotic treatment with bisphosphonates such as alendronate and risedronate, the changes in BMD in this study appear rather small. On the other hand, the rates of fracture reduction differ considerably between the treatment groups, at least when vertebral fractures or the combined data of all fractures are considered. The risk reduction of 61% observed for alfacalciol in vertebral fractures is in the range of results found with bisphosphonate treatment of GIOP [28, 29] in which the specific drugs were also compared with combined vitamin D and calcium therapy. This paradox of relatively small increase in BMD and higher reduction of fracture incidence was discussed already in a recent study on raloxifene [30]. In that elegant statistical analysis of a large study on postmenopausal osteoporosis, the extent of treatment-associated changes in BMD accounted for only 4% of the observed vertebral fracture risk reduction. The change in BMD seems to be a poor predictor of fracture outcome for raloxifene and alfacalcidol. Protection against further destruction of the trabecular microarchitecture by the regulation of increased bone remodeling and resorption and improvement of bone quality and therefore strength are more important for fracture prevention, especially in GIOP.
Our study shows alfacalcidol to be an efficient agent in the therapy of GIOP. It was superior to vitamin D in terms of bone mass gain and fracture risk reduction. The controversy concerning treatment with alfacalcidol—greater cost—is answered: plain vitamin D is definitely insufficient for the management of GIOP.
Bisphosphonates are very effective in preserving BMD and preventing fractures. However, oral bisphosphonates may have gastrointestinal side effects, especially in combination with nonsteroidal anti-inflammatory drugs, and the long-term efficacy and safety of bisphosphonates remain an open issue. In addition, that treatment is much more expensive than with alfacalcidol. D-hormone analogs might be interesting candidates for combination with bisphosphonates or selective estrogen receptor modulators such as raloxifene, since in animal studies it has been proven that the key factor in GIOP, e.g., reduced bone quality, could be significantly improved by a combined therapy [31, 32].
Due to its pleiotropic efficacy on bone, muscles, and the immune system and its excellent tolerability, long-term safety, simple and patient-friendly mode of administration (which all promote long-term patient compliance), and moderate daily costs, physiological alfacalcidol is an important treatment option in patients with GC/inflammation-induced osteoporosis.
Notes
Alpha D3 produced by TEVA. Brand names in Germany: Bondiol, Doss
References
Schacht E (1999) Rationale for treatment of involutional osteoporosis in women and for prevention and treatment of corticosteroid-induced osteoporosis with alfacalcidol. Calcif Tissue Int 65:317–327
Cantorna MT, Hayes CE, DeLuca HF (1998) 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 128:68–72
DeLuca HF, Cantorna MT (2001) Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585
Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, Pavlica L, Stefanovic D (1999) Disease modifying and immunomodulatory effects of high dose 1α(OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 17:453–456
Hein G, Oelzner P (2000) Vitamin D-Metabolite bei rheumatoider Arthritis: Befunde–Hypothesen–Konsequenzen. Z Rheumatol 59 [Suppl 1]:I/28–I/32
Scharla SH, Schacht E, Bawey S, Kamilli I, Holle D, Lempert UG (2003) Pleiotropic effects of 1α-hydroxyvitamin D (alfacalcidol) in patients with rheumatoid arthritis. Bone [submitted]
Van Cleemput J, Daenen W, Geusens P, Dequeker J, van de Werf F, Vanhaecke J (1996) Prevention of bone loss in cardiac transplant recipients. Transplantation 61:1495–1499
Reginster JY, Kuntz D, Verdickt W, Wouters M, Guillevin L, Menkès CJ, Nielsen K (1999) Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos Int 9:75–81
Lakatos P, Nagy Z, Kiss L, Horvath C, Takacs I, Foldes J, Speer G, Bossanyi A (2000) Prevention of corticosteroid-induced osteoporosis by alfacalcidol. Z Rheumatol 59 [Suppl 1]:I 48–52
Sambrook PN, Birmingham J, Kelly PJ, Kempler S, Pocock NA, Eisman JA (1993) Prevention of corticosteroid osteoporosis: a comparison of calcium, calcitriol and calcitonin. N Engl J Med 328:1747–1752
Sambrook PN, Henderson NK, Keogh A, Macdonald P, Glanville A, Spratt P, Bergin P, Ebeling P, Eisman J (2000) Effect of calcitriol on bone loss after cardiac or lung transplantation. J Bone Miner Res 15:1818–1824
Adachi JD, Bensen WG, Bianchi F, Cividino A, Pillersdorf S, Sebaldt RJ, Tugwell P, Gordon M, Steele M, Webber C, Goldsmith CH (1996) Vitamin D and calcium in the prevention of corticosteroid induced osteoporosis: A 3 year follow up. J Rheumatol 23:995–1000
Buckley LM, Leib ES, Cartularo KS, Vacek PM, Cooper SM (1996) Calcium and vitamin D3 supplementation prevents bone loss in the spine secondary to low-dose corticosteroids in patients with rheumatoid arthritis. Ann Intern Med 125:961–968
Lau WK-H, Baylink DJ (2001) Treatment of 1,25(OH)2D3 (D-hormone) deficiency/resistance with D-hormone and analogs. Osteologie 10:28–39
Shane E, Addesso V, Namerow P, Maybaum S, Staron R, Lo S, Zucker M, Pardi S, Mancini D (2002) Prevention of bone loss after cardiac transplantation with alendronate or calcitriol: efficacy and safety. J Bone Miner Res 17:S135
American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis (2001) Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum 44:1496–1503
Richy F, Reginster JY (2002) Efficacy of D-hormones in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int [Suppl 3]:S21
Orimo H, Shiraki M, Hayashi Y, Hoshino T, Onaya T, Miyazaki S, Kurosawa H, Nakamura T, Ogawa N (1994) Effects of 1α-hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcif Tissue Int 54:370–376
Hayashi Y, Fujita T, Inoue T (1992) Decrease of vertebral fracture in osteoporotics by administration of 1α-hydroxy-vitamin D3. J Bone Miner Metab 10:50–54
Tilyard MW, Spears GF, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326:357–362
Gallagher JC, Fowler SE, Detter JR, Sherman SS (2001) Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 86:3618–3628
Boland R (1986) Role of vitamin D in skeletal muscle function. Endocr Rev 7:434–448
Ebert-Dümig R, Jovanovic M, Köhrle J, Jakob F (2001) Human 25(OH) vitamin D3-1α-hydroxylase promoter is regulated by TNFα-in HepG2 and HEK-293 cell lines. Exp Clin Endocrinol Diabetes 109 [Suppl 1]:S26
Oelzner P, Muller A, Deschner F, Huller M, Abendroth K, Hein G, Stein G (1998) Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62:193–198
Haug CJ, Aukrust P, Haug E, Morkrid L, Muller F, Froland SS (1998) Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab 83:3832–3838
Lempert UG, Minne HW, Albrecht B, Scharla SH, Matthes F, Ziegler R (1989) 1,25-dihydroxy-vitamin D3 prevents the decrease of bone mineral appositional rate in rats with inflammation-mediated osteopenia (IMO). Bone Miner 7:149–158
Shiraishi A, Higashi S, Ohkawa H, Kubodera N, Hirasawa T, Ezawa I, Ikeda K, Ogata E (1999) The advantage of alfacalcidol over vitamin D in the treatment of osteoporosis. Calcif Tissue Int 65:311–316
Saag KG for the Glucocorticoid-Induced Osteoporosis Intervention Study Group (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339:292–299
Cohen S, Levy RM, Keller M, Boling E, Emkey R, Greenwald M, Zizic T, Wallach S, Sewell K, Lukert B, Achselrod D, Chines A (1999) Risedronate therapy prevents corticosteroid-induced bone loss—a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Sarkar S, Mitlak BH, Wong M, Stock IL, Black DM, Harper KD (2002) Relationships between bone mineral density and incident vertebral fracture risk with raloxifen therapy. J Bone Miner Res 17:1–10
Ito M, Azuma Y, Takagi H, Komoriya K, Ohta T, Kawaguchi H (2002) Curative effect of combined treatment with alendronate and 1α-hydroxyvitamin D3 on bone loss by ovariectomy in aged rats. Jpn J Pharmacol 89:255–266
Erben RG, Mosekilde L, Thomsen JS, Weber K, Stahr K, Leyshon A, Smith SY, Phipps R (2002) Prevention of bone loss in ovariectomized rats by combined treatment with risedronate and 1α,25-dihydroxyvitamin D3. J Bone Miner Res 17:1498–1511
Wei LJ, Lachin JM (1984) Two-sample asymptotically distribution-free tests for incomplete multivariate observations. J Am Stat Assoc 79:653–661
Thall PF, Lachin JM (1988) Analysis of recurrent events: nonparametric methods for random interval count data. J Am Stat Assoc 83:339–347
Lachin JM (1992) Some large-sample distribution-free estimators and tests for multivariate partially incomplete data from two populations. Stat Med 11:1151–1170
Colditz, GA, Miller JN, Mosteller F (1988) Measuring gain in the evaluation of medical technology: the probability of a better outcome. J Technol Assessment Health Care 4:637–642
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ringe, J.D., Dorst, A., Faber, H. et al. Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid-induced osteoporosis. Rheumatol Int 24, 63–70 (2004). https://doi.org/10.1007/s00296-003-0361-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-003-0361-9