Abstract
In this paper, the Cu2+ ion-imprinted polymer (Cu2+-IIP) was prepared in the homogeneous phase by the ion-imprinted technology. Polyethyleneimine was used as the basic bone which was rich in amino-functionalized groups to coordinate with template Cu2+ and the 1,2-bibromoethane was used as a crosslinker. The chemical structure and surface morphology of the imprinted polymer were characterized by Fourier transform infrared spectroscopy (FTIR) and the scanning electron microscopy (SEM). Batch adsorption studies were performed to evaluate the adsorption capacity, the adsorption isotherm and the adsorption selectivity of Cu2+-IIP. The maximum adsorption capacity of Cu2+-IIP was 83.3 mg/g, significantly more than most of the reported adsorbents and also about 2.4 times that of non-imprinted polymer (Non-IIP) in this work. In addition, Cu2+-IIP performed good adsorption selectivity towards Cu2+ with the interference of Ni2+, Zn2+, or Pb2+. These results indicated that Cu2+-IIP is a promising absorbent for the real wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the global industrialization, the water contamination caused by metal ions becomes a general problem for the human health, ecological conditions, the modern agriculture and irrigation systems [1,2,3,4,5]. Therefore, the effective removal of toxic heavy metals by appropriate treatment technologies is a crucial issue for a long time [6].
Among the heavy metals, Cu2+ is not acutely toxic to humans. But its extensive use and increasing level in the environment may cause serious health problems [7]. So it was enrolled in the 13 toxic metal species on the EPA’s priority pollutant list [8]. The conventional methods of Cu2+ removal from the wastewater are chemical precipitation, ion exchange, electrolysis, reverse osmosis processes and adsorption on activated carbon [9]. But these methods are industrially applicable for the non-preferential separation. The inherent lack of selectivity is a major drawback [10]. To remove or separate a specific metal ion from a mixture, a selective separation method is required.
Molecular imprinting technique, as selective sorbents for a particular chemical form of the given element, has become a powerful method for the preparation of sturdy materials that have the ability to recognize a specific chemical species [11, 12]. For metal ions, the molecular imprinting can be exactly interpreted as the ionic imprinting. Metal ions used as template molecules, the selectivity of the ionic imprinted polymer (IIP) enhances because of the memory effect of the polymer on the imprinted ions from the specific interaction of ligands with the metal ions as well as the coordination geometry, the coordination number, the size and the charge of metal ions. There are a number of published papers related to the preparation of imprinted polymers for the selective adsorption of Cu2+. As early as 1976, Nishide et al. [13] prepared a chelate resin through crosslinking a complex of poly(4-vinylpyridine) with a metal ion (Cu2+, Fe3+, Co2+, Zn2+, Ni2+, and Hg2+) as a template. The resin comparatively adsorbed the metal ion which was used as a template. Afterwards, to improve the adsorption capacity, selectivity, rapid uptake kinetics and reduce costs, various of imprinted methods were developed, such as grafting functional groups, which can coordinate with metal ion on the silica gel surface [14,15,16], some composite sorbents containing natural biopolymer as chitosan [17,18,19], Cu2+ imprinting polymer nanoparticles [20,21,22] or microspheres [23], and hydrogel [24, 25].
Among these, amino-functionalized metal ion-imprinted sorbents [26,27,28] were paid great attention, because amino groups easily form coordination bonds with Cu2+. Liu et al. [29] found that the Cu2+ adsorption capacity increased obviously through injecting Congo Red rich in –NH2 into the crosslinked chitosan hydrogels.
Therefore, polyethyleneimine (PEI), a kind of water-soluble polyamine with a large quantity of amino groups, naturally gathered intense interest of researchers as an ideal trapping agent for metal ions [30,31,32]. An and Gao [33] reported that PEI/SiO2 had the high adsorption amount for plumbum ions even in the presence of the high concentration of alkaline-earth metal ions. Deng et al. [34] synthesized a novel Cu2+ imprinted membrane by pressure-driven depositing poly(ethyleneimine) (PEI) onto the surface of a polyacrylonitrile (PAN) ultrafiltration (UF) membrane combined with ion imprinting technique. The imprinted membrane exhibited the good specificity and recognition to the Cu2+. The structure, effective equilibrium constant, stoichiometric ratio, and the reaction energy change of complex formation of Cu2+ and PEI were investigated by Kislenko and Oliynyk [35], and the complex formation processing within the sorbents imprinted with Cu2+ was investigated in ESR method by Zagorodni [36]. All these researches provided the mature conditions for using PEI as the chelating agent to prepare Cu2+ imprinted sorbents.
Although each has its own advantages, the adsorption capacity of reported similar imprinted sorbents is still low [37,38,39], which could not meet the demand of the waste water treatment, especially at the high metal ion concentration. The low capacity may attribute to the limited grafting amount of chelating agents or the low compatibility between the body surface and chelating agents [40].
In this study, PEI long chain as the basic bone, Cu2+ as the imprinted ion, and 1,2-bibromoethane as the cross linker, a Cu2+ imprinted polymer, containing abundant amino groups were synthesized by the ion-imprinted technology with no any initiator condition. The whole reaction was carried out in the homogeneous phase and synthesis procedures are rather brief. Here, PEI long chain was employed as the basic bone (functional monomer) rather than being grafted to other substrates, which naturally increased the amino-group amounts largely. The synthesized imprinted sorbent had high adsorption capacity due to the specific cavities from the imprinted template and the chelation of the abundant amino groups. The imprinted sorbent was characterized by Fourier transform infrared spectroscopy (FTIR) and the scanning electron microscopy (SEM). The adsorption kinetics, isotherm, and selectivity of the Cu2+ imprinted polymer were also investigated.
Experimental
Materials
50.09 wt% linear PEI was purchased from Xiya Reagent-Puruidi Instrument Co., Ltd. (Chengdu, China). All other necessary chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Wuhan, China). 1,2-Dibromoethane was chemical grade and the others were analytical grade. All solutions were prepared using the distilled water.
Instruments and apparatus
An IRIS INTREPID IIXSP model inductively coupled plasma (ICP-OES, Thermo Electron Co. America) was used for the determination of all metal ions. The instrumental parameters of ICP-OES were in accordance with the manufacturer’s instructions. The pH values were measured by PHS-3C pH meter (Shanghai Precision & Scientific Instrument Co., Ltd., Shanghai, China). FTIR spectra (4000–400 cm−1) were recorded using a Magna-560 spectrometer (Thermo Nicolet Co., USA). SEM images were obtained by a scanning electron microscope (JEOL, JSM-5610LV). All adsorption experiments were accomplished in a model SHZ-82(A) Vapour-bathing Constant Temperature Vibrator (Beijing Ke Ning Bio-Tech. Center, Beijing).
Preparation of polyamine Cu2+ ion-imprinted polymer
The preparation procedure includes three steps as shown in Fig. 1.
The first step is the preparation of Cu2+-PEI complex. 12 g of 50.09% PEI was dissolved in 60 mL DMSO. Then, 8.4 g of stoichiometric Cu(NO)3·3H2O was added into the DMSO solution under mechanical stirring. The resulting reaction mixture was stirred about 1 h at room temperature.
The second step is the cross-linking reaction. With vigorous stirring, 6 mL 1,2-bibromoethane and excessive anhydrous K2CO3 powder were added to the above solution at 60 °C. At this temperature, the cross-linking reaction was carried out about 4 h. Then, the reaction was continued at 120 °C for 24 h. The solid polymer was obtained by the filtration.
The third step is the elution of imprinted Cu2+ in the polymer. The polymer was washed by distilled water and ethanol in sequence. Then, the polymer was soaked in 0.1 M HCl solution to remove the Cu2+, and followed by washed with plenty of distilled water till the solution is neutral. The polymer was dried in a vacuum oven at 60 °C about 24 h. Finally, the dried polymer was further ground. The particles of 0.1–0.3 mm size were selected as the experiment Cu2+ imprinted adsorbent (Cu2+-IIP).
For comparison, non-imprinted polymer (Non-IIP) was prepared according to the above approach with an exception that Cu2+ was not added.
Adsorption experiments
Adsorption kinetics
In this experiment, nine capped conical flasks containing 50 mL 50 mg/L Cu2+ ion solutions (C 0) at the pH 6 and 0.015 g Cu2+-IIP were shaken in a vibrator at a rotating rate of 130 rpm at 25 °C. One of the flasks was taken out at the adsorption time of 10, 20, 30, 40, 50, 60, 80, 100, and 120 min, respectively. The solid was filtered and the concentration of Cu2+ (C t) in the filtrate was measured by ICP. The adsorption capacity (Q t) was calculated by the following equation:
where V was the volume of solution and m was the weight of Cu2+-IIP.
The effect of pH on adsorption
0.015 g of Cu2+-IIP or Non-IIP was added into capped conical flasks containing 50 mL 50 mg/L Cu2+ solution at different pH. These flasks were shaken at 130 rpm for 4 h in the Vibrator. The temperature was set at 25 °C. Then, the absorbents were filtered and the concentration of Cu2+ in the filtrate was measured by ICP. The adsorption capacity (Q) was calculated by the following equation:
where C 0 and C e represented the initial and equilibrium concentration of Cu2+, respectively. V was the volume of solution and m was the weight of Cu2+-IIP or Non-IIP.
The effect of initial concentration of Cu2+ on adsorption
The effect of the initial concentration of Cu2+ on the adsorption was carried out by mixing 0.015 g Cu2+-IIP or Non-IIP and 50 mL of the solution containing various initial concentrations of Cu2+ at pH 6. These mixtures were shaken in the Vibrator at 130 rpm for 4 h at 25 °C. Then, polymers were filtered and the concentration of Cu2+ in the filtrate was measured by ICP. The adsorption capacity (Q) was also calculated by Eq. (2).
Selectivity of Cu2+ imprinted polymer
To evaluate the selectivity of the synthesized Cu2+-IIP, batch of static adsorption experiments was carried out. The binary mixture solutions of Cu2+/Ni2+, Cu2+/Zn2+, or Cu2+/Pb2+ were prepared. In these solutions, the concentration of each metal ion was 0.4 mmol/L. 50 mL mixture solution with pH = 6 was treated by adding 0.015 g Cu2+-IIP or Non-IIP in each of 100 mL conical flasks. These flasks were shaken in the Vibrator at 130 rpm for 4 h at 25 °C. After reaching the adsorption equilibrium, the concentrations of metal ions were also measured by ICP. Distribution coefficients (K d) for Ni2+, Zn2+, and Pb2+ with respect to Cu2+ were calculated by the following equation [41]:
where K d is the distribution coefficient (mL/g), and C i and C e are the initial and equilibrium concentration of metal ion, respectively. V is the total volume of the aqueous solution and m is the weight of adsorbent.
The selectivity coefficient (k) for the Cu2+ in the presence of competitor ions can be obtained from the following equation:
where Xm+ is the competitor ions (Ni2+, Zn2+, and Pb2+). The larger k is, the better the selectivity of the absorbent for Cu2+ is.
A comparison of the k values between the imprinted and non-imprinted samples can further illustrate the imprinting effect and selectivity on the special metal ion for a given sorbent. The relative selectivity coefficient (k′) can be defined by the following equation:
where k imprinted and k Non-imprinted represent the selectivity coefficients of the Cu2+-IIP and Non-IIP, respectively. k′ represents the difference of metal adsorption affinity and recognition of sites to the imprinted Cu2+ for the Cu2+-IIP with respect to Non-IIP [42].
Results and discussion
Preparation and characterization of Cu2+ imprinted polymer
The homogeneous reaction is much easier to be controlled than the heterogeneous reaction. Therefore, DMSO was selected as solvent to ensure the reaction carried out in a homogeneous system. Moreover, the organic solvent can prevent the hydrolysis of Cu2+ resulted by the adding of anhydrous K2CO3, which could increase the imprinted efficiency of Cu2+.
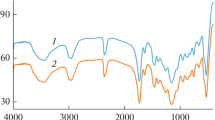
The FTIR spectra of PEI, Non-IIP, Cu2+-IIP, and Cu2+-loaded IIP were shown in Fig. 2. The broad band of PEI ranging from 3300 to 3500 cm−1 can be assigned to the presence of –NH-group. The presence of three peaks at about 2943, 2835, and 1473 cm−1 can be attributed to asymmetric stretching vibration, symmetric stretching vibration, and in-plane bending vibration of –C–CH2–C– group, respectively [43]. The 1643 and 1566 cm−1 are the bending vibration of –N(R)H and primary amine in PEI. Whereas the adsorption band between 3300 and 3500 cm−1 becomes narrow in Non-IIP, Cu2+-IIP, and Cu2+-loaded IIP due to –NH2 and –NH participating in the cross-linking reaction. Simultaneously, the peak at 1566 cm−1 became much lower in Non-IIP than the raw material PEI and disappeared in Cu2+-IIP and Cu2+-loaded IIP [44]. This result further indicated that –NH2 and –NH participated in the cross-linking reaction. As expected, the peak at 2943, 2835, and 1473 cm−1 was basically not changed. The shift from 3417 cm−1 of Cu2+-IIP to 3409 cm−1 of Cu2+-loaded Cu2+-IIP indicated that coordination between N atom and Cu2+ and the similar shift from 1658 to 1655 cm−1 corresponding to the bending vibration of –N(R)H group was also observed, which are due to the decreasing the electron density of C–N group [41, 45]. These transforms suggested that chelation action was involved and dominated in the adsorption progress, as reported by literatures [29, 46, 47].
SEM images (Fig. 3) show that the surface of Non-IIP particles is almost smooth and little porous. However, Cu2+-IIP has a rough surface and a large number of porous. The porous could be attributed to the removing of Cu2+ after cross-linking reaction. These imprinted cavities favor to absorbing Cu2+ in the solution, because the mass transfer rate of metal ions towards the polymer surface was improved [48].
Adsorption of Cu2+ from aqueous solutions
Adsorption kinetics
The time to reach adsorption equilibrium is an important parameter for an adsorbent. Many applications, such as purification of product and deionization of waste water, require a fast absorption process. The kinetics curve of adsorption of Cu2+ on Cu2+-IIP was illustrated in Fig. 4. The adsorption capacity was quickly up to about 40 mg/g at the first 10 min. This fast adsorption was probably due to the abundant active sites on Cu2+-IIP, which could bind to Cu2+ by the chelation. Then with the adsorption proceeding, the adsorption sites decreased gradually. The adsorption capacity increased slowly after 10 min and nearly did not increase after adsorption time of 60 min. This indicated the adsorption nearly reached equilibrium. This sorption rate was higher than some other imprinted sorbents [5, 18, 49]. To ensure complete adsorption, a long enough adsorption time (4 h) was applied in all following adsorption experiments.
Effect of pH
The adsorption capacity of Cu2+-IIP and Non-IIP at different pH was given in Fig. 5. The adsorption capacity increased with the increase of pH, in according with results reported by Gao et al. [14] and Sgawky [50]. The low uptake of Cu2+ at pH 2–3 is because the amino groups (NH2) on the PEI chains were protonated to NH3 +, which induced an electrostatic repulsion for Cu2+ [18]. With increasing of pH, the functional groups (NH2) deprotonated, benefiting to Cu2+ binding. Hence, the sorption enhanced. At pH range of 5–6, the adsorption capacity increased slowly. The adsorption experiments were also carried out at pH 6.5 and 7.0, respectively. However, blue precipitate was found in the solution of Cu2+, because copper oxides or hydroxides formed at pH > 6 [19, 28]. That means that both adsorption and precipitation are responsible for the removal of Cu2+, and the solution above pH 6 is not suitable for adsorption experiments. To avoid the formation of precipitation, the pH 6 was chosen for the total adsorption experiments.
Identical experiments with Non-IIP were run for comparison. Obviously, the sorption capacity of Cu2+-IIP is much greater than that of Non-IIP.
Adsorption isotherms
Cu2+ adsorption isotherm of Cu2+-IIP and Non-IIP was presented in Fig. 6. As the increase of the initial concentrations of Cu2+ from 30 to 90 mg/L, the equilibrium adsorption capacity of Cu2+-IIP and Non-IIP all increased. When concentrations of Cu2+ were up to 70 mg/L, the adsorption become very slowly. However, a direct comparison showed that Cu2+-IIP adsorbed more Cu2+ from the solution than Non-IIP due to the high affinity of the imprinted polymer to template ion leaded by a mass of cavities whose shape, size, and spatial arrangement of functional groups are complementary to the template Cu2+ after leaching.
The capacity comparison with the other reported absorbents is shown in Table 1. The adsorption capacity of Cu2+-IIP in this work is about 83.3 mg/g, which is obviously superior to those of other similar adsorbents reported by literature [15, 18, 19, 21, 28, 51,52,53]. In addition, the adsorption capacity of Non-IIP is nearly reach the same or even higher capacity of imprinted adsorbents in Table 1. That is contributed to the specific cavities from the imprinted template and the chelation of the abundant amino groups.
In general, it is important to study the adsorption isotherm for the design of an adsorbent. Isotherm curves could describe a relationship between the ion concentration in the solution and the amount of ion adsorbed on absorbent when the adsorption system is at equilibrium. The equilibrium is usually described by Langmuir isotherm or Freundlich isotherm.
Langmuir isotherm is based on the assumption that surface active sites existing on the solid material are equivalent and distant to each other, so that there are no interactions between molecules adsorbed to adjacent sites. The ions are adsorbed on a monolayer on the solid surface. Langmuir model could be expressed by the following equations:
where Q is the amount of adsorbed Cu2+ on the ion-imprinted polymers (IIP) particles at equilibrium (mg/g), C e is the equilibrium concentration in solution (mg/L), and Q max and K L represent the maximum sorption capacity of adsorbent (mg/g) and affinity constant.
Freundlich isotherm could be expressed by the following equations [45].
where Q is the amount of adsorbed Cu2+ on the IIP particles at equilibrium (mg/g), K F is the Freundlich constant, and n is the Freundlich exponent. Freundlich model is empirically highly applicable for adsorbents having heterogeneous surfaces.
The equilibrium data in Fig. 6 were fitted by Langmuir isotherm and Freundlich isotherm, respectively. The plots were corresponding to be displayed in Figs. 7 and 8 and the parameters along with the regression coefficients were presented in Table 2. It can be seen that Langmuir isotherm fitted the experimental data better than Freundlich adsorption, because the correlation coefficient (R 2) was high (0.9727) and the value of Q m (101.6 mg/g) for Cu2+-IIP is close to the experimental value (83.3 mg/g). Therefore, it could be concluded that the adsorbed Cu2+ ions onto the Cu2+-IIP showed a monolayer adsorption behavior.
The maximum adsorption capacity (Q m) corresponds to the maximum quantity of Cu2+ that the adsorbent can adsorb at monolayer coverage. At this point, all the active sites had been occupied by ions, and the polymer could not adsorb any further Cu2+ even if the Cu2+ concentration in the solution was increased. This further explained the adsorption isotherms results that the adsorption capacity was no longer increased when Cu2+ concentration reached a certain value.
By comparing the adsorption capacity of the Cu2+-IIP and Non-IIP, the maximum adsorption capacity of Cu2+-IIP was obviously much higher than Non-IIP. Higher adsorption capacity can be explained by affinity constant (K L). K L is related to the energy of adsorption, which represents the affinity between the solute and the adsorbent. The higher value of K L for Cu2+-IIP (0.0700 L/mg) indicates that it has better affinity to adsorb Cu2+ than Non-IIP (0.0277 L/mg). Furthermore, the affinity between Cu2+ and Cu2+-IIP can also be expressed by dimensionless separation factor R L defined as follows:
Here, R L indicates the favorability and the capacity of the adsorption system. The value of R L between 0 and 1 represents favorable adsorption. The values of R L were shown Table 3. All R L were within 0 and 1, indicating that this adsorption system was favorable [54]. In general, this is a favorable adsorption system, having high affinity to template Cu2+ for Cu2+-IIP than Non-IIP.
The adsorption selectivity
The above experiments’ results clearly illustrate that imprinted polymer Cu2+-IIP can enhance the recognition for Cu2+. However, the most applications require ion-imprinted polymer to be able to selectively bind the specific metal ion from a mixture that contains various competitors. To evaluate the selectivity of Cu2+-IIP, Ni2+ and Zn2+ were chosen as competitors because of their same positive charge (+2) and similar ionic radius (Cu2+: 73 pm; Ni2+: 69 pm; Zn2+: 74 pm) [42]. Since Pb2+ was a typical poisonous ion, it was also chosen as competitive metal ion. Table 4 summarized the data of the adsorption capacity (Q m), the selectivity coefficient (k), and the relative selectivity coefficient (k′). The adsorption capacity showed that Cu2+-IIP absorbed more Cu2+ than Non-IIP in all mixture. This is an expected result due to template effect. Random distribution of ligands in Non-IIP leads to no specificity in rebinding affinity for metal ions. However, chelation of ligands with template ions could create activated sites and cavities formed after removal of template ions which could match the imprint ion well in size and coordination geometries [20]. A comparison of adsorption capacity (Q m) and k values showed that the adsorption ability of Cu2+-IIP and Non-IIP for Cu2+ is stronger than that for Ni2+ or Zn2+. This may be due to the larger stability constants of Cu2+ coordinating with N atom than that of Ni2+ or Zn2+ [55]. It is noted that the adsorption capacity of Pb2+ was much higher than that of Cu2+ for imprinted polymer, which is because the unit of the adsorption capacity is mg/g and the atomic mass of plumbum is heavier than that of copper. If the unit of the adsorption capacity is mmol/g, the adsorption capacity of Cu2+ would become higher, indicating that Cu2+-IIP had more superior selectivity for Cu2+ than Pb2+. This could be further verified by the value of the relative selectivity coefficient k′. The relative selectivity coefficient is an indicator to express metal binding affinity of recognition sites to the imprinted Cu2+ ions. Obviously, k′ is the largest for Cu2+/Pb2+ in three binary metal systems. The reason for this is the cavities imprinted by Cu2+ did not fit Pb2+ in size. The values of k′ for Cu2+/Ni2+ and Cu2+/Zn2+ are also higher than the literatures [37, 53] reported. From the above results, it can be conclude that the imprinting cavity for Cu2+ has good recognition memory effect and high binding selectivity.
Conclusion
The new Cu2+ imprinted polymer for selective removal of Cu2+ was synthesized in the homogeneous phase by ion-imprinted technology. The adsorption kinetics revealed that this sorption is a fast dynamic behavior. Compared with Non-IIP, the relative selectivity of Cu2+-IIP is higher, suitable for the selective removal of Cu2+ from the mixture system. This proved that the template ion played an imprinted role in the synthesis. This imprinted polymer exhibits not only a better selectivity but also a higher capacity (83.3 mg/g) for the metal ion templates than the non-imprinted analogues. This is markedly superior as an absorbing material for absorbing Cu2+. In addition, the best Cu2+ uptake was found at pH = 6, which is promising for real wastewater treatment.
References
Yannopoulos S, Lyberatos G, Theodossiou N, Li W, Valipour M, Tamburrino A, Angelakis A (2015) Evolution of water lifting devices (pumps) over the centuries worldwide. Water 7:5031–5060
Valipour M (2015) Surface irrigation simulation models a review. Int J Hydrol Sci Technol 5:51–70
Wang JJ, Ding L, Wei J, Liu F (2014) Adsorption of copper ions by ion-imprinted simultaneous interpenetrating network hydrogel: Thermodynamics, morphology and mechanism. Appl Surf Sci 305:412–418
Monier M, Ibrahim AA, Metwally MM, Badawy DS (2015) Surface ion-imprinted amino-functionalized cellulosic cotton fibers for selective extraction of Cu(II) ions. Int J Biol Macromol 81:736–746
Jiang Y, Kim D (2011) Effect of solvent/monomer feed ratio on the structure and adsorption properties of Cu2+-imprinted microporous polymer particles. Chem Eng J 166:435–444
Wang JQ, Lu XK, Ng PF, Lee KI, Fei B, Xin JH, Wu JY (2015) Polyethylenimine coated bacterial cellulose nanofiber membrane and application as adsorbent and catalyst. J Colloid Interface Sci 440:32–38
Hu R, Gou H, Mo ZL, Wei XJ, Wang YW (2015) Highly selective detection of trace Cu2+ based on polyethyleneimine-reduced graphene oxide nanocomposite modified glassy carbon electrode. Ionics 21:3125–3133
Im HJ, Yang YH, Allain LR, Barnes CE, Dai S, Zl Xue (2000) Funtionalized sol–gels for selective copper(II) separation. Environ Sci Technol 34:2209–2214
Al Qodah Z (2006) Biosorption of heavy metal ions from aqueous solutions by activated sludge. Desalination 196:164–176
Hoai NT, Yoo DK, Kim D (2010) Batch and column separation characteristics of copper-imprinted porous polymer micro-beads synthesized by a direct imprinting method. J Hazard Mater 173:462–467
Wang ZQ, Wang M, Wu GH, Shen YY, He CY (2010) Ion imprinted sol-gel nanotubes membrane for selective separation of copper ion from aqueous solution. Microchim Acta 169:195–200
Gao RX, Cui XH, Hao Y, He GY, Zhang M, Tang YH (2016) Preparation of Cu2+-mediated magnetic imprinted polymers for the selective sorption of bovine hemoglobin. Talanta 150:46–53
Nishide H, Deguchi J, Tsushida E (1976) Selective adsorption of metal ions on crosslinked poly(vinylpyridine) resin prepared with a metal ion as a template. Chem Lett 5:169–174
Gao B, An F, Zhu Y (2007) Novel surface ionic imprinting materials prepared via couple grafting of polymer and ionic imprinting on surfaces of silica gel particles. Polymer 48:2288–2297
Wu G, Song G, Wu D, Shen Y, Wang Z, He C (2010) Synthesis of ion-imprinted mesoporous silica gel sorbent for selective adsorption of copper ions in aqueous media. Microchim Acta 171:203–209
Li M, Feng C, Li M, Zeng Q, Gan Q, Yang H (2015) Synthesis and characterization of a surface-grafted Cd(II) ion-imprinted polymer for selective separation of Cd(II) ion from aqueous solution. Appl Surf Sci 332:463–472
Xiang G, Zhang Y, Jiang X, He L, Fan L, Zhao W (2010) Determination of trace copper in food samples by flame atomic absorption spectrometry after solid phase extraction on modified soybean hull. J Hazard Mater 179:521–525
Liu H, Yang F, Zheng Y, Kang J, Qu J, Chen JP (2011) Improvement of metal adsorption onto chitosan/Sargassum sp. Composite sorbent by an innovative ion-imprint technology. Water Res 45:145–154
Shi Y, Zhang Q, Feng L, Xiong Q, Chen J (2014) Preparation and adsorption characters of Cu(II)-imprinted chitosan/attapulgite polymer. Korean J Chem Eng 31:821–827
Shamsipur M, Besharati-Seidani A, Fasihi J, Sharghi H (2010) Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 83:674–681
Roushani M, Abbasi S, Khani H (2015) Synthesis and application of ion-imprinted polymer nanoparticles for the extraction and preconcentration of copper ions in environmental water samples. Environ Monit Assess 187:219
Motevalizadeh SF, Khoobi M, Sadighi A, Khalilvand-Sedagheh M, Pazhouhandeh M, Ramazani A, Faramarzi MA, Shafiee A (2015) Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. J Mol Catal B Enzym 120:75–83
Dam AH, Kim D (2008) Metal ion-imprinted polymer microspheres derived from copper methacrylate for selective separation of heavy metal ions. J Appl Polym Sci 108:14–24
Aflaki Jalali M, Dadvand Koohi A, Sheykhan M (2016) Experimental study of the removal of copper ions using hydrogels of xanthan, 2-acrylamido-2-methyl-1-propane sulfonic acid, montmorillonite: kinetic and equilibrium study. Carbohydr Polym 142:124–132
Wang J, Li J (2015) Cu2+ adsorption onto ion-imprinted composite hydrogels: thermodynamics and mechanism studies. Polym Bull 72:2143–2155
Zhan Y, Luo X, Nie S, Huang Y, Tu X, Luo S (2011) Selective separation of Cu(II) from aqueous solution with a novel Cu(II) surface magnetic ion-imprinted polymer. Ind Eng Chem Res 50:6355–6361
Luo X, Huang Y, Deng F, Luo S, Zhan Y, Shu H, Tu X (2012) A magnetic copper(II)-imprinted polymer for the selective enrichment of trace copper(II) ions in environmental water. Microchim Acta 179:283–289
Peng W, Xie Z, Cheng G, Shi L, Zhang Y (2015) Amino-functionalized adsorbent prepared by means of Cu(II) imprinted method and its selective removal of copper from aqueous solutions. J Hazard Mater 294:9–16
Liu Y, Kang YR, Huang DJ, Wang AQ (2012) Cu2+ removal from aqueous solution by modified chitosan hydrogels. J Chem Technol Biotechnol 87:1010–1016
Sun M, Ding B, Yu J (2012) Sensitive metal ion sensors based on fibrous polystyrene membranes modified by polyethyleneimine. RSC Adv 2:1373–1378
Sui DP, Fan HT, Li J, Li Y, Li Q, Sun T (2013) Application of poly (ethyleneimine) solution as a binding agent in dgt technique for measurement of heavy metals in water. Talanta 114:276–282
Abolmaali SS, Tamaddon A, Najafi H, Dinarvand R (2014) Effect of l-histidine substitution on sol–gel of transition metal coordinated poly ethyleneimine: synthesis and biochemical characterization. J Inorg Organomet 24:977–987
An F, Gao B (2007) Chelating adsorption properties of PEI/SiO2 for plumbum ion. J Hazard Mater 145:495–500
Deng H, Gao L, Zhang S, Yuan J (2012) Preparation of a copper ion selective membrane by surface-modified molecular imprinting. Ind Eng Chem Res 51:14018–14025
Kislenko VN, Oliynyk LP (2002) Complex formation of polyethyleneimine with copper(II), nickel(II), and cobalt(II) ions. J Polym Sci Part A: Polym Chem 40:914–922
Molochnikov LS, Kovalyova EG, Zagorodni AA, Muhammed M, Sultanov YM, Efendiev AA (2003) Coordination of Cu(II) and Ni(II) in polymers imprinted so as to optimize amine chelate formation. Polymer 44:4805–4815
Kang C, Li W, Tan L, Li H, Wei C, Tang Y (2013) Highly ordered metal ion imprinted mesoporous silica particles exhibiting specific recognition and fast adsorption kinetics. J Mater Chem A 1:7147–7153
Wang J, Li Z (2015) Enhanced selective removal of Cu(II) from aqueous solution by novel polyethylenimine-functionalized ion imprinted hydrogel: behaviors and mechanisms. J Hazard Mater 300:18–28
Li Z, Li J, Wang Y, Wei Y (2014) Synthesis and application of surface-imprinted activated carbon sorbent for solid-phase extraction and determination of copper (II). Spectrochim Acta A Mol Biomol Spectrosc 117:422–427
Zhou X, Laroche F, Lamers GEM, Torraca V, Voskamp P, Lu T, Chu F, Spaink HP, Abrahams JP, Liu Z (2012) Ultra-small graphene oxide functionalized with polyethylenimine (PEI) for very efficient gene delivery in cell and zebrafish embryos. Nano Res 5:703–709
Esen C, Andac M, Bereli N, Say R, Henden E, Denizli A (2009) Highly selective ion-imprinted particles for solid-phase extraction of Pb2+ ions. Mater Sci Eng C 29:2464–2470
Ren Y, Zhang M, Zhao D (2008) Synthesis and properties of magnetic Cu(II) ion imprinted composite adsorbent for selective removal of copper. Desalination 228:135–149
Saegusa T, Ikeda H, Fujii H (1972) Crystalline polyethylenimine. Macromolecules 5:108
Jia J, Wu A, Luan S (2014) Synthesis and investigation of the imprinting efficiency of ion imprinted nanoparticles for recognizing copper. Phys Chem Chem Phys 16:16158–16165
Demiralay EÇ, Andac M, Say R, Alsancak G, Denizli A (2010) Nickel(II)-imprinted monolithic columns for selective nickel recognition. J Appl Polym Sci 117:3704–3714
Akar T, Tunali S (2005) Biosorption performance of botrytis cinerea fungal by-products for removal of Cd(II) and Cu(II) ions from aqueous solutions. Miner Eng 18:1099–1109
He J, Chen JP (2014) Cu(II)-imprinted poly(vinyl alcohol)/poly(acrylic acid) membrane for greater enhancement in sequestration of copper ion in the presence of competitive heavy metal ions: material development, process demonstration, and study of mechanisms. Ind Eng Chem Res 53:20223–20233
Yilmaz V, Hazer O, Kartal S (2013) Synthesis, characterization and application of a novel ion-imprinted polymer for selective solid phase extraction of copper(II) ions from high salt matrices prior to its determination by faas. Talanta 116:322–329
Deng H, Zhao S, Meng Q, Zhang W, Hu B (2014) A novel surface ion-imprinted cation-exchange membrane for selective separation of copper ion. Ind Eng Chem Res 53:15230–15236
Shawky HA (2009) Synthesis of ion-imprinting chitosan/pva crosslinked membrane for selective removal of Ag(I). J Appl Polym Sci 114:2608–2615
Luo X, Luo S, Zhan Y, Shu H, Huang Y, Tu X (2011) Novel Cu (II) magnetic ion imprinted materials prepared by surface imprinted technique combined with a sol–gel process. J Hazard Mater 192:949–955
Kuras MJ, Wieckowska E (2015) Synthesis and characterization of a new copper(II) ion-imprinted polymer. Polym Bull 72:3227–3240
Chen CY, Yang CY, Chen AH (2011) Biosorption of Cu(II), Zn(II), Ni(II) and Pb(II) ions by cross-linked metal-imprinted chitosans with epichlorohydrin. J Environ Manage 92:796–802
Li CX, Gao J, Pan JM, Zhang ZL, Yan YS (2009) Synthesis, characterization, and adsorption performance of Pb(II)-imprinted polymer in nano-TiO2 matrix. J Environ Sci 21:1722–1729
Laatikainen M, Sirola K, Paatero E (2007) Binding of transition metals by soluble and silica-bound branched poly(ethyleneimine). Colloids Surf A: Physicochem Eng Aspects 296:191–205
Acknowledgements
The author is graceful to the National Natural Science Foundation of China (Grant No. 51273155).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, JX., Li, X. & Zhang, CC. The synthesis and adsorption performance of polyamine Cu2+ imprinted polymer for selective removal of Cu2+ . Polym. Bull. 74, 3487–3504 (2017). https://doi.org/10.1007/s00289-017-1905-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-1905-6