Abstract
A study about the chemical structure, morphology and electric conductivity of polyfuran spherical particles synthesized by rf plasma glow discharges is presented in this work. This kind of particles is difficult to obtain with other syntheses at lower energy. The particles had average diameter that increased linearly with the energy of synthesis, from 172 to 456 nm with 2.91 nm/W growth rate. The chemical structure of the particles was analyzed by XPS relating the formation energy of the polymeric chemical groups with their C1s and O1s atomic orbital energies. The analysis indicated that almost all alpha C configurations of the initial furan molecules transformed into unions of monomers to construct linear polymers. On the other hand, almost all beta configurations remained unreacted in the furan rings and those that reacted transformed into the networked unions of the polymeric structure, approximately 10 %. The percentages of fragmentation, hydrogenation and resonance groups in the structure were also calculated. The particles thermally degraded in one phase from 200 to 600 °C, centered at 425 °C, suggesting that all synthesized particles have similar structure. The electric conductivity of the particles was between 10−11 and 10−09 S/m, increasing with the temperature, in which it is probable that the contact resistance among particles rule the transference of charges. The electronic activation energy was in the interval of semiconductors, from 0.031 to 3.83 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
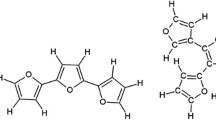
Furan (Fu) is an aromatic heterocycle formed by C4H4O; the molecule is shown in Fig. 1a with red, gray and white spheres representing O, C and H atoms, respectively. The electronic layer is shown as a surrounding network in red and blue colors which belong to the negative and positive polarization that is a consequence of the electronegative difference among the furan atoms, C = 2.5, O = 3.5 and H = 2.1, which have a highest difference of 1.4. This polarization makes furan molecules more reactive under electric fields than other heteroaromatic pentacycles as pyrrole or thiophene, which have an electronegative difference of 0.9 (N = 3.0) and 0.4 (S = 2.5), respectively. Due to the symmetry in the furan molecule, there are only two different C chemical states, 50 % due to the α configuration (C=CH–O), in the proximity of the O atom, and 50 % due to the β configuration (C=CH–C) in the others; see Fig. 1b. The condensed chemical formulas in Fig. 1b represent the atomic neighborhood of each C atom marked in bold.
Polyfuranes are arrangements of furan rings with different configurations, Fig. 1c. When they are constructed with linear segments or with a low cross-linking, the morphology of the polymer can be bi-dimensional forming films; however, when cross-linking increases, the growing of the polymer tends to be three dimensional forming particles. Cross-linking during the synthesis can be obtained by plasma due to the continuous collisions of accelerated particles which break the C–H bonds of furan molecules creating radicals that neutralize them, forming networked structures. The energy of the collisions has to be high enough to break mainly C–H bonds and not the furan backbone bonds such as C–C, C–O and C=C, which are stronger in the furan configuration because of the ring conjugation. In these conditions, plasmas can be used to synthesize and cross-link polymers simultaneously forming particles with furan or with many other molecules.
By conventional synthesis, polyfuran (PFu) has been homopolymerized and copolymerized with pyrrole and thiophene among other compounds [1–6]. However, there are practically no studies on the synthesis of PFu, which have reported the formation of particles, mainly the formation of thin films on different surfaces [7]. Comparatively with other syntheses, the syntheses by plasma occur with collisions among accelerated particles by electrical fields in gas phase whose mass efficiency is very small compared with liquid-phase polymerizations [8–12], so almost only films of different thicknesses can be obtained with this technique. Gok y Oksuz made a comparison of chemical and plasma syntheses of PFu films considering the possibility of particle formation using chemical synthesis [10]. However, the formation of polymer particles of furan has not been reported, but only particles derived from other pentagonal heterocycles, pyrrole and thiophene [13–18]. Because many applications of PFu have been directed to photoelectronics in transistors and light emission diodes, different works on electric conductivity have been published [19–23]. This paper presents the synthesis of plasma particles of polyfuran and their chemical structure and morphological properties. The chemical analysis includes the evolution of the main chemical states of PFu in the particles as a function of the synthesis energy.
Experimental
PFu particles were synthesized with glow discharges at 13.56 MHz, 10−1 mbar, power between 20 and 120 W during 180 min in a tubular vacuum glass reactor of 1500 cm3 with stainless steel flanges and electrodes of 7 cm diameter separated by 10 cm between them. The flanges have three access ports to support the electrodes and the inlets of chemical reagents. The pressure was reduced with an Alcatel Pascal 2010C1 vacuum pump coupled with an Alcatel LNT 25S gas condenser cooled with liquid nitrogen. The reactor was connected to a radio frequency power source Cesar Dressler. Furan (Aldrich, 99 %) was introduced into the reactor from a sealed container at 10 °C, because its boiling temperature was close to the room temperature (T b = 32 °C). The vapor flow to the reactor was manually controlled through pressure variations.
Morphologic analyses of the particles were performed by scanning electron microscopy (SEM) using a JEOL JSM-5900LV apparatus, thermal analyses were done with a TA Instrument SDT Q600 equipment and the surface chemical analyses of the polymers were performed using X-ray photoelectron spectroscopy (XPS) with a Thermo K-Alpha apparatus. The electrical conductivity was calculated through the resistance measured with a device of Cu parallel electrodes. The particles were located between the electrodes with slight compression. This is a volumetric measure, because the charges have to travel from one particle to another until they reach the other electrode. This means that the contact area between particles is critical in the transference of charges and may rule the process.
Results and discussion
Morphological analysis
Figure 2 shows the SEM micrographs of particles synthesized at different powers with diameter from 70 to 3000 nm. The particles have a spherical profile regardless of the power of synthesis; some particles join in groups of two or three and others are grouped in clusters of different sizes joined by electrostatic attraction. Figure 3a shows the harmonic diameter distributions as a function of the power of synthesis; 280 particles were measured in each case. The tendency of the mean diameter is to have a linear increase with the energy of synthesis, from 172 to 456 nm with 2.91 nm/W growth rate; see Fig. 3b. The width of the distribution increases also with the energy of synthesis, giving wider distributions at higher energy.
Thermal analysis
Figure 4 shows the thermal decomposition of polyfuran particles as a function of the power of synthesis. The thermograms show that there are only small differences between them. In the range from 20 to 100 °C, the particles lose mass up to 2 %, mainly moisture, which indicates low hygroscopy. Between 100 and 200 °C, the mass loss goes up to 7 %, which can be from low molecular weight compounds such as small fragments and oligomers. At 200 °C, the decomposition of the PFu molecular structure starts and ends approximately at 600 °C, with a mass loss of 80 %, depending on the power of synthesis. The center of this decomposition is at 425 °C. After 600 °C, the material is composed only of pyrolyzed residues. From a global point of view, the thermal decomposition indicates that the structure of the particles is mainly composed of very similar polyfuran structures, independently of the power of synthesis.
Superficial atomic energy states
The superficial chemical structure is important for any type of application because it will be in direct contact with the surrounding medium. The elemental content of the superficial layers of PFu particles obtained by XPS survey spectra scanned from 0 to 1100 eV binding energy (BE) is shown in Fig. 5. BE was obtained by the expression: BE = hv – KE − W, in which KE is the kinetic energy of the released electrons as a consequence of an incident X-ray beam with an hv energy and W is the work spent in the process. The relationship among these variables is an energy balance in the releasing process. XPS spectrometers calculate these variables in different ways giving the BE distribution of survey and detailed spectra of each element. Each orbital energy distribution has a distinctive maximum BE which is used to identify and quantify the participation of the chemical elements in the material [24, 25]. It is important to remark that XPS analyses are done only on the exterior layers of the samples, with a depth of approximately 1 nm.
Figure 5 shows that the PFu particles synthesized in this work are composed of C, O, N and Si. C and O are part of the furan structure, but N and Si can be attributed to the residual atmosphere and surface contamination by the glass on which the particles were synthesized. It is interesting to note that N residual atoms participate in the polymers at 60 W and higher power. The Table inside Fig. 5 shows the atomic elemental percentage and the atomic C/O ratios on the surface. The C/O stoichiometric ratio in the furan molecule is 4, four carbon atoms for each oxygen atom; however, the synthesized particles have atomic ratios in the 4.63–12.9 interval, much higher than the stoichiometric case, indicating some kind of fragmentation and carbonization in the outer layers of the particles. A more detailed discussion about this effect is presented in the following sections.
The main surface atomic energy states of the particles were studied through their C1s and O1s orbitals; see Figs. 6a and 7a that show the energetic distribution of C1s and O1s orbitals of PFu particles synthesized at 40 W. This power was taken as an example to fit the energetic distribution in several interior curves and to find their main chemical states. In the particles synthesized at different powers, only the final percentage of chemical states is presented, because all the analyses are similar; see Figs. 6b and 7b. The energy distributions obtained in the XPS spectrometer were adjusted with Gaussian curves, in which the width at half maximum (FWHM) was based on data handbooks for advanced peak fitting of monochromatic XPS spectra [26]. The atomic chemical states were assigned, considering the probable bonding possibilities of each element in its atomic neighborhood, because the energetic balance in the atoms is closely influenced by its atomic environment.
The C1s energetic distribution was adjusted with five or six Gaussian curves with FWHM = 1.0 ± 0.1 eV in the I (c/s) vs BE (eV) format; see Fig. 6a. The curves are identified by their maximum BE and associated with a state or combination of chemical states whose formation energy is very close, in which the % area is equivalent to the % of participation in the polymers. In Figs. 6a and 7a, the chemical states are inserted in each curve with the respective approximated formation energy in eV calculated in the aromatic furan configuration. Each curve is numbered in this format starting with the lowest BE, from the left. The states are expressed in a condensed manner, but they are pointed to a typical chemical structure of cross-linked PFu (X-PFu) inserted in the graphs, to identify them.
Curves 2, 3 and 4 in Fig. 6a can be identified in the chemical structure of X-PFu. Curve 2 is associated with the C=CH–C configuration (beta in Fig. 1b) and can be identified with C of furan that almost did not react during the synthesis, because its percentage in the monomer is 50 % and in the polymer 46.4 %. The small percentage that reacted is partially associated with curve 4, represented by the formula C=C–C2, which is the union of two furan molecules by dehydrogenation of the beta (C=CH–C) configuration. The participation of this chemical state reduces as the power of synthesis increases; see Fig. 6b, suggesting an increasing dehydrogenation with the energy applied to the synthesis.
The other C chemical states in the monomer, C=CH–O (alpha in Fig. 1b), cannot be identified with the curves of the BE distribution, because its formation energy lies between curves 1 and 2. This means that almost the entire C=CH–O configuration in furan reacted during the polymerization to form other chemical states, as that on curve 3, C=CO–C, which is the union of two furan molecules through the alpha configuration.
Curve 1 represents the chemical configuration of C2–C–O2, according to its formation energy and cannot be associated with the chemical structures of X-PFu or furan. This suggests some kind of molecular oxidation during the synthesis, which at 40 W is approximately 15 % of the total C chemical states. However, in Fig. 6b, the evolution of curves 1 and 3 with the power of synthesis indicates that when one chemical state reduces, the other increases suggesting that their structures are closely related in the particles. Curve 5 can be identified with some molecular resonant segments of X-PFu, C=C=C and O=C=C, 5 % at 40 W, with a participation that slightly increases with the energy applied to the synthesis. Curve 6 appears only after 60 W and can be associated with triple bonds, C≡C–N, which suggests that N atoms participate in the synthesis at those energies. Triple bonds can be associated in C with the highest oxidized configurations.
O1s energetic distributions were adjusted with three Gaussian curves with FWHM = 1.4 ± 0.1 eV, see Fig. 7a. As C1s, each curve was assigned with a state or combination of states, according to its formation energy. Curve 1 corresponds to hydrogenated groups in the polymers, C–O–H, probably hydrogen bonds formed due to the electrostatic attraction between H and O in the structure, because they are not part of the PFu molecule. Curve 2 is identifiable with the oxygen position in the furan rings in the polymer networks, C–O–C, which in general increases with the power of synthesis, from approximately 50 to 65 %; see Fig. 7b. Curve 3 can be associated with resonance groups in the polymers, C=O, due to the electronic conjugation in the furan rings. The evolution of curves 1 and 3 with the power of synthesis indicates that when one participation reduces, the other increases suggesting also that their structures are closely related in the particles, as curves 1 and 3 in C1s.
From a global point of view, this analysis indicates that the particles grew using alpha positions in the furan rings and that beta positions were used in approximately 10 % of the total C configurations, see the evolution of curve 4, C=C–C2, in Fig. 6b. Linear polyfuran usually grows using alpha configurations, so any additional beta configuration used to join furan molecules can be seen as indicators of cross-linking in the polymer, which in this work reached up to 10 % of the total C configurations, considering that canonic linear PFu would have 50 % of beta configurations. Another interesting point is resonance in PFu, in which at least two resonance arrangements were identified, approximately 5 % in C, C=C=C or O=C=C, and an average of 30 % in O atoms as C=O. This effect suggests different resonances in the PFu structure, at least more than the effect of the conjugated union of carbon atoms.
Electric conductivity
Figure 8 shows the electric conductivity of polyfuran particles as a function of temperature, from 35 to 100 °C. To reduce the influence of moisture in the conductivity, the particles were heated up to 100 °C and slowly cooled to room temperature or to the limit of the sensing instrument; the data presented here were collected during the cooling stage. The electric conductivity was calculated between 10−11 and 10−09 S/m, increasing linearly in a semilog scheme with the power of synthesis. Some particles presented two slopes, which suggest two different mechanisms of charge transference. The conductivity is located in the region of non-conductor materials, maybe because of a high contact resistance among particles, which apparently rules the transference of charges. Because of this effect, no association could be found between the chemical structure and the conductivity. The electronic activation energy (E a) calculated with the Arrhenius equation varied from 0.031 to 3.83 eV, in the interval of organic semiconductors, except those particles with E a >2 eV in which the conductivity highly changed with temperature.
Conclusions
Polyfuran spherical particles were synthesized by plasma at power from 20 to 120 W with average diameter increasing linearly from 172 to 456 nm with 2.91 nm/W growth rate. The main thermal degradation of the particles occurred from 200 to 600 °C, centered at 425 °C with a similar decomposition profile, which suggests that the particles had similar structure with some residues of the chemical reactions, as fragments of furan and oligomers. The superficial chemical structure of the particles was analyzed by XPS, finding that almost all alpha C configurations in the initial furan molecules, C=CH–O, reacted during the polymerization to produce chemical unions of furan molecules, C=CO–C, and other oxidized states. On the contrary, almost all C configurations in beta positions, C=CH–C, remained unreacted and those that reacted produced furan ring unions, C=C–C2, approximately 10 %, which indicates a slight networked structure in the particles. In a similar analysis of C1s orbitals in polypyrrole particles, the percentage of networking in the structure was calculated up to 10 %, additionally to the networking in linear polymers which is considered 50 % [25].
The analysis of the oxygen orbitals in PFu particles indicated that more than half of oxygen atoms have the chemical configuration of furan rings, C–O–C, and that the other percentage is divided between hydrogen bonds, C–O–H and a C=O union, probably as a resonance. Triple bonds, C≡C–N, found in the polymeric structure were considered an evidence of fragmentation giving high oxidized groups due to the high energy collisions among the particles in the plasma. These groups were found only at the energy of synthesis higher than 60 W in which N atoms also participated and were considered impurities in the particles.
The electric volumetric conductivity of the particles was between 10−11 and 10−09 S/m increasing with temperature with activation energy from 0.031 to 3.83 eV. The conductivity is in the region of non-conductor materials, maybe because of a high contact resistance among particles which rule the transference of charges; however, E a is located in the interval of organic semiconductors. In these conditions, no direct association could be found with the structure of the particles.
References
Balbas A, Gonzalez-Tejera MJ, Tortajada J (2001) Influence of the electron correlation on computed properties of furan oligomers. J Mol Struct 572:141–150. doi:10.1016/S0166-1280(01)00581-4
Shilabin AG, Entezami AA (2000) Electrochemical behavior of conducting polyfuran derivatives containing pyrrole, thiophene and ethylenic spacers. Eur Polym J 36:2005–2020. doi:10.1016/S0014-3057(99)00262-1
Salzner U, Lagowski JB, Pickup PG, Poirier RA (1998) Comparison of geometries and electronic structures of polyacetylene, polyborole, polycyclopentadiene, polypyrrole, polyfuran, polysilole, polyphosphole, polythiophene, polyselenophene and polytellurophene. Synth Met 96:177–189. doi:10.1016/S0379-6779(98)00084-8
Wan XB, Zhang W, Jin S, Xue G, You QD, Che B (1999) The electrochemical copolymerization of pyrrole and furan in a novel binary solvent system. J Electroanal Chem 470:23–30. doi:10.1016/S0022-0728(99)00205-3
Demirboga B, Onal AM (1999) Electrochemical polymerization of furan and 2-methylfuran. Synth Met 99:237–242. doi:10.1016/S0379-6779(98)01509-4
Talu M, Kabasakaloglu M, Yıldırım F, Sarı B (2001) Electrochemical synthesis and characterization of homopolymers of polyfuran and polythiophene and biopolymer films polyfuran–polythiophene and polythiophene–polyfuran. Appl Surf Sci 181:51–60. doi:10.1016/S0169-4332(01)00355-5
Zuñiga R, Cruz GJ, Olayo MG, Sanchez-Mendieta V, Gomez LM, Gonzalez-Torres M, Gonzalez-Salgado F, Morales J (2015) Synthesis and superficial characterization of plasma polyfuran thin films. Polym Bull 72:839–850. doi:10.1007/s00289-015-1309-4
Kumar DS (1999) Dielectric properties of plasma polymerized furan thin film capacitors. Mater Lett 4:1–4. doi:10.1016/S0167-577X(99)00093-2
Kumar DS (2000) On the mechanism of electrical conduction in plasma polymerized furan films. J Mater Sci. 35:4427–4430. doi:10.1023/A:1004873410933
Gok A, Oksu L (2007) Atmospheric pressure plasma deposition of polyfuran. J Macromol Sci Part A Pure Appl Chem 44:1095–1099. doi:10.1080/10601320701524021
Satulu V, Galca AC, Aldica GV, Dinescu G (2010) Polymer-like thin films obtained by RF plasma polymerization of pentacyclic monomers. J Optoelectron Adv Mater. 12(3):631–636
Uygun A, Oksuz L, Yavuz AG, Gulec A, Sen S (2011) Characteristics of nanocomposite films deposited by atmospheric pressure uniform RF glow plasma. Curr Appl Phys 11:250–254. doi:10.1016/j.cap.2010.07.017
Yang P, Zhang J, Guo Y (2009) Synthesis of intrinsic fluorescent polypyrrole nanoparticles by atmospheric pressure plasma polymerization. Appl Surf Sci 225:6924–6929. doi:10.1016/j.apsusc.2009.03.016
Cao J, Matsoukas T (2004) Synthesis of hollow nanoparticles by plasma polymerization. J Nanoparticles Res. 6:447–455. doi:10.1007/s11051-004-2716-x
Wang J, Neoh KG, Kang ET (2004) Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 446:205–217. doi:10.1016/j.tsf.2003.09.074
Cruz GJ, Olayo MG, López OG, Gómez LM, Morales J, Olayo R (2010) Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer 51:4314–4318. doi:10.1016/j.polymer.2010.07.024
Vasquez-Ortega M, Ortega M, Morales J, Olayo MG, Cruz GJ, Olayo R (2014) Core-shell polypyrrole nanoparticles obtained by atmospheric pressure plasma. Polym Int. doi:10.1002/pi.4756
Jobanputra MC, Durstock MF, Clarson SJ (2003) Investigation of plasma polymerized benzene and furan thin films for application in opto-electronic devices. J Appl Polym Sci 87:523–528. doi:10.1002/app.11450
Atta NF, El-Kady MF, Galal A (2009) Palladium nanoclusters-coated polyfuran as a novel sensor for catecholamine neurotransmitters and paracetamol. Sens Actuators B Chem. 141:566–574. doi:10.1016/j.snb.2009.07.002
Saha Sardar P, Ghosh S, Biswas M, Ballav N (2008) Highly conductive polyfuran-13X zeolite-polyaniline composite. Polym J 40(12):1199–1203. doi:10.1295/polymj.PJ2008172
Sen S, Bardakci B, Yavuz AG, Gok AU (2008) Polyfuran/zeolite LTA and adsorption properties. Eur Polym J 44:2708–2717. doi:10.1016/j.eurpolymj.2008.05.018
Amiri A, Baghayeri M, Kashmari M (2016) Magnetic nanoparticles modified with polyfuran for the extraction of polycyclic aromatic hydrocarbons prior to their determination by gas chromatography. Microchim Acta 183(1):149–156. doi:10.1007/s00604-015-1622-5
Turkaslan EB, Aktan T, Oksuz L, Oksuz AU (2016) Use of polyfuran/chitosan composite films deposited by atmospheric pressure plasma glow discharges as glucose sensors. Asian J Chem 28(5):941–946. doi:10.1423/ajchem.2016.19093
Gonzalez-Torres M, Olayo MG, Cruz GJ, Gomez LM, Sanchez-Mendieta V, Gonzalez-Salgado F (2014) XPS study of the chemical structure of plasma biocopolymers of pyrrole and ethylene glycol. Adv Chem ID 965920:1–8. doi:10.1155/2014/965920
Gomez LM, Cruz GJ, Olayo MG, Gonzalez-Torres M, Gonzalez-Salgado F, Lopez-Gracia OG (2014) Analysis of crosslinking in polypyrrole particles synthesized by plasma. Polym Bull 71(12):3275–3287. doi:10.1007/s00289-014-1249-4
Crist BV (1998) Advanced peak-fitting of monochromatic XPS spectra. J Surf Anal. 4:428–434
Acknowledgments
The authors thank Jorge Perez for the support in the SEM analyses and to CONACyT for the partial financial support to this work with the Projects 130190 and 154757.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olayo, M.G., Zúñiga, R., González-Salgado, F. et al. Structure and morphology of plasma polyfuran particles. Polym. Bull. 74, 571–581 (2017). https://doi.org/10.1007/s00289-016-1730-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1730-3