Abstract
This work presents a study about the synthesis of polyfuran films by plasma and their structural, morphological, electrical and hydrophilic characteristics. The syntheses were carried out at low pressure with resistive electrical glow discharges. The films have thickness in the 7–61 μm interval with a linear tendency as a function of the power of synthesis. The functional groups of the monomer, –C=C– and C–O, and other as C=O produced during the polymerization, were found in the polymers probably as dehydrogenation of =C–H groups in the monomer. The films have contact angles between 52° and 92° for distilled water, but with the addition of salts in KR solutions, the angles slightly increased reducing the hydrophilicity of the system. The conductivity of polyfuran films was calculated in the range of 10−9–10−11 S/m with two trends, one with high electronic activation energy at low temperature, and another with low activation energy at high temperature. The electronic activation energy in the first trend varied between 1.01 and 1.9 eV and in the second trend between 0.26 and 0.08 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
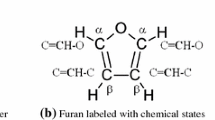
Pyrrole (Py), thiophene (Th) and furan (Fu) are heterocyclic conjugated monomers with 4 carbon atoms and a fifth element which can be nitrogen, sulfur or oxygen, respectively. Some plasma polymers of pyrrole (PPy) have shown biocompatibility because of their amine groups similar to those in the human body, polythiophene (PTh) has been distinguished by its electrochromic properties, however, polyfuran (PFu) has not been extensively studied even though it may have many potential applications due to its oxygenated groups in conjugated cyclic structures [1]. As PPy and PTh, PFu synthesized by plasma can also organize in linear and crosslinked configurations, see Fig. 1. The presence of oxygenated groups influences the hydrophilicity of PFu and might be an important factor in its biocompatibility. So far, the potential applications of PFu have been related to its electrochromic properties and redox capacity in transistors, light emitting diodes and sensors [2–4]. Only few reports have been published in the synthesis of PFu as homopolymer [5–9], however, copolymers with PFu have been obtained based on furan/pyrrole combinations [10], furan/2-methylfuran [11] and PFu/PTh films [12].
The plasma synthesis of PFu, whose polymer can be named PPFu, has been even less studied than the chemical synthesis. Kumar in 1999 and 2000 synthesized PPFu thin films to study the effect of temperature on their electrical conductivity [13, 14]. Gok et al. in 2007 synthesized PPFu by atmospheric pressure plasma, and found that the polymer formed on glass substrates can be used as support for biosensors [15]. The main characteristic of plasma polymers is that they can be synthesized by collisions of accelerated particles in electric fields only with the monomer and some of their fragments, depending on the applied energy, producing clean and sterile polymers. In those conditions, PPy has been reportedly formed by partially crosslinked segments including fragments created by recombination of radicals during the polymerization [16]. To study potential applications of PPFu, this work studies the synthesis by plasma of polyfuran films and their electrical and hydrophilic properties with fluids with salt concentration and composition similar to the extracellular fluids of the human body.
Synthesis of PPFu
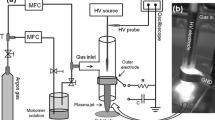
The PPFu syntheses were carried out in a 1,500 cm3 vacuum tubular glass reactor with stainless steel electrodes of 7 cm diameter as shown in Fig. 2. The reactor ends have stainless steel flanges with three access ports to introduce two electrodes with a central separation of 6 cm to apply radiofrequency (RF) electric discharges between them. The reactor was connected to an Alcatel Pascal 2010 C1 vacuum pump and to an Alcatel LNT 25 S liquid nitrogen condenser for the residual gases of the chemical reactions, pressure was 10−1 mbar measured with a Pirani gauge 945 MKS Instruments HPS. The electric glow discharges were set at 13.56 MHz with a Dressler Cesar 136 power generator coupled with a matching network resistance in which one electrode was grounded and the other connected to the RF output, the net power applied between the electrodes varied between 20 and 100 W. The reaction time was 180 min, during this time films were produced with sufficient thickness for the analyses presented in this work, contact angle, conductivity, etc. In other syntheses of plasma polymers, it has been established that the thickness of films is a linear function of the synthesis time [16–18] or the power applied to the synthesis [19].
Only furan (Aldrich, ≥99 %) and the residual atmosphere in the reactor were the chemical agents participating in the synthesis, there were no carrier gases or dopants. The monomer was kept at 10 °C in order to prevent a rapid evaporation of furan (T boil = 32 °C) to the reactor with the room temperature variations. The reactor walls were cleaned and prepared to serve as a support for the synthesis of PPFu. The polymers were obtained as thin films adhered to the reactor walls, which were washed in situ, swelled with distilled water and carefully separated from the walls with a thin spatula. The films without substrates were later used for the different analyses. Contact angle analysis of PPFu needed a special support, so glass sample holders were used between the electrodes where the polymer was simultaneously synthesized. These supported films were dedicated to contact angle analysis and were not detached.
Materials and methods
The thickness of the films was obtained with a Mitutuyo Micrometer with 1 μm resolution. The morphology was analyzed in a JSM-5900LV Jeol scanning electron microscope (SEM). The PPFu structure was studied with a FT-IR i5 Thermo Scientific infrared spectrophotometer applying 100 scans in attenuated reflectance mode in the 4,000–550 cm−1 interval. The surface of PPFu was analyzed by X-Ray photoelectron spectroscopy (XPS) with a Thermo K-Alpha apparatus with a monochromatic X-ray Al source (1,486.6 eV). The contact angle was measured on the surface of the polymers with drops of distilled water and a Krebs–Ringer (KR) solution whose concentration was in mM: NaCl = 118, KH2PO4 = 1.3, KCl = 4.7, CaCl2 = 2.5, MgSO4 = 1.17 and NaHCO3 = 25.0. KR solutions have salts in concentration similar to those in the human body and can be used to recreate some human fluids. In this work, they were used to study the hydrophilicity of PPFu with fluids of human salt content. For this study, drops of 2 µL on the same spot were deposited, so that the volume of the drop was cumulative from approximately 5 to 30 μL. A Ramé-Hart 250 goniometer was used in this task. The electric conductivity of PPFu was calculated using the resistance of the polymers measured in an arrangement of two parallel plates of Cu with an OTTO MX-620 high resistance meter in the 55°–100 °C interval. The temperature was measured with a Mastech Mas-345 digital multimeter.
Results and discussion
Thickness of films
The PPFu average thickness as a function of the power of synthesis is presented in Fig. 3. Each value represents the average of 12 measurements. The thickness was measured in the 7–61 μm interval with a linear tendency as a function of the power of synthesis. The growth rate was 0.63 μm/W.
Morphology
PPFu superficial images in Fig. 4 show that the surfaces have bubbles with diameter 0.25–7.5 µm randomly distributed. As the polymerization occurs in gas phase, the bubbles can be caused by droplets of monomer vapor trapped in the process. The PPFu synthesized at 40 W has additionally some spherical microparticles on the surface. There is not a clear effect of the power of synthesis on the film surface, only on the thickness. The size of the bubbles on the SEM images was measured at different magnifications with approximately 250 samples. A normal distribution was used to find the harmonic mean, which was between 0.97 and 2.4 µm, increasing with the power of synthesis, except the distribution calculated at 80 W, which shows a different behavior, see Fig. 5. The width of the distribution also increased with the power of synthesis. Protrusions, bubbles or crests in general have a direct impact on the hydrophilicity or hydrophobicity of the surfaces [19].
Structure of the polymers
Figure 6 shows the infrared spectra of Fu and PPFu synthesized in this work taken by attenuated total reflectance (ATR), which is based mainly on the surface of the films, bubbles included. All signals were normalized to fit the 0–100 % interval with the most intense peak. In general, all polymer spectra are similar, but different from Fu, in which the most important peaks associated with =C–H groups, 985 and 738 cm−1, reduced their intensity probably because many H are replaced by other furan rings to form chains or crosslinked networks. The absorptions of Fu that survived in the polymers can be found in 1,060 and 1,374 cm−1. The first one has been observed in plasma copolymers of ethylene glycol, which also have oxygen in its structure as furan and is related with C–O groups [20]. The second one has been observed in plasma polymer-like thin films of PPFu belonging to C=C in plane ring vibrations [21]. The 1,060 cm−1 peak can also be assigned to Si–O groups in the polymers, however, as this absorption may arise from superficial contamination with the glass reactor walls where the polymers were synthesized, they were not considered as part of PPFu. The superficial content of Si can be seen in Table 1.
One of the absorptions in the polymers that increase due to the polymerization at low power (20–60 W) is centered at 2,362 cm−1, which corresponds to multiple bonds involving combinations of C=O and C=C [22]. Another important absorption in PPFu can be found at 1,712 cm−1 which also belongs to C=O and/or C=C that was also seen in other polymers synthesized by plasma as polystyrene [23], polypyrrole [16], polyaniline [18], polyallylamine and polyethylene glycol [24] which are associated with different aspects of oxidation, dehydrogenation and recombination. At higher power, one of the most intense absorptions is centered on 2,931 cm−1 which belongs to aliphatic C–H groups and is an indicative of fragmentation of Fu molecules, which increases with the power. The absorption at 1,450 cm−1 belongs to conjugated =C– groups, which increases with the energy of the plasma discharges, probably forming long conjugated segments as a function of the power of synthesis.
Superficial elemental analysis
A superficial elemental analysis of PPFu was obtained by XPS. The signals detected were C1s, O1s and small traces of N1s and Si2p that can be due to superficial contamination, see Table 1. Nitrogen can be due to atmospheric interaction, but Si can arise from the glass of the reactor where the polymers were synthesized. As the films were separated with a thin spatula, it is possible that small scratches could have been made to the glass, especially in the sample synthesized at 80 W, which has the highest Si percentage. C and O are part of the Fu structure in which the stoichiometric C/O atomic ratio is 4, 1 oxygen for every 4 carbon atoms, however, in PPFu this ratio is lower than 4 varying from 1.26 to 3.95, increasing in general terms with the power of synthesis, up to almost reaching the stoichiometric Fu ratio. This effect suggests a higher reactivity of the monomer and intermediate compounds with the power of synthesis, which may induce a higher polymerization degree in the polymer. However, when surfaces are exposed to the atmospheric oxygen or humidity, oxidation increases, reducing the C/O ratio, which in some way alters the tendency of the ratios as in the case of the sample synthesized at 80 W. Thus, the ratios show a balance between the chemical structure and the atomic distribution of oxygen on the surface.
Contact angle
An analysis of the advancing contact angles of PPFu with deionized water and a Krebs–Ringer solution was done to evaluate the hydrophilicity of the polymers. For this study, drops of approximately 2 µL were deposited on the surface and later added consecutively to the initial drop. With this technique, the influence of the protrusions on the surface with the contact angles can be evaluated, because as the drop increases its volume, the protrusions have less dimensional influence on the contact angles. A photograph of each drop on the surface was taken to measure the tangent of the drop with respect to the surface. Two sides of the drop were averaged to calculate the tangent. The resulting contact angles are presented in Fig. 7, where each value represents the average of 10 measurements.
The contact angles varied from 52° to 92° with a tendency to stabilize at larger drop volumes. This could be due to the dimension of the bubbles on the surface, which although are several times smaller than the drop diameter, influence the contact angles in such a way that are less significant as the drop becomes larger. Figure 7 shows also that the angles are not a function of the power of synthesis. The lowest angles were measured in PPFu synthesized at 20 and 60 W, and the highest angles belonged to PPFu synthesized at 40 W.
For distilled water, the averaged angles are between 52º and 92º and with the addition of salts in the KR solution, the angles reduce their variation with averages from 56° to 92°. An imaginary line at 90º marks the limit of hydrophilicity, surfaces with greater angles can be considered hydrophobic [25]. In the hydrophilic zone, surfaces with angles lower than 45º can be considered markedly hydrophilic, and in the opposite case are considered low hydrophilic. From a global point of view, contact angles of PPFu are located in the low hydrophilic zone, particularly the samples synthesized at 40 W which increase up to 90º and may be due to the presence of a small amount of spherical microparticles on the surface that do not appear on the other PPFu, see Fig. 4b. As the contact angles increase, it is more difficult to wet the surface and may take longer to interact with cells when these materials are implanted in the human body.
Electric conductivity
The electric volumetric conductivity of PPFu as a function of temperature in the 55°–100 °C interval is shown in Fig. 8a. The conductivity was calculated with the resistance of the polymers measured in capacitive arrangements with the sample in the middle of two Cu electrodes. The values were between 8 × 10−11 and 3.4 × 10−9 S/m increasing one order of magnitude as a function of temperature in each power of synthesis. The electronic activation energy (Ea) in eV of PPFu was also calculated, the values are at the side of each power line in the graph.
Most PPFu has two conductivity trends, one with high Ea at low temperature and another with low Ea at higher temperature. The lowest and highest Ea are 0.08 and 1.25 eV, respectively, which locates PPFu among organic semiconductors [26]. This behavior suggests at least two mechanisms for the transference of electric charges, which can be due to intrinsic factors as the chemical structure and to extrinsic factors as the absorbance of humidity in the polymers [27].
The variation of Ea expressed in Fig. 8b shows that Ea decreases as a function of the power of synthesis, rapidly between 20 and 40 W and slower, almost linear, at higher power. This behavior could be due to a progressive variation in the structure of the polymers with the power of synthesis; consequently, intrinsic factors may be dominant in the first transference of charges. The power of synthesis also influences the temperature of the inflection points in which another mechanism of transference of charges appears, see Fig. 8c. The temperature of the inflection points increase linearly as a function of the power of synthesis, which also suggests that intrinsic factors in PPFu determines the point in which the first mechanism reduces substantially its influence, because the second Ea is much lower than the first Ea, 0.08, 0.082 and 0.26 eV. In PPFu synthesized at 20 W, only one mechanism for transference of charges was detected, with Ea of 1.9 eV. In PPFu synthesized at 100 W, the inflection point is barely observed at 94 °C.
Conclusions
To prepare heterocyclic polymer thin films, polyfuran was synthesized by plasma as compact layers with bubbled surfaces whose mean diameter was between 0.97 and 2.4 µm, film thickness and bubble diameter increased with the power of synthesis. In plasmas, the chemical reactions start in gas phase and end on the surfaces of the reactor promoted by the constant collisions of the monomers with accelerated electrons and ions by electrical fields. In these conditions, the chemical structure of PPFu has a combination of the initial oxygenated heterocycles and other segments formed by dehydrogenation and recombination of radicals produced in the plasma. However, the atomic C/O ratio indicates that as the power of synthesis increase, the polymers tend to have the stoichiometric ratio of furan, which suggests that the polymerization increases with the energy of synthesis.
To evaluate the hydrophilicity of PPFu films, the contact angles with distilled water and KR solutions were measured on the surface resulting between 52º and 92º increasing with the addition of salts at low drop volume. The results suggest that PPFu has low hydrophilicity probably because of the bubbled surfaces. The conductivity of polyfuran has two trends, one with high electronic activation energy at low temperature and another with low activation energy at high temperature, which suggests two mechanisms for transferring electric charges in which the intrinsic factors related with the chemical structure are dominant in the first transference of charges. The temperature, in which starts a second mechanism of transference of charges, increases linearly with the power of synthesis. This suggests that the structure determines the position of this point. The activation energy is between 0.08 and 1.25 eV, in the range of organic semiconductors.
References
Carrillo I, Fernández-Martín F, Barba C, Sánchez de la Blanca E, González-Tejera MJ, Hernández-Fuentes I (1999) Thermal stability of polyfuran/pechlorate doped films. Polym Bull 43:269–276. doi:10.1007/s002890050562
Acebedo DF, Miras MC, Barbero CA (2005) Solid support for high-throughput screening of conducting polymers. J Comb Chem 7:513–516
Abou-Elenien GM, El-Maghraby AA, El-Abdallah GM (2004) Electrochemical relaxation study of polythiophene as conducting polymer (I). Synth Met 146:109–119. doi:10.1016/j.synthmet.2004.04.020
Hotta S (1997) Handbook of organic conductive molecules and polymers 2, Conductive polymers: synthesis and electrical properties. Wiley, New York, p 309
Glenis S, Benz M, LeGoff E, Schindler JL, Kannewurf CR, Kanatzidis MG (1993) Polyfuran: a new synthetic approach and electronic properties. J Am Chem Soc 115:12519–12525. doi:10.1021/ja00079a035
Wan X, Yan F, Jin S, Liu X, Xue G (1999) Low potential electrochemical synthesis of polyfuran and characterization of the obtained free-standing film. Chem Mater 11:2400–2407. doi:10.1021/cm9900453
Balbas A, Gonzalez-Tejera MJ, Tortajada J (2001) Influence of the electron correlation on computed properties of furan oligomers. J Mol Struct 572:141–150. doi:10.1016/S0166-1280(01)00581-4
Shilabin AG, Entezami AA (2000) Electrochemical behavior of conducting polyfuran derivatives containing pyrrole, thiophene and ethylenic spacers. Eur Polym J 36:2005–2020. doi:10.1016/S0014-3057(99)00262-1
Salzner U, Lagowski JB, Pickup PG, Poirier RA (1998) Comparison of geometries and electronic structures of polyacetylene, polyborole, polycyclopentadiene, polypyrrole, polyfuran, polysilole, polyphosphole, polythiophene, polyselenophene and polytellurophene. Synth Met 96:177–189. doi:10.1016/S0379-6779(98)00084-8
Wan XB, Zhang W, Jin S, Xue G, You QD, Che B (1999) The electrochemical copolymerization of pyrrole and furan in a novel binary solvent system. J Electroanal Chem. 470:23–30. doi:10.1016/S0022-0728(99)00205-3
Demirboga B, Onal AM (1999) Electrochemical polymerization of furan and 2-methylfuran. Synth Met 99:237–242. doi:10.1016/S0379-6779(98)01509-4
Talu M, Kabasakaloglu M, Yıldırım F, Sarı B (2001) Electrochemical synthesis and characterization of homopolymers of polyfuran and polythiophene and biopolymer films polyfuran/polythiophene and polythiophene/polyfuran. Appl Surf Sci 181:51–60. doi:10.1016/S0169-4332(01)00355-5
Kumar DS (1999) Dielectric properties of plasma polymerized furan thin film capacitors. Mater Lett 4:1–4. doi:10.1016/S0167-577X(99)00093-2
Kumar DS (2000) On the mechanism of electrical conduction in plasma polymerized furan films. J Mater Sci 35:4427–4430. doi:10.1023/A:1004873410933
Gok A, Oksuz L (2007) Atmospheric pressure plasma deposition of polyfuran. J Macromol Sci Part A Pure Appl Chem 44:1095–1099. doi:10.1080/10601320701524021
Cruz GJ, Morales J, Olayo R (1999) Films obtained by plasma polymerization of pyrrole. Thin Solid Films 342:119–126. doi:10.1016/S0040-6090(98)01450-3
Cruz GJ, Morales J, Castillo-Ortega MM, Olayo R (1997) Synthesis of polyaniline films by plasma polymerization. Synth Met 88:213–218. doi:10.1016/S0379-6779(97)03853-8
Olayo MG, Cruz GJ, López S, Morales J, Olayo R, (2010) Conductivity and activation energy in polymers synthesized by plasmas of thiophene. J Mex Chem Soc 54(1):18–23. http://www.redalyc.org/articulo.oa?id=47516172004
Colin E, Olayo MG, Cruz GJ, Carapia L, Morales J, Olayo R (2009) Affinity of amine-functionalized plasma polymers with ionic solutions similar to those in the human body. Prog Org Coat 64:322–326. doi:10.1016/j.porgcoat.2008.08.033
Gonzalez-Torres M, Cruz GJ, Olayo MG, Gomez LM, Lopez OG (2012) Plasma copolymerization of pyrrole and ethylenglycol to obtain porous polymers. Superficies y Vacío 25(3):179–182. http://smcsyv.fis.cinvestav.mx/supyvac/25_3/SV25317912.pdf
Satulu V, Mitu B, Galca AC, Aldica GV, Dinescu G (2010) Polymer-like thin films obtained by RF plasma polymerization of pentacyclic monomers. J Optoelectron Adv Mater 12(3):631–636
Cruz GJ, Olayo MG, Lopez OG, Gomez LM, Morales J, Olayo R (2010) Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer 51:4314–4318. doi:10.1016/j.polymer.2010.07.024
Morales J, Olayo MG, Cruz GJ, Herrera-Franco P, Olayo R (2006) Plasma modification of cellulose fibres for composite materials. J Appl Polym Sci 101:3821–3828. doi:10.1002/app.24085
Gómez LM, Morales P, Cruz GJ, Olayo MG, Palacios JC, Morales J, Olayo R (2009) Plasma copolymerization of ethylene glycol and allylamine. Macromol Symp 283–284:7–12. doi:10.1002/masy.200950902
Shackelford JF (1995) Ciencia de Materiales para Ingenieros, Ed. Hispanoamérica, México
Malhotra BD (2002) Handbook of poymers in electronics. Rapra Technology Limited, UK
Palacios JC, Olayo MG, Cruz GJ, Chavez-Carvayar JA (2014) Meyer-Neldel rule in plasma polythiophene thin films. Open J Polym Chem 4:31–37. doi:10.1016/j.polymer.2010.07.024
Acknowledgments
The authors wish to thank Jorge Pérez for their support in the SEM analysis and to CONACyT for the partial financial support to this work with the 130190 and 154757 projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zúñiga, R., Cruz, G.J., Olayo, M.G. et al. Synthesis and superficial characterization of plasma polyfuran thin films. Polym. Bull. 72, 839–850 (2015). https://doi.org/10.1007/s00289-015-1309-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1309-4