Abstract
This work presents a study of the chemical structure, thermal degradation and electric conductivity of spherical particles synthesized as a result of intense crosslinking of pyrroles in plasma glow discharges. A new method to calculate crosslinking, hydrogenation and fragmentation in polymers was developed in this task based on XPS calculations. The results indicated that the percentages of hydrogenated states in polypyrrole particles varied in the 37.1–46.6 % and 55.5–58.5 % intervals for C and N, respectively. The participation of crosslinked states increased from 48.5 to 59.8 % with the energy of synthesis considering C atoms and decreased from 24 to 6 % for N atoms. The particles have two thermal degradations, the first between 115 and 400 °C and the second with mean temperature degradation of 535 °C. The electric conductivity of the particles was in the range of 10−5–10−10 S/m with activation energy between 1.96 and 2.34 eV. This behavior can be associated with the crosslinking percentages in the particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When all C and N atoms of pyrrole molecules join with other similar molecules and these with others, and so on, the resultant crosslinked structure forms planes whose curvature depends on the space left among the pyrrole molecules. The smallest possible space is a pentagonal outline that theoretically could generate a spherical particle with a minimum diameter of approximately 3.88 Å (Fig. 1a). If a hexagonal outline is used, the minimum particle diameter would be slightly bigger, about 7.09 Å (Fig. 1b), and so forth. As the number of outline sides increases, more space is left among pyrrole rings and the spherical profile tends to disappear producing plane surfaces instead of particles. This effect depends on the hydrogenation and crosslinking degree of the structure to form particles or films with different physicochemical properties.
The name pyrrolenes is applied to these polypyrrole (PPy) particles because of their similarity to fullerenes, which are formed by crosslinked benzene rings with pentagonal outlines forming spheres (C60) or hexagonal ones forming cylinders. Pyrrolenes unlike fullerenes are formed with C and N atoms with a crosslinked pyrrole configuration which produce a slight polarization in the structure due to the difference in electronegativity between C (2.5) and N (3.0), and to the difference between the bonding energies of C–C (3.6 eV) and C–N (3.04 eV), (see colored zones in Fig. 2). Thus, the presence of N in this type of structures increases the chemical reactivity of pyrrolenes compared with that of fullerenes.
These kinds of polypyrrole particles have been synthesized by oxidation with different precursors in liquid phase (sol–gel and emulsion) and in gas phase (plasma). In this last technique, molecular dehydrogenation by the constant collisions between particles can be the main step in the formation of particles, producing crosslinking with the neutralization of radicals with other excited molecules. If the crosslinking is small, the result can be mainly a horizontal growth (Fig. 3a) generating thin films, but if crosslinking is high, the growth can be tridimensional generating pyrrolene particles (Fig. 3b). Thus, depending on the synthesis conditions, the reactions can produce PPy films and/or particles. Both could be used as biocompatible materials, because of their aromatic and aliphatic amines and because the polymerization is not affected by impurities, since only the monomer and possibly other dopants specifically added to the synthesis participate in the chemical reactions [1, 2].
Using plasma techniques, hollow-centered spheres, solid spheres and rods of PPy nanoparticles have been obtained in Ar [3, 4]. PPy hemispherical particles were synthesized at voltages greater than 1,100 V [5]. PPy/I particles were synthesized using air plasma with diameters between 35 and 350 nm with electric conductivity in the 10−9–10−6 S/m interval as a function of relative humidity [6]. Wang and colleagues [7] compared plasma and chemical PPy finding that as the power of synthesis increased the films which had a smoother surface. On the other hand, chemically synthesized PPy formed particles with sizes from 0.1 to 0.5 µm in aggregates of different sizes.
In chemical synthesis, other authors have synthesized PPy spherical nanoparticles via emulsion using ammonium peroxydisulfate [8–11] with diameters of 20 [8], 60–90 [9] and 500 [11] nm and conductivity of 6,190 S/m [9] and 10−2 S/m [10]. In these conditions, PPy has a thermal degradation temperature of 218 °C, whereas PPy obtained using FeCl3 and ammonium persulfate presented degradation between 220 and 298.4 °C [10–14]. In other chemical synthesis, PPy particles were obtained with sizes less than 1,000 nm and electric conductivity around 125 S/m [15–18].
In none of these works, the structure of PPy particles was studied to find the main chemical states in the particles. So, this work presents a study on the structure, morphology, thermal and electric behavior of particles derived from pyrrole synthesized by plasma. The chemical structure is studied by XPS techniques to follow the main atomic states in the particles. A method to calculate crosslinking in polymers with the data obtained by XPS was developed as a result of these analyses. Up to now, the evaluation of crosslinking has been done analyzing qualitatively, the evolution of specific chemical groups as C=C double bonds through IR spectroscopy [19] or following the proton signals in NMR spectrometry [20]. However, the evaluation of crosslinking presented here is more quantitative than the other techniques.
Experimental
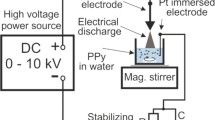
Pyrrolenes were synthesized by resistive plasmas at 13.56 MHz, 10−1 mbar with 40, 80 and 120 W during 180 min [21]. Morphological analysis was performed on a JEOL JSM-5900LV and a JEOL JEM 2100 F scanning and transmission electron microscopes, respectively. The structure of pyrrolenes was analyzed with a Thermo K-Alpha X-ray photoelectron spectrometer with a monochromated X-ray Al source (1,486.6 eV). The orbital energy distribution was fitted using Gaussian curves. The thermal behavior of pyrrolenes was obtained using a Linseis STA PT1600 thermogravimetric analyzer with a heating rate of 10 °C/min. The electric conductivity of pyrrolenes was calculated using the resistance of the material measured in an arrangement of two parallel plates of Cu with an OTTO MX-620 high resistance meter in the 20–100 °C interval. The temperature was measured with a Mastech Mas-345 digital multimeter.
Results
Morphology
Figure 4 shows SEM and TEM micrographs of pyrrolenes synthesized by plasma at 40 W, at a higher power of synthesis the micrographs are similar. The particles are spherical, smooth and form random agglomerates (Fig. 4a). Pyrrolenes synthesized at 40 W have diameters between 131 and 510 nm, at 80 W the diameters are between 61 and 350 nm and at 120 W are between 76 and 310 nm. Transmission electron micrographs (TEM) are shown in Fig. 4b which presents internal homogeneous spherical particles. They were dispersed in ultrasonic agitation for 20 min, however, after the treatment many agglomerates still remained. Figure 4b shows particles synthesized at 40 W with diameter between 70 and 110 nm. Pyrrolenes synthesized at 80 and 120 W are grouped in agglomerates with sizes from 125 to 335 nm.
Superficial atomic energy states
The surface of materials is important for most applications, as it will be in direct contact with the external medium; in biomaterials, it is particularly important since it interfaces with cells. The surface of pyrrolenes has C, N, O and Si atoms, see Table 1 which shows the atomic percentage. C and N atoms form the pyrrole structure, but O and Si atoms were originated in oxidation and glass contamination during synthesis. The O content rises with the power of synthesis indicating that the material increases oxidation with this variable [22].
The main superficial energetic atomic states of pyrrolenes were studied through C1s and N1s orbitals, see Figs. 6a and 7a, respectively. The detailed spectra were adjusted using Gaussian curves with the FWHM (Full Width at Half Maximum) parameters obtained from the Crist work [23]. Each curve has a maximum BE (binding energy) which can be associated with at least one chemical state. Figures 6b and 7b show the percentage of participation with the associated chemical states for C1s, and N1s orbitals, respectively.
The atomic chemical states were assigned considering all possible bonds of each atom, C=4 and N=3. The energy of the states is the sum of all chemical bonds in that configuration reported in the literature [24, 25]. In the discussion of states, the atom studied in the chemical state is highlighted in bold and all other atoms in the same formula are considered attached to it.
Carbon chemical states
In Fig. 5a, a scheme of a pyrrole molecule is presented. If the carbons are numbered clockwise in the figure, only two chemical states can be identified, C=CH–N in carbons 1 and 4, C=CH–C, in carbons 2 and 3, both with participation of 50 %. In a typical linear chain, see Fig. 5b, C=CH–C remains untouched, but C=CH–N tends to be substituted for C=CC–N, which represents one type of union between pyrroles. If crosslinking in polypyrroles (X) is defined as the union of pyrroles, a very long linear chain would have X = 50 %. Note that in a chain with only 3 pyrrole rings X = 33 %, with 5 rings X = 40 %, with 10 rings X = 45 %, with 100 rings X = 49.5 %, and so on.
As more pyrroles substitute H atoms in the structure, X increases in the same way as in linear chains, with five pyrrole rings, X = 40 %, see Fig. 5c. However, the percentage of C=CH–C reduces, from 50 % in linear chains to 33 % in a 3-ring scheme. In the 5-ring structure, as hydrogenation reduces, crosslinking increases and C=CH–C states transform in C=CC–C, resulting in X > 50 %, see Fig. 5d which shows a 5-ring highly crosslinked structure with X = 70 %. Thus, X > 50 % indicates that dehydrogenation in polypyrroles generates segments with highly crosslinked structures as in Fig. 5d. This is important because in the following discussion, the main chemical states in polypyrrole particles are identified and their percentages calculated to estimate hydrogenation and crosslinking.
The detailed C1s spectrum of pyrrolenes synthesized at 80 W is presented in Fig. 6a as an example of the method employed to identify chemical states and to calculate their percentages. Figure 6b presents the condensed results of other pyrrolenes synthesized at 40 and 120 W. The C1s distribution energy was adjusted with Gaussian curves using FWHM = 1.0 ± 0.1 eV resulting in six curves in all polymers. Each curve is identified by its maximum BE value and can be associated with at least one chemical state with the characteristic that as BE increases, higher oxidation states are obtained. The formation energy of those chemical states follows an inverse trend, as oxidation or dehydrogenation increases, the formation energy reduces.
The first curve in Fig. 6a with maximum BE at 284.1 eV (6.3 %) represents the lowest oxidation configuration in pyrrolenes and was assigned to C3–C–H [26], which corresponds to fragments of pyrrole rings produced by the constant collisions of particles during the synthesis, The formation energy of this state is approximately 15.1 eV. The second curve with maximum area of the entire C1s orbital has its maximum BE at 284.8 eV (34.5 %) and was assigned to C=CH–C with a formation energy of 14.32 eV. This state corresponds to C atoms of pyrrole in positions 2 and 3, see Fig. 5a, which survived the chemical reactions without modification.
The next three curves involve different unions of pyrrole molecules and represent crosslinking in pyrrolenes. The C=C–C2 state [27] was assigned to the curve with maximum BE at 285.5 eV (22.4 %) and corresponds to C joining two pyrrole rings through positions 2 and 3. This state has formation energy of 13.62 eV. The curve with maximum BE at 286.2 eV (21.1 %) was assigned to C=CC–N, with formation energy of 13.06 eV, and corresponds to crosslinking of pyrrole rings via positions 1 and 4. The maximum BE at 287.0 eV (12.0 %) was assigned to the C=CN–N state with formation energy of 12.5 eV and is another indication of crosslinking formed by the union of two pyrrole rings through one N atom. The final curve belongs to the most oxidized chemical state in the particles, C≡C–N with an energy of 11.46 eV, and was assigned to the curve with maximum BE at 288.0 eV (3.8 %). This is not part of the polypyrrole structure and may have originated from pyrrole fragments.
Figure 6b shows a comparison between the percentages of participation of each state at different power of synthesis. X-axis represents the maximum BE of the adjusted curves and each point is the chemical state found at that BE. Pyrrolenes have three types of states: hydrogenated (H), crosslinked (X) and multiple (M). Hydrogenated states are those having at least one hydrogen atom bonded to the polypyrrole structure, crosslinked states are formed by the union of two pyrrole rings. Multiple states are those with double or triple bonds that do not belong to the polypyrrole structure and may be formed as fragments during the synthesis.
The participation of hydrogenated states varies between 46.6 and 37.1 % (H in Fig. 6b), indicating that these states slightly decrease with the increases of energy applied during the synthesis. In a similar but inverse way, the percentage of crosslinked states increased from 48.5 to 59.8 % with the energy of synthesis (X in Fig. 6b). Another important study in plasma polymers is that fragmentation always happens during synthesis and that can be seen in the participation of M states in the polymers. In this work, the participation of multiple bonds seems almost independent of the energy applied to the synthesis, between 4.9 and 3.1 % (M in Fig. 6b). The method used here can be applied to other polymers to calculate crosslinking, hydrogenation or the evolution of other chemical states.
Nitrogen chemical states
The N1s distribution energy of pyrrolenes obtained at 40, 80 and 120 W was adjusted with three Gaussian curves using FWHM = 1.3 ± 0.1 eV, see Fig. 7a that shows the N1s spectrum of particles synthesized at 80 W and Fig. 7b that has the condensed results of pyrrolenes synthesized at 40 and 120 W. The first curve with maximum at 398.5 eV and 16.9 % area was assigned to C=N–H, which has a formation energy of 10.38 eV. This state may be formed with partially dehydrogenated segments of pyrrole rings. The curve with maximum BE at 399.5 eV and 58.5 % area was assigned to the atomic configuration of N in the monomer, C–NH–C. This configuration has formation energy of 10.08 eV and the percentage of area indicates that only 41.5 % of N atoms participate in the chemical reactions. The curve centered in 400.4 eV, 24.6 % area was assigned to C–N–C2 with formation energy of 9.12 eV. This curve corresponds to tertiary amines, which can be formed by the chemical bonding of two pyrrole rings via one N atom. This is an expression of crosslinking in pyrrolenes. In a typical linear chain, the union between pyrroles occurs with C atoms; however, segments with highly crosslinked structures involving C and N atoms could also be created. This is the case of C–N–C2.
In a similar analysis to C1s in Figs. 6b and 7b show a comparison between the percentages of H, X and M chemical states at 40, 80 and 120 W for N1s orbitals in pyrrolenes. The participation of H respect to the power of synthesis is between 55.5 and 58.5 %, which indicates that it is almost independent of the applied energy. On the other hand, the participation of X decreases from 24 to 6 % with the increase of energy in the synthesis, which indicates that fragmentation increases after 80 W. This is complemented with the increase of M from 16.9 to 36.3 % in the same interval of energy, suggesting that M and X are linked in these transformations.
Thermal analysis
Thermal degradation of pyrrolenes is presented in Fig. 8 with two schemes, %m vs T and dm/dT vs T. The graph shows similar tendencies for pyrrolenes synthesized at 40 and 80 W, with a slight difference in the material synthesized at 120 W. All pyrrolenes lose moisture between 3.3 and 5.3 % mass in the 20–115 °C range. After 115 °C initiates the first loss of the material, up to 400 °C, and the second loss ends at 675 °C. The central temperature in the first degradation is approximately 263 and 535 °C for the second one. The first degradation may be constituted from hydrogenated segments and fragments with low molecular weight originated in the synthesis, with approximately 25 % mass. In this context, the second degradation refers to the main crosslinked network with approximately 60 % mass. However, after 700 °C, all pyrrolenes were reduced to ashes.
Electric conductivity
Figure 9 shows the electric conductivity of pyrrolenes synthesized by plasma at 40, 80 and 120 W as a function of temperature in a heating–cooling cycle between 20 and 100 °C. As the material is formed by particles, it was compressed to reduce the contact resistance among particles. The electric conductivity of the particles in the cycle was between 10−10 and 10−5 S/m. The material behaves like an organic semiconductor, as its conductivity increases with temperature. During the heating step, solid symbols in the plot, the conductivity is susceptible to the moisture and gases absorbed in the material. In the cooling step, after the release of gases and moisture, open symbols, the electric conductivity of the particles has a more homogeneous behavior with temperature. These values can be considered as the intrinsic volumetric conductivity of the particles. In general terms, the electric conductivity of pyrrolenes increases with the percentage of crosslinking in carbon atoms, the greatest electric conductivity of pyrrolenes was obtained at 120 W. The activation energy was calculated under an Arrhenius scheme using the conductivity in the cooling steps resulting in the 1.96 to 2.34 eV interval, which is commonly found in organic semiconductors.
Discussion and conclusions
Pyrrolenes synthesized by plasma are spherical particles of polypyrroles forming agglomerates in which the percentage of hydrogenation, crosslinking and fragmentation were calculated with a new method based in analyses of the atomic chemical states by means of XPS. In general, the method consists of identifying and calculating the percentage of those chemical states forming the crosslinked structure, which in polypyrroles are the union of pyrrole rings. The total percentage of those states represents the crosslinked percentage. A similar reasoning was applied to calculate hydrogenation states. The percentage of fragmentation was calculated as the sum of all chemical states with double or triple bonds in the particles not belonging to the initial monomers.
The results indicated that the superficial hydrogenated states (H) decreased 10 % in C, but increased 2 or 3 % in N in the power of synthesis studied. The group formed with crosslinked states (X) in C increased approximately 12 %, but decreased approximately 20 % in N in the same interval of power. The percentage of a third group containing the most oxidized states (M) formed with multiple bonds in structures with fragments of pyrrole rings was almost independent of the energy of synthesis in C, with changes between 2 and 3 %, but increased approximately 15 % in N. All these numbers suggest that pyrrole rings tend to form crosslinked structures through C atoms, but that the possible fragments occur through the C–N groups with a tendency to form multiple bonds with the power of synthesis. Similar analyses can be applied to study the structure evolution of other polymers as a function of the main variables in the process, and it is possible to follow the participation of each atomic chemical state.
In terms of the thermal stability, the particles show the same behavior in the first two synthesis (40 and 80 W), and different at higher power, which can be associated with the fragmentation and crosslinking percentages of nitrogen chemical bonds in polypyrroles that follows a similar tendency, see Fig. 7b. The electric conductivity shows another possible association with the percentages of hydrogenation and crosslinking in the particles, which changes gradually with the power of synthesis, without being apparently affected by the fragmentation, see Fig. 6b. The method presented in this work can be applied to other polymers to calculate crosslinking, hydrogenation, fragmentation or the evolution of different chemical states as a function of the variables in each specific process.
References
Cruz GJ, Morales J, Olayo R (1999) Films obtained by plasma polymerization of pyrrole. Thin Solid Films 342:119–126
Morales J, Olayo MG, Cruz GJ, Olayo R (2002) Synthesis by plasma and characterization of bilayer aniline-pyrrole thin films doped with iodine. J Polym Sci Pol Phys 40:1850–1856
Cao J, Matsoukas T (2004) Synthesis of hollow nanoparticles by plasma polymerization. J Nanopart Res 6:447–455
Yang P, Zhang J, Guo Y (2009) Synthesis of intrinsic fluorescent polypyrrole nanoparticles by atmospheric pressure plasma polymerization. Appl Surf Sci 225:6924–6929
Paosawatyanyong B, Tapaneeyakorn K, Bhanthumnavin W (2010) AC plasma polymerization of pyrrole. Surf Coat Tech 204:3069–3072
Cruz GJ, Olayo MG, Lopez OG, Gomez LM, Morales J, Olayo R (2010) Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer 51:4314–4318
Wang J, Neoh KG, Kang ET (2004) Comparative study of chemically synthesized and plasma polymerized pyrrole and thiophene thin films. Thin Solid Films 446:205–217
Gupta ND, Banerjee D, Dasb NS, Chattopadhyay KK (2011) Kinetics of micelle formation and their effect on the optical and structural properties of polypyrrole nanoparticles. Colloid Surf A 385:55–62
Reung-U-Rai A, Prom-Jun A, Prissanaroon-Ouajai W, Ouajai S (2008) Synthesis of highly conductive polypyrrole nanoparticles via microemulsion polymerization. JOM J Min Met Mat S 18:27–31
Wei S, Mavinakuli P, Wang Q, Chen D, Asapu R, Mao Y, Haldolaarachchige N, Young DP, Guo A (2011) Polypyrrole-titania nanocomposites derived from different oxidants. J Electrochem Soc 158:K205–K212
Xie X, Zhou X (2011) Well encapsulated hollow borosilicate glass sphere@ polypyrrole composite with low density, designable thickness and conductivity. Colloid Surf A 386:158–165
Zhu J, Wei S, Zhang L, Mao Y, Ryu J, Mavinakuli P, Karki AB, Young DP, Guo Z (2010) Conductive polypyrrole/tungsten oxide metacomposites with negative permittivity. J Phys Chem C 114:16335–16342
Mavinakuli P, Wei S, Wang Q, Karki AB, Dhage S, Wang Z, Young DP, Guo Z (2010) Polypyrrole/silicon carbide nanocomposites with tunable electrical conductivity. J Phys Chem C 114:3874–3882
Guo Z, Shin K, Karki AB, Young DP, Kaner RB, Hahn HT (2009) Fabrication and characterization of iron oxide nanoparticles filled polypyrrole nanocomposites. J Nanopart Res 11:1441–1452
Noh KA, Kim DW, Jin CS, Shin KH, Kim JH, Ko JM (2003) Synthesis and pseudo-capacitance of chemically-prepared polypyrrole powder. J Power Sources 124:593–595
Geng W, Li N, Li X, Wang R, Tu J, Zhang T (2007) Effect of polymerization time on the humidity sensing properties of polypyrrole. Sensor Actuat B Chem 125:114–119
Ghamouss F, Brugere A, Anbalagan AC, Schmaltz B, Luais E, Tran-Van F (2013) Novel glycerol assisted synthesis of polypyrrole nanospheres and its electrochemical properties. Synthetic Met 168:9–15
Hoshina Y, Zaragoza-Contreras EA, Farnood R, Kobayashi T (2012) Nanosized polypyrrole affected by surfactant agitation for emulsion polymerization. Polym Bull 68:1689–1705
Bortolato SA, Thomas KE, McDonough K, Gurney RW (2012) Evaluation of photo-induced crosslinking of thymine polymers using FT-IR spectroscopy and chemometric analysis. Polymer 53:5285–5294
Gauthier MA, Luo J, Calvet D, Ni C, Zhu XX, Garon M, Buschmann MD (2004) Degree of crosslinking and mechanical properties of crosslinked poly(vinyl alcohol) beads for use in solid-phase organic synthesis. Polymer 45:8201–8210
Gomez LM, Olayo MG, Cruz GJ, Lopez-Gracia OG, Gonzalez-Torres M, de Jesus C, Gonzalez-Salgado F (2012) Effect of energy in the size of pyrrole-derived particles synthesized by plasma. Superficies y Vacío 25:88–91
Olayo R, Ríos C, Salgado-Ceballos H, Cruz GJ, Morales J, Olayo MG, Alcaraz-Zubeldia M, Alvarez AL, Mondragon R, Morales A, Diaz-Ruiz A (2008) Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J Mater Sci Mater M 19:817–826
Crist BV (1998) Advanced peak-fitting of monochromatic XPS spectra. J Surf Anal 4:428–434
Cotton FA, Wilkinson G (1980) Quimica inorganica avanzada. Limusa, Mexico
Dean JA (1999) Lange´s handbook of chemistry. McGraw-Hill, New York
Olayo MG, Gonzalez-Salgado F, Cruz GJ, Gomez LM, Garcia-Rosales G, Gonzalez-Torres M, Lopez-Gracia (2013) Chemical structure of TiO organometallic particles obtained by plasma. Adv Nanopart 2:229–235
González-Torres M, Olayo MG, Cruz GJ, Gómez LM, Sánchez-Mendieta V, González-Salgado F (2014) XPS study of the chemical structure of plasma biocopolymers of pyrrole and ethyleneglycol. Adv Chem. ISSN 2356-6612 (print), ISSN 2314-7571 (online) (in press)
Acknowledgments
The authors wish to thank Jorge Pérez for his help in the SEM micrographs, Rafael Basurto for his support in the XPS analysis and CONACYT for the partial financial support to this work with the projects 130190 and 154757.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomez, L.M., Cruz, G.J., Olayo, M.G. et al. Analysis of crosslinking in polypyrrole particles synthesized by plasma. Polym. Bull. 71, 3275–3287 (2014). https://doi.org/10.1007/s00289-014-1249-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1249-4