Abstract
Large-scale screening of 848 culturable soil and endophytic filamentous fungi and yeasts for the ability to mobilize inorganic and organic P compounds was performed. Five strains of filamentous fungi having the highest level of phosphate-mobilizing ability were selected: Penicillium bilaiae Pb14, P. bilaiae C11, P. rubens EF5, Talaromyces pinophilus T14, and Aspergillus sp. D1. These strains in vitro actively solubilized Ca, Al, and Fe phosphates and Ca phytate. The amount of mobilized P negatively correlated with pH of the medium and positively correlated with fungal biomass. The proposed mechanisms for P mobilization were acidification of the medium, organic acid release, and phosphatase activity. The fungi decreased pH of the medium from 7.0 to 2.3–5.0. Ten different organic acids were produced by fungi with pyruvic acid being a major component. Acid phosphatase activity varied from 0.12 EU to 0.84 EU, and alkaline phosphatase activity varied from 0.08 EU to 0.61 EU depending on the strain. Available P concentration in soil was increased by 13–28% after introduction of the fungi. The fungi also produced phytohormones auxins, salicylic acid, and abscisic acid. All the strains, except Aspergillus sp. D1, promoted elongation and increased biomass of barley seedlings grown in soil. Shoot P concentration increased by 17–26% after inoculation with P. bilaiae Pb14, T. pinophilus T14, and Aspergillus sp. D1. It was concluded that the selected fungal strains promoted plant growth due to P mobilization and phytohormone production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is the essential macronutrient required for synthesis of vital cell compounds and energy exchange in plants. Despite the high total P content in soil, its bioavailability is a limiting factor for plant growth because approximately 95–99% of P is in insoluble forms and unavailable for plants [1, 2].
The problem of soil P deficiency is partially solved by applying mineral fertilizers. However, only about 20% of P fertilizers is assimilated by plants, whereas the remainder is rapidly converted into insoluble complexes with aluminum (Al) and iron (Fe) in acidic soils (especially those with pH lower than 5.0) and with calcium (Ca) in alkaline soils (pH above 7.0) [2, 3]. An alternative approach is the mobilization of phosphates from insoluble compounds using the phosphate-mobilizing microorganisms. Many soil microorganisms including bacteria, fungi, and algae are able to mineralize insoluble mineral and organic P compounds, making them available for plants [3,4,5]. The microbial solubilization of inorganic P compounds is due to various mechanisms which is associated mainly with the acidification of the medium that happens through the release of organic acids [1, 2]. Di-and tricarboxylic acids were found to be more effective in phosphate solubilization compared to monobasic and aromatic acids [2, 3]. Another principal mechanism of microbial P mineralization is the production of acid and alkaline phosphatases that hydrolyze organic phosphates. Acid phosphatases can be released also by plants, whereas alkaline phosphatases are mostly of microbial origin [6].
Microbial phosphate mobilization is considered as one of the main mechanisms related to plant growth promotion [1, 4]. In recent years, numerous reports presented a considerable information on phosphate-mobilizing fungi (PMF). However, most of them are focused primarily on phosphate mobilization in vitro [6,7,8,9,10,11,12,13,14,15,16], and information on their effects on soil and plants is insufficient [17,18,19,20,21]. Furthermore, there are only few reports mentioning not only plant growth characteristics in general (plant biomass, height, etc.), but also parameters related to P uptake by plants [18, 19]. Many of studies investigated P mobilization from inorganic P sources [7,8,9, 18, 22], predominantly tricalcium phosphate [10,11,12,13, 17, 23]. Other P compounds are also examined, but on a very small scale. Despite numerous reports on filamentous PMF isolated from a wide range of soils [8,9,10,11,12,13,14,15,16,17, 19,20,21], information on their presence in plant tissues (endophytic traits) is very limited [7, 22]. Little attention also received phosphate-mobilizing yeasts [22, 24]. Thus, a more detailed study on the characteristics and mechanisms of phosphate mobilization from different P sources by both groups of PMF, as well as their application for increasing soil and plant P availability is needed to expand and deepen existing knowledge.
Different approaches, such as cultivation-dependent techniques and cultivation-independent high-throughput DNA sequencing technologies, are used to investigate composition and diversity of fungal communities [25]. Although culture-based techniques reveal a portion of the actual diversity, they are significant for isolation and characterization of pure fungal cultures, including those with the potential for application as inoculants.
The present study focuses on the large-scale screening of culturable soil and endophytic filamentous fungi and yeasts for phosphate mobilization and characterization of the mechanisms underlying both inorganic P solubilization (Ca phosphate, Al phosphate, Fe phosphate) and organic P mineralization (Ca phytate). Subsequent evaluation of fungi for phytohormone production and plant growth promotion in combination with P uptake in vivo allowed selecting the most efficient strains with potential for biotechnological application.
Materials and Methods
Plant and Soil Sampling
Seven plant species were sampled in May–June 2019 during vegetative growth phase from agricultural fields located in Turgen, Almaty region, Kazakhstan (43° 27′ N 77° 34′ E, 817 m a.s.l.): soybean (Glycine max (L.) Merr.), barley (Hordeum vulgare L.), alfalfa (Medicago sativa L.), rapeseed (Brassica napus L.), safflower (Carthamus tinctorius L.), sweet clover (Melilotus officinalis (L.) Pall.), and sainfoin (Onobrychis viciifolia Scop.). Five plants located at 10-m intervals from each other were collected per each plant species. The root–soil system of each plant was gently shaken by hands to remove and collect the bulk soil free of roots. Each sample was put separately in sterile plastic bag and brought to the laboratory on the same day to be processed immediately. Plant and soil characteristics are summarized in Table S1 (Supplementary material Table S1).
Soil characteristics were determined according to analytical procedures, described in [26]. Soil texture was determined by the hydrometer method. Soil pH was measured in the soil/water suspensions at a ratio of 1:5 (w/v) using an ionomer I-160 M (“Antech,” Belarus). Soil total C and total N were determined by dry combustion procedure using a Multi N/C 3100 TOC analyzer (Analytik Jena, Germany). Total P was extracted by digestion with 60% HClO4 after pretreatment with HNO3 followed by estimation of extracted P using ascorbic acid molybdenum blue method [27]. Available P was determined by extraction with 0.5 M sodium bicarbonate solution followed by colorimetric measurement using ascorbic acid molybdenum blue method [28]. Soil type was classified according to the World Reference Base for Soil Resources [29].
Fungal Isolation
Soil fungi were isolated from the bulk soil samples by serial dilution technique [30] using Sabouraud dextrose agar and Czapek Dox agar. The media with different compositions and pH values were used to reveal more fungal diversity. Sabouraud dextrose agar is commonly used for isolation of fungi and, especially, yeasts. Czapek Dox agar is predominantly used for isolation of filamentous fungi. Briefly, bulk soil samples were air-dried and passed through 2-mm sieve. Then 10 g of each soil sample was suspended in 90 mL sterile water, shaken at 120 rpm for 60 min, and settled for 10 min. Subsequently suspensions were serially diluted, 200 μL of each dilutions (10 −3, 10 −4, 10 −5, and 10 −6) were plated on Petri dishes and incubated at 25 °C for 7 days. The individual fungal colonies were selected and transferred into fresh dishes for purification.
Endophytes were isolated from roots, stems, and leaves by modified fragment plating technique [7] using potato dextrose agar (PDA), which is the most suitable and therefore frequently used medium for isolation of endophytic fungi. Twenty mature leaves, five mature stems, and ten coarse roots were obtained from each plant species with the exception of barley, from which ten leaves were collected. The samples of healthy plant parts were washed under running tap water and then cut into 0.5-cm pieces (50 pieces for each plant organ of each plant species). These segments were surface sterilized by dipping in 70% ethanol for 1 min and 3% sodium hypochlorite for 2 min followed by finally rinsing in sterile distilled water for 2 min to remove any epiphytic microbes. The sterilized segments were placed in Petri plates containing PDA medium supplemented with 50 mg L−1 streptomycin and 50 mg L−1 tetracycline to avoid contamination by endophytic bacteria. Cultures were incubated at 25 °C for 14 days and checked daily. To verify the surface sterilization, 100 µL of water used for final rinse was plated on PDA as well.
All the isolated fungal strains were stored in the local Collection of Effective Agricultural Fungal Strains (CEAFS, Department of Biology and Biotechnology, al-Farabi Kazakh National University, Almaty, Kazakhstan). The most effective strains were deposited to the Republican Collection of Microorganisms (RCM, Nur-Sultan, Kazakhstan, http://www.rcm.kz/) and/or Russian Collection of Agricultural Microorganisms (RCAM, St.-Petersburg, Russian Federation, http://www.arriam.ru/kollekciya-kul-tur1/). Accession numbers of these strains are shown in Table S2 (Supplementary material Table S2).
Fungal Identification
The standard biochemical and morphological tests were used for preliminary identification of yeasts under study [31]. Preliminary identification of filamentous fungi was based on the morphological characteristics and growth rate [32,33,34]. The distribution of taxa was expressed using the relative frequency (RF), which is calculated as the number of isolates of one genus divided by the total number of isolates and expressed as percentage [35]. All fungal strains isolated from 35 soil samples and 35 plants were taken into account when calculating RF.
Five strains of filamentous fungi having the highest level of phosphate-mobilizing ability were selected as the most promising for the study and identified by sequencing the nuclear ribosomal internal transcribed region (ITS). Fungal cultures were grown in Sabouraud dextrose liquid medium at 25 °C. Fungal mycelium (approximately 10 mm diameter) of three-day-old culture was used for DNA extraction. DNA was extracted and purified as described by Wilson [36]. The following PCR primers were used: ITS1-5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4-5′-TCCTCCGCTTATTGATATGC-3′ [37]. The amplification was carried out in a final volume of 50 µl reaction mixtures containing PCR buffer (Fermentas, USA), 25 mM of MgCl2, 0.8 mM of dNTPs mix, 1.0 U of Taq DNA polymerase (Fermentas, USA), 10.0 pmol of each forward and reverse primers, and 2 µL of DNA template. PCR was carried out using the following conditions: after an initial denaturation step for 4 min at 95 °C, 30 cycles followed by 25 s at 95 °C, 30 s at 54 °C, and 40 s at 72 °C, and a final extension at 72 °C for 7 min. Electrophoresis was carried out with 1% agarose gel in TAE under 100 V for 60 min. The DNA fragments were stained with Blue Green Loading Dye and visualized using UV transilluminator. The enzymatic technique with Exonuclease I (Fermentas, USA) and Alkaline Phosphatase (Fermentas, USA) was used to purify obtained PCR products. Sequencing was performed in 3730xl DNA Analyzer (Applied Biosystems. USA) using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) following the manufacturer’s protocol. The length of the sequences was as follows: P. bilaiae Pb14 -573 bp, P. bilaiae C11—542 bp, P. rubens EF5—584 bp, T. pinophilus T14—544 bp, and Aspergillus sp. D1—585 bp.
All sequences were aligned using pair-wise Clustal W alignment and compared with the related sequences of the type strains available in the GenBank database using BLAST analysis. Phylogenetic trees were constructed using the Maximum likelihood method in Mega-X software package [38]. Bootstrap analysis with 1000 replicates was carried out to estimate the support of clusters.
The obtained sequences were deposited to the NCBI GenBank database under accession numbers MT364469 (Penicillium bilaiae Pb14), MT364470 (Penicillium bilaiae C11), MT364482 (Penicillium rubens EF5), MT364484 (Talaromyces pinophilus T14), and MT364485 (Aspergillus sp. D1).
Phosphorus Mobilization In Vitro
The fungal strains were qualitatively screened for P-mobilizing ability by culturing on plates with Pikovskaya’s (PKV) agar medium containing (g L−1) glucose, 10; yeast extract, 0.5; (NH4)2SO4, 0.5; MgSO4·7H2O, 0.1; KCl, 0.2; NaCl, 0.2; MnSO4·4H2O, 0.02; FeSO4·7H2O, 0.02; P source (Ca phosphate or Ca phytate), 5.0; agar, 15; and pH 7.0. The plates were spot inoculated and incubated at 25 °C for 7 days. PMF were recognized by the formation of visible halo zones around colonies. The phosphate solubilization index (PSI) was calculated as a ratio of the total diameter (colony + halo zone) to the colony diameter [19].
To determine soluble P content quantitatively in growth cultures, Erlenmeyer flasks (250 mL) were filled with 50 mL of liquid PKV medium, inoculated with fungal mycelium, and incubated at 180 rpm at 25 °C for 14 days. The uninoculated medium was used as the control. Fungal biomass was obtained by filtering cultures on a Whatman No.1 paper, dried for 48 h at 80 °C, and weighted. To remove insoluble P compounds, the filtrates were centrifuged at 9000×g for 20 min. The supernatants were assayed for P content by spectrophotometric measurement at 405 nm using the ascorbic acid molybdenum blue method [27]. The pH of supernatants was measured using an ionometer I-160 M (Antech, Belarus). The solubilization efficiency (SE) was calculated as a percentage of solubilized P from the amount of supplied insoluble P [6]. Quantitative estimations on P solubilization/mineralization by five selected fungal strains were carried out for 14 days at two-day interval. Five of the most promising strains were additionally tested for solubilization of hard-to-solubilize P sources AlPO4 and FePO4·4H2O as described above.
Extracellular Phosphatase Activity
Fungal strains were grown in liquid PKV medium for 7 days and supernatants were obtained as described above. Phosphatase activities were measured in the supernatants spectrophotometrically at 405 nm using 23 mM p-nitrophenylphosphate (pNPP) as the substrate. Sodium acetate buffer (0.1 M, pH = 5.5) was used for acid phosphatase assay. Universal buffer (0.1 M, pH = 9.0) was used to detect alkaline phosphatase activity. The reaction mixture was incubated at 37 °C for 1 h. The reaction was terminated by adding 0.5 M NaOH. An enzymatic unit (EU) was defined as the amount of enzyme produced per gram of dry mycelium that hydrolyzed 1 μmol pNPP per min in 1 mL of supernatant [6].
Organic Acid Production
PKV liquid medium supplemented with 0.5% Ca phosphate (Ca3(PO4)2) was used for organic acids detection. Cultures were incubated at 180 rpm at 25 °C for 7 days. Then the culture media were centrifuged at 11,000×g for 15 min. Eleven low-molecular weight organic acids mainly involved in the microbiological mobilization of P [2, 3, 7] were determined. For this purpose, the supernatants and uninoculated media (as control) were concentrated at 45 °C on rotary vacuum evaporator Heidolph Hei-VAP Precision (Heidolph Instruments GMBH & CO KG, Germany) to dryness at suspended in 1 mL of Milli-Q water. The quantitative composition of organic acids was evaluated using a Waters ACQUITY UPLC H-Class ultra-performance liquid chromatography (UPLC) system (Waters, USA). Organic acids were separated in 10 mM orthophosphoric acid on a Waters ACQUITY CSH C18 (1.7 µm, 2.1 × 75 mm) column (Waters, USA) at flow rate 0.1 mL min−1, column temperature 24 °C, and detected with Waters eλPDA detector at a wavelength of 220 nm.
Phytohormone Production
The fungal strains were grown in Sabouraud dextrose liquid medium amended with 1% L-tryptophan. To determine phytohormones (auxins, abscisic acid, salicylic acid, gibberellic acid GA3), fungal cultures were prepared as described above and the supernatants were acidified to pH 3.0 with 0.4 N hydrochloric acid and extracted with equal volumes of ethyl acetate. The organic phase containing phytohormones were evaporated to dryness under vacuum at 35 °C and suspended in 0.5 mL of 18% acetonitrile. The all obtained samples were filtered through 0.22-μm Costar® Spin-X® microtubes with nylon membrane filters (Sigma-Aldrich Int. GMBH) prior to chromatographic analysis. Uninoculated medium was used as a control. Phytohormones were separated on Waters ACQUITY UPLC BEH RP18 Shield (1.7 µm, 2.1 × 50 mm) column (Waters, USA) in a mixture of 0.1% formic acid (A) and acetonitrile supplied with 0.1% formic acid (B) at flow rate 0.3 mL min−1 with isocratic elution in 18% B for 5 min, followed by washing with 80% B for 2 min and conditioning the column for 3 min at 18% B. Auxins and salicylic acid were detected with fluorescence detector (λex = 280 nm, λem = 350 nm for auxins; λex = 300 nm, λem = 405 nm for salicylic acid). Gibberellic acid GA3 and abscisic acid were detected with Waters eλPDA detector at wavelength of 208 and 265 nm, respectively.
Preparation of Inoculum
To prepare inoculum, PMF were cultivated on PKV agar medium for 7 days at 25 °C (each strain separately). Spores were collected by scraping the surface of a sporulating culture into sterile water supplemented with Triton X-100, centrifuged at 9000×g for 1 min, and resuspended in sterile distilled water. The concentration of spores was adjusted to 107 spore mL−1 by microscopic enumeration using hemocytometer.
In Vitro Assay of Fungal P Mobilization in Soil
Surface soil (0–15 cm) was collected from uncultivated area in Turgen, Almaty region, Kazakhstan (43° 27´ N 77° 34´ E, 817 m a.s.l.). The soil was classified as Kastanozem (dark chestnut) [29] with light loam texture and had the following characteristics: bulk density 1.23 g cm−3, total C 2.65%, total N 1.8 g kg−1, total P 1.7 g kg−1, available P 12.2 mg kg−1, available K 535.3 mg kg−1, CaCO3 7.7%, and pH (H2O) = 8.4. Soil characteristics were determined according to analytical procedures [26] as described above. The soil was air-dried, passed through 2-mm sieve, and then stored at 4 ℃.
The unsterile soil was placed in plastic pots (300 g per pot, 5 pots per treatment) of 65 × 65 × 100-mm size. There were six treatments: control, P. bilaiae Pb14, P. bilaiae C11, P. rubens EF5, T. pinophilus T14, and Aspergillus sp. D1. Twenty milliliters of fungal spore suspension prepared as above mentioned were added into each pot and thoroughly mixed with soil. Sterile distilled water was used as a control. Pots were arranged according to a completely randomized design (CRD). Soil moisture was maintained at 60% of the whole water holding capacity (WHC) by weighing the pots and replacing the lost moisture with sterile distilled water.
Soil samples (10 g) were taken from the top layer with a sterile spatula and analyzed for available P and pH every 10 day for 20 days. Sodium bicarbonate solution (0.5 M, pH = 8.5) was used for extraction soluble P [28]. The extracted P was measured by colorimetric ascorbic acid molybdenum blue method [27]. The soil pH was measured using an ionometer I-160 M (Antech, Belarus).
Pot Experiment
Barley (cv. Baysheshek) seeds were kindly provided by agro-industrial firm “Turgen,” Almaty region. Seeds were surface sterilized by soaking in 75% ethanol for 5 min, in 1% sodium hypochlorite for 10 min, followed by thoroughly rinsing in sterile distilled water. Unsterile Kastanozem soil (same as used in study on P mobilization in soil) was used for the experiment. Pots of 65 × 65 × 100-mm size were filled with dry soil (300 g per pot) and then the soil was moistened up to 60% of WHC with distilled water. Seeds were placed in pots (15 seeds per pot, 5 pots per treatment) and each seed was immediately inoculated with 1 mL of spore suspension containing 107 spore mL−1. Inoculum was prepared as above mentioned. Control seeds were treated with sterile water. There were six treatments: control, P. bilaiae Pb14, P. bilaiae C11, P. rubens EF5, T. pinophilus T14, and Aspergillus sp. D1. Pots were randomly arranged according to a CRD of one factor (type of treatment listed above) with five replicates per each treatment. The pots were incubated in a growth chamber at 22 °C under a 16-h/8-h light/dark cycle at a 100 µmol m−2 s−1 photon flux density. All pots were equally irrigated every three days. After incubation for 14 days the plants were harvested and the length and dry weight of roots and shoots were determined. The shoot P concentration was determined as described above.
Statistical Analysis
A CRD was used for the experiments. All the data are presented as mean values and standard deviation (SD) of three or five replicates (depending on the experiment). The data were processed by the standard methods of one-way and two-way analysis of variance (ANOVA) and Pearson simple linear correlation using the software Statistica version 10.0 (TIBCO Software Inc., CA, USA). Tukey’s honestly significant difference (HSD) test (P < 0.05) was performed to estimate significant differences between means. Prior to analysis, the equality of the sample variances and data normality were verified using the Levene’s test and Shapiro–Wilk test, respectively. When normality of variances was not confirmed, data were log transformed.
Results
In Vitro Screening of Fungi for Phosphate-Mobilizing Ability
A total of 848 fungal strains (653 strains of filamentous fungi and 195 of yeasts) were isolated. Among them, 528 strains were isolated from soil samples and 320 from plants (Supplementary material Table S3).
Results of preliminary identification of the studied fungi showed that yeasts were represented by the genera Aureobasidium, Candida, Cryptococcus, Lipomyces, Metschnikowia, Rhodotorula, and Saccharomyces. Representatives of genera Rhodotorula, Aureobasidium, and Metschnikowia were the most frequently isolated yeasts with RF of 34%, 20%, and 17%, respectively (Fig. 1a).
The filamentous fungi belonged to the genera Aspergillus, Beauveria, Cladosporium, Fusarium, Metarhizium, Mucor, Penicillium, Talaromyces, and Trichoderma. Among them Penicillium, Aspergillus, and Fusarium were the most frequently isolated genera with RF of 27%, 24% and 20%, respectively (Fig. 1b).
The formation of halo zones was observed around colonies of various fungal strains grown on PKV solid medium supplemented with Ca phosphate or/and Ca phytate. The percent of Ca phytate-mineralizing strains was higher than Ca phosphate-solubilizing ones for all types of fungi and origin of isolation (Supplementary material Table S4). Both phytate-mineralizing and phosphate-solubilizing ability were more often found in filamentous fungi than in yeasts. The percent of phosphate-mobilizing filamentous fungi was higher among strains originated from soil than those isolated from plants, although such effect was not observed for yeasts (Supplementary material Table S4).
A total of 115 fungal strains, mostly filamentous fungi, mobilized both inorganic and organic P (Supplementary material Table S4). Among them 12 strains had PSI above 1.2 for both P sources, therefore they were further tested quantitatively for P mobilization capacity using a liquid medium (Supplementary material Table S5). In most cases, the solubilization efficiency of organic P was higher than inorganic P. Only Aspergillus sp. D5 demonstrated the same activity for both P sources and vice versa Aspergillus sp. D1 was more efficient in solubilizing Ca phosphate than Ca phytate. A decrease in pH of the culture medium was observed in all treatments (Supplementary material Table S5). The P solubilization efficiency negatively correlated with the final pH of the medium (for Ca phosphate r = − 0.81, P = 0.01, n = 108 and for Ca phytate r = − 0.38, P = 0.02, n = 108).
Except studies with Ca phosphate and Ca phytate, the experiments with other hard-to-dissolve P sources like Al phosphate and Fe phosphate were carried out to confirm that five selected strains are true P mobilizers. Four selected fungal strains originated from soil (P. bilaiae Pb14, P. bilaiae C11, T. pinophilus T14, Aspergillus sp. D1) and one strain P. rubens EF5 was isolated from barley roots (Supplementary material Table S5). The solubilization efficiency of Ca phosphate was higher than Al and Fe phosphates. However, there was one exception with the strain P. rubens EF5, which showed the same efficiency for all inorganic P compounds, whereas the mobilization efficiency of organic source of P (Ca phytate) was significantly higher than inorganic P sources (Table 1). Both factors “fungal strain” (F = 33.3; P = 0.0001) and especially “P source” (F = 55.4; P = 0.0001) had the significant effect on the values of P solubilization efficiency. In addition, available P solubilization efficiency was influenced by the interaction between these factors (F = 56.3; P = 0.0001).
Colony morphology of five filamentous fungi contributing to the maximum increase in the amount of P solubilized in the medium is shown in Fig. 2. These strains were selected for further study and identified by the ITS-region sequences.
Identification of the Selected Fungi
Strains Pb14 and C11 had 99.82% ITS-region similarity with the type strain Penicillium bilaiae NRRL 339 (Supplementary material Table S6), while the sequence of isolate EF5 had 100% similarity with the type strain Penicillium rubens CBS 129667 (Supplementary material Table S7). The ITS-region of the isolate T14 was most similar (100%) to the Talaromyces pinophilus CBS 631.66 (Supplementary material Table S8). Comparison of the ITS-region sequences of the isolate D1 showed 100% identity with Aspergillus foetidus CBS 121.28, Aspergillus niger ATCC 16888, and Aspergillus welwitschiae CBS 139.54 (Supplementary material Table S9).
Phylogenetic analysis based on ITS-region sequences showed that fungal isolates Pb14 and C11 and type strain Penicillium bilaiae NRRL 339 are forming the cluster with support 44% (Fig. 3a). The isolate EF5 is clustering with Penicillium rubens CBS 129667 with support 81% (Fig. 3b). Strains T14 and Talaromyces pinophilus CBS 631.66 are clustering together with support 57% (Fig. 3c). The fungal isolate D1 and Aspergillus foetidus CBS 121.28, Aspergillus niger ATCC 16888, Aspergillus welwitschiae CBS 139.54, and Aspergillus awamori CBS 557.65 combined into cluster with support 92% (Fig. 3d).
Phylogenetic trees of the studied fungal strains and the type strains of closely related species. a The strains related to Penicillium bilaiae Pb14 and Penicillium bilaiae C11. b the strains related to Penicillium rubens EF5. c the strains related to Talaromyces pinophilus T14. d the strains related to Aspergillus sp. D1
Analysis of the ITS-region sequencing confirmed morphological identification of all isolated fungi and allowed to specify the taxonomic position of these strains as follows: strains Pb14 and C11 were assigned to P. bilaiae, the strain EF5 to P. rubens, and the strain T14 to T. pinophilus, respectively. The strain D1 was assigned as Aspergillus sp.
Characterization of P Mobilization Processes
The amount of solubilized P gradually increased till the maximum values reached on the 8–10 days. The maximum solubilized P concentration in the medium amended with Ca phosphate was recorded for Aspergillus sp. D1 followed by T. pinophilus T14 and P. bilaiae Pb14. Strains P. bilaiae C11 and P. rubens EF5 in a less degree demonstrated the ability to solubilize Ca phosphate (Fig. 4a). In the medium with Ca phytate all investigated strains, except P. rubens EF5, were very efficient in P mineralization. The amount of P solubilized by P. rubens EF5 was less compared to other strains (Fig. 4b).
Phosphorus mobilization by fungal strains during different incubation periods. Phosphorus concentration (a), pH values (c), and fungal biomass (e) in PKV medium supplemented with Ca phosphate. Phosphorus concentration (b), pH values (d), and fungal biomass (f) in PKV medium supplemented with Ca phytate. Data are presented as means ± SD. Different letters at the same incubation day indicate statistically significant differences between strains according to Tukey HSD test (P < 0.05; n = 3)
Phosphate mobilization was accompanied by a decrease in pH of the medium for both P forms (Figs. 4c,d). The pH decreased during 4–6 days and remained stable until the end of incubation period. Strain Aspergillus sp. D1 demonstrated significantly greater drop in the pH compared to the other strains. The uninoculated media had 997 µg P ml−1 and 1046 µg P ml−1 in the presence of Ca phosphate and Ca phytate, respectively, and no changes were observed in soluble P and pH during experiments (data not shown).
The change of the pH of the medium was closely related to the phosphate-mobilizing ability of the fungi. Significant negative correlations were found between the pH and the amount of soluble P in the medium at the eighth day, when soluble P content reached maximum, for Ca phosphate (r = − 0.85, n = 45, P = 0.043) and for Ca phytate (r = − 0.56, n = 45, P = 0.02).
The fungal biomass increased up to 6 days of incubation (Figs. 4e,f). A positive correlation between phosphate mobilization and fungal biomass was found for Ca phosphate (r = + 0.73, n = 45, P = 0.03) and for Ca phytate (r = + 0.83, n = 45, P = 0.02) treatments.
Activity of Extracellular Phosphatases
The studied fungi produced acid and alkaline phosphatases growing with both Ca phosphate (Fig. 5a) and Ca phytate (Fig. 5b). All the strains showed 1.3–3.1 times higher phosphatase activity grown on the medium with Ca phytate in comparison with Ca phosphate. A maximal activity of both phosphatases in experiments with Ca phosphate and Ca phytate was recorded for T. pinophilus T14. Acid phosphatase activity in most cases was higher (1.2–1.9 times) than alkaline phosphatase in all treatments (Fig. 5). No detectable enzyme activity was found in uninoculated controls (data not shown).
Production of Organic Acids and Phytohormones
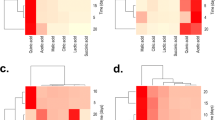
Ten of eleven measured organic acids were produced by the fungi during their growth in the PKV medium with Ca phosphate, but propionic acid was not produced (Table 2). Fumaric, malic, pyruvic, and oxalic acids were detected in all supernatants, whereas other acids were produced only by some strains. Pyruvic acid was the most quantitatively produced organic acid by all strains.
All the studied strains produced indole-3-acetic acid (Table 3). Indole-3-lactic acid was the most quantitatively produced phytohormone with the maximum amount recorded for P. bilaiae C11. Abscisic acid was produced by P. bilaiae C11 and T. pinophilus T14 only. Some strains also produced indole-3-carboxylic and salicylic acids. Gibberellic acid GA3 was not detected in any supernatant.
Phosphorus mobilization In Soil and Soil pH
Significant increase in the mobilized soil P was observed for all strains already from 10th day (Table 4). Strains T. pinophilus T14 and Aspergillus sp. D1 contributed to the further P mobilization up to 20th day, whereas other strains maintained the same values until the end of the experiment. Available P content was affected by two main factors: “treatment” (F = 19.7; P = 0.002) and “days after treatment” (F = 28.4; P = 0.0001). The interaction between these factors was also important (F = 5.8; P = 0.0001). Soil pH was influenced by the “treatment” (F = 10.7; P = 0.0001), “days after treatment” (F = 9.4; P = 0.0005), and interaction between these main factors (F = 4.9; P = 0.0002). The influence of each of the main factors taken separately was greater than the interaction between them. The greatest increases in P availability and decreases in soil pH were obtained by application of P. bilaiae Pb14 and Aspergillus sp. D1 (Table 4).
Effects of Phosphate-Mobilizing Fungi on Barley Growth
The maximum percentage of seed germination was found after inoculation with P. bilaiae Pb14 (Table 5). Treatments with all strains, except Aspergillus sp. D1, promoted shoot and root elongation. Strain P. bilaiae Pb14 enhanced shoot dry weight by 23% and root weight by 32%. Treatment with P. rubens EF5 also increased root weight by 27%.
Strain Aspergillus sp. D1 significantly increased shoot P concentration and P content by 26% and 35%, respectively. Treatments with P. bilaiae Pb14 and T. pinophilus T14 also contributed to the increase in shoot P concentration by 22% and 17%, respectively (Table 5).
Discussion
The results showed that the phosphate-mobilizing trait is more common in filamentous fungi than in yeasts. Information on phosphate mobilization by yeast is very limited compared to fungi, but there are examples of using yeasts as bio-inoculants to improve the P nutrition of plants [22, 24]. Here, we compared fungi and yeasts on this basis using a wide range of species. In addition, percent of filamentous PMF isolated from soil was significantly higher as compared to those isolated from plants (endophytes). It might be speculated that endophytes utilize easily accessible P from plants and therefore the P mobilization trait is of less importance for them. Little is known about comparison of the soil and endophytic PMF and the role of P mobilization interaction between partners inside plants. Such studies were conducted for bacteria only [39]. Here, for the first time we demonstrated such possibility comparing PMF isolated from soil and plants. The ability of endophytes to mobilize phosphates may be due to their need for P during the saprophytic existence. On the other hand, given high integration of endophytes with the host plant, the latter can attract such microorganisms and use P mobilized in the rhizosphere.
There were no strains having high level of P-mobilizing ability isolated from tissues of Brassica napus (Brassicaceae) and Carthamus tinctorius (Asteraceae), as well as from soils where these crops were cultivated (data not shown). This finding allows presuming that plant species affect the abundance of PMF. It is known that the host plant plays a major role in structuring the composition of its microbial communities and their functional properties [40]. For example, Spohn et al. [41] showed that glucose and alanine, the most common components of root exudates, increased both microbial biomass and organic P mineralization rates. But further studies are needed to understand the observed phenomenon.
The number of strains mobilizing organic P was higher than those mobilizing inorganic P. Organic P, containing both carbon and P sources, might be a more efficient nutrient explaining this phenomenon. Information about such speculation is limited, although it was shown that fungi are very efficient particularly in Ca phytate mineralization [15, 16]. It is also known that the P solubilization occurs more actively in the presence of available carbon [12]. In most cases, the strains forming large halo zones on agar plates also had high ability to mobilize P compounds in liquid medium. However, some strains had relatively low PSI values but actively mobilized P in liquid medium. This observation might be due to different diffusion rates of organic acids secreted by fungi cultivated in agar plates. Similar to our results, no or small halo zones were observed in the solid media for yeasts [22] and bacteria [42] that actively solubilized P in liquid media. However, this phenomenon was not previously described for filamentous fungi. The obtained results allow presuming that the size of visible halo zones on agar plates is not sufficient criterion for selection of active P-mobilizing fungi and it should be accompanied with experiments on liquid cultures. Taking into account the amount of solubilized P, five most active strains were selected and arranged by this criterion in order: Aspergillus sp. D1 > T. pinophilus T14 > P. bilaiae Pb14 > P. bilaiae C11 > P. rubens EF5. The amounts of mobilized P were similar or much higher than those described previously for A. niger [14, 23], some species of Talaromyces [6, 15], and Penicillium [11, 19], including P. bilaiae [43].
Agricultural soils in Almaty region, where the phosphate solubilizers will be used, have alkaline conditions with average pH value of 8.3. These soils are calcareous (CaCO3 content is about 7.7%) and rich in humus with soil organic matter content values ranging from 4.2 to 4.7% [44, 45]. In such soils, most P is immobilized in forms of sparingly soluble complexes of Ca phosphates and phytate. Taking into account the characteristics of the target soil, Ca phosphate and Ca phytate were used in the screening experiment. However, to identify true P solubilizers among bacteria it was important to use hard-to-dissolve P sources, like Al phosphate or Fe phosphate [5]. Here we applied this approach for search and characterization of P-solubilizing fungi. The selected five strains demonstrated ability to solubilize all examined P sources that confirmed them as true P-mobilizing fungi. Therefore these strains can be used for phosphate mobilization in both alkaline and acidic soils. The fungal capacity of phosphate mobilization from different P compounds in most cases can be presented in order: Ca phytate ˃ Ca phosphate ˃ Al phosphate ˃ Fe phosphate.
Unlike most previous studies, our work was focused on characterization of fungal P mobilization processes in dynamics. We showed that this approach is useful for assessing the interrelations between the measured parameters, understanding the mechanisms of P mobilization. Fungal growth was accompanied with a decrease in pH of the medium that significantly dropped from 7.0 to 2.3–5.0 depending on the strain and P source already from the 4th day of incubation (Figs. 4c, d). This result is in line with other findings with yeasts [22] and fungi [11, 19]. During the incubation period the growth of fungi significantly increased, the pH of the medium decreased, and the soluble P also increased. Here, for the first time we described significant correlation between the amount of soluble P and fungal biomass, suggesting that the mobilized P was important P source for fungi.
The lowering in pH is usually related to production of organic acids by the PMF, which could either dissolve phosphate as a result of anion exchange or can chelate Ca, Fe, or Al ions associated with the insoluble phosphates [2, 3, 7]. Some recent studies have shown that the types and amounts of organic acids varied with incubation temperature and time and greatly affected by P sources [7, 9, 21]. In our experiments the most quantitatively secreted organic acid was pyruvic acid produced particularly by Aspergillus sp. D1. There are only few reports mentioning pyruvic acid production by PMF [46]. Oxalic, malic, and succinic acids were reported as the major organic acids produced by PMF in the presence of Ca phosphate, while citric acid is predominantly produced in the presence of Fe phosphate [7, 9]. In accordance with these reports, the studied strains also secreted these organic acids during the solubilization of Ca phosphate. However, here no correlations were found between the amount of mobilized P and production of organic acids. Our results showed that Aspergillus sp. D1 and T. pinophilus T14 were more efficient in solubilizing Ca phosphate compared to other strains. Strain Aspergillus sp. D1 was the most active organic acid producer and significantly decreased pH of the media, whereas T. pinophilus T14 showed a maximal activity of phosphatases in the presence of Ca phosphate and Ca phytate. The obtained results suggest that the resulting P mobilization depends on a combination of factors, namely composition and the amount of organic acids produced, changing pH of the medium (due to the release of the organic acids themselves, proton accompanying respiration or ammonium assimilation), and production of phosphatases [3].
The production and release of phosphatases is the important mechanism involved in organic P mineralization by microorganisms [1,2,3]. These enzymes degrade soil organic P compounds resulting in enhancement of the P bioavailability for plants [2]. In the present study, fungal phosphatase activity significantly varied depending on the P source and fungal strain (Fig. 5). The activity values were similar or much higher (up to 3.1 times) than the values described in previous studies with other fungal strains [6, 15]. However, there are no data available in the previous reports about phosphatase activity of species P. rubens, P. bilaiae and T. pinophilus. Notably, production of phosphatases was revealed both in the presence and absence of enzyme substrate (organic P). This observation allows presuming the simultaneous occurrence of constitutive and P-induced mechanisms related to production of phosphatases that could be attributed to adaptation to the environment. Our results also showed that acid phosphatase activity was 1.2–1.9 times higher than alkaline ones (Fig. 5). This was probably due to the decreased pH of the medium. It is known that an optimum pH for acid phosphatase activity is around 4–6, while for alkaline it is 8–10 and pH controls the phosphatase activity [6].
Little is known about mobilization of insoluble P from soils by fungal species P. rubens, P. bilaiae, T. pinophilus, and Aspergillus genus. Here we showed that inoculation with these fungi markedly improved availability of P in soil by 13–28% compared to the untreated control.
Previous studies showed that application of phosphate-mobilizing microorganisms enhanced germination and seedling vigor, improved growth, nutrient content, and yield of different crops [17,18,19,20,21,22, 47]. As for barley, such effects were described for bacteria only [48, 49], whereas our study expands this information to five fungal species. Significant promotion of seed germination, stimulation of plant growth, and increase in shoot P concentrations and contents were observed in inoculated plants compared to the uninoculated control. This finding suggests that the inoculated plants can consume P mobilized by the studied fungi. However, there was one exception with the strain Aspergillus sp. D1. Although this strain increased significantly the amount of available P in the soil by 28% and shoot P concentration in plants by 26%, it did not stimulate seed germination and plant growth. It is possible that Aspergillus sp. D1 produces some compounds alleviating its beneficial effects on plants due to P solubilization and phytohormone production. But further studies are needed to understand this phenomenon better. These observations suggest that the higher phosphate-mobilizing ability of microorganisms alone is not always sufficient for plant growth promotion.
Information about phytohormones produced by P-mobilizing fungi is very limited and only few studies have examined this trait [50, 51]. Our study indicated the presence of auxins, salicylic acid, and abscisic acid in fungal culture media (Table 3). Auxins stimulate root growth, branching, and root hair development and thus indirectly enhance plant P acquisition in soil [52]. The strain T. pinophilus T14 actively produced abscisic acid, which plays significant role in many cellular processes and adaptation of plants to environmental stresses [53, 54], including P deficiency [55]. We propose that plant growth promotion by the studied fungal strains attributed not only to the P mobilization but also to other beneficial traits, such as production of phytohormones.
Conclusion
A large-scale screening of culturable fungi for the ability to mobilize phosphates revealed important patterns, namely: (1) the phosphate-mobilizing property is more inherent in filamentous fungi than in yeasts; (2) soil filamentous mobilizers are more common than endophytes; and (3) the ability to mobilize organic P (Ca phytate) is more abundant as compared to inorganic P (Ca phosphate). The strains of filamentous fungi P. bilaiae Pb14, P. bilaiae C11, P. rubens EF5, T. pinophilus T14, and Aspergillus sp. D1 showing high ability to mobilize both inorganic and organic phosphates were selected. The P solubilization and mineralization processes carried out by the selected fungi were related to decrease in pH of the medium, organic acid production, and activity of phosphatases. The pot experiment revealed the potential of these fungal strains to increase soil P availability and uptake by barley. However, improvement of plant growth was evident only for four of five strains, suggesting that the ability to mobilize phosphates is not sufficient for the selection of plant growth-promoting phosphate mobilizers. Further pot and field trials be conducted to test efficiency of application of these strains (except Aspergillus sp. D1) in natural environment as bio-inoculants.
Data Availability
The data and materials supporting this study are available from the corresponding author on request.
Code Availability
Not applicable.
References
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Kalayu G (2019) Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron 2019:1–7. https://doi.org/10.1155/2019/4917256
Jones DL, Oburger E (2011) Solubilization of phosphorus by soil microorganisms. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action. Soil biology 26. Springer, Heidelberg, pp 169–198. https://doi.org/10.1007/978-3-642-15271-9_7
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus. https://doi.org/10.1186/2193-1801-2-587
Bashan Y, Kamnev AA, de-Bashan LE (2013) Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49:465–479. https://doi.org/10.1007/s00374-012-0737-7
Della Mónica IF, Godoy MS, Godeas AM, Scervino JM (2018) Fungal extracellular phosphatases: their role in P cycling under different pH and P sources availability. J Appl Microbiol 124(1):155–165. https://doi.org/10.1111/jam.13620
Adhikari P, Pandey A (2019) Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 9:2–9. https://doi.org/10.1016/j.rhisph.2018.11.002
Bakri MM (2019) Tri-calcium and zinc phosphates solubilization by Aspergillus niger and its relation to organic acids production. BioNanoSci 9:238–244. https://doi.org/10.1007/s12668-019-0604-1
Jiang Y, Tian J, Ge F (2020) New Insight into carboxylic acid metabolisms and pH regulations during insoluble phosphate solubilisation process by Penicillium oxalicum PSF-4. Curr Microbiol 77(12):4095–4103. https://doi.org/10.1007/s00284-020-02238-2
Doilom M, Guo JW, Phookamsak R, Mortimer PE, Karunarathna SC, Dong W, Liao CF, Yan K, Pem D, Suwannarach N, Promputtha I, Lumyong S, Xu JC (2020) Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front Microbiol 11:585215. https://doi.org/10.3389/fmicb.2020.585215
Pandey A, Das N, Kumar B, Rinu K, Trivedi P (2008) Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World J Microbiol Biotechnol 24:97–102. https://doi.org/10.1007/s11274-007-9444-1
Stefanoni Rubio PJ, Godoy MS, Della Mónica IF, Pettinari MJ, Godeas AM, Scervino JM (2016) Carbon and nitrogen sources influence tricalcium phosphate solubilization and extracellular phosphatase activity by Talaromyces flavus. Curr Microbiol 72(1):41–47. https://doi.org/10.1007/s00284-015-0914-7
Zhang J, Feng L, Ouyang Y, Hu R, Xu H, Wang J (2020) Phosphate-solubilizing bacteria and fungi in relation to phosphorus availability under different land uses for some latosols from Guangdong, China. CATENA 195:104686. https://doi.org/10.1016/j.catena.2020.104686
Li C, Li Q, Wang Z, Ji G, Zhao H, Gao F, Su M, Jiao J, Li Z, Li H (2019) Environmental fungi and bacteria facilitate lecithin decomposition and the transformation of phosphorus to apatite. Sci Rep. https://doi.org/10.1038/s41598-019-51804-7
Oliveira CA, Alves VM, Marriel IE, Gomes EA, Scotti M, Carneiro NP, Guimarães CT, Schaffert RE, Sa NM (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 41:1782–1787. https://doi.org/10.1016/j.soilbio.2008.01.012
Yadav RS, Tarafdar JC (2003) Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolyzing different organic P compounds. Soil Biol Biochem 35:1–7. https://doi.org/10.1016/j.soilbio.2008.01.012
Anil K, Lakshmi T (2010) Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz J Microbiol 41(3):787–795
López JE, Gallego JL, Vargas-Ruiz A, Peña-Mosquera AL, Zapata-Zapata AD, López-Sánchez IJ, Botero-Botero LR (2020) Aspergillus tubingensis and Talaromyces islandicus solubilize rock phosphate under saline and fungicide stress and improve Zea mays growth and phosphorus nutrition. J Soil Sci Plant Nutr 20:2490–2501. https://doi.org/10.1007/s42729-020-00315-w
Morales A, Alvear M, Valenzuela E, Castillo CE, Borie F (2011) Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertiliser. J Soil Sci Plant Nutr 11(4):89–103. https://doi.org/10.4067/S0718-95162011000400007
Qiao H, Sun XR, Wu XQ, Li GE, Wang Z, Li DW (2019) The phosphate-solubilizing ability of Penicillium guanacastense and its effects on the growth of Pinus massoniana in phosphate-limiting conditions. Biol Open 8(11):bio046797. https://doi.org/10.1242/bio.046797
Wang J, Zhao YG, Maqbool F (2021) Capability of Penicillium oxalicum y2 to release phosphate from different insoluble phosphorus sources and soil. Folia Microbiol (Praha) 66(1):69–77. https://doi.org/10.1007/s12223-020-00822-4
Kuo CY, Fu SF, Chou FC, Chen RY, Chou JY (2018) Phosphate-solubilizing characteristics of yeasts. Mycosphere 9(6):1117–1131. https://doi.org/10.5943/mycosphere/9/6/4
Wang X, Wang C, Sui J, Liu Z, Li Q, Ji C, Song X, Hu Y, Wang C, Sa R, Zhang J, Du J, Liu X (2018) Isolation and characterization of phosphofungi, and screening of their plant growth-promoting activities. AMB Expr. https://doi.org/10.1186/s13568-018-0593-4
Ramya P, Gomathi V, Devi RP, Balachandar D (2021) Pichia kudriavzevii—a potential soil yeast candidate for improving soil physical, chemical and biological properties. Arch Microbiol 203(7):4619–4628. https://doi.org/10.1007/s00203-021-02447-8
Zheng H, Qiao M, Xu J, Yu Z (2021) Culture-based and culture-independent assessments of endophytic fungal diversity in aquatic plants in Southwest China. Front Fungal Biol 2:27. https://doi.org/10.3389/ffunb.2021.692549
Pansu M, Gautheyrou J (2006) Handbook of soil analysis. Springer, Heidelberg. https://doi.org/10.1007/978-3-540-31211-6
Murphy J, Riley JP (1962) A Modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. of Agriculture, Washington
Anjos L, Gaistardo C, Deckers J, Dondeyne S, Eberhardt E, Gerasimova M, Harms B, Jones A, Krasilnikov P, Reinsch T, Vargas R, Zhang G (2015) World Reference Base for Soil Resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. FAO, Rome
Waksman SA (1927) Principles of soil microbiology. William & Wilkins, Baltimore
Kurtzman CP, Fell JW, Boekhout T (2011) The yeasts: a taxonomic study, 5th edn. Elsevier Science, Burlington
Ramírez C (1982) Manual and atlas of the penicillia. Elsevier Biomedical Press, Amsterdam
Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi, 4th edn. Amer Phytopathological Society, St Paul Minn
Domsch K, Gams W, Traute-Heidi A (1995) Compendium of soil fungi. Ubrecht & Cramer Ltd, Germany
Huang WY, Cai YZ, Hyde KD, Corke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 33:61–75
Wilson K (2001) Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. https://doi.org/10.1002/0471142727.mb0204s56
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, London, pp 315–322
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Mehta P, Walia A, Shirkot CK (2015) Functional diversity of phosphate solubilizing plant growth promoting rhizobacteria isolated from apple trees in the trans Himalayan region of Himachal Pradesh, India. Biol Agr Hort 31(4):265–288. https://doi.org/10.1080/01448765.2015.1014420
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Spohn M, Ermak A, Kuzyakov Y (2013) Microbial gross organic phosphorus mineralization can be stimulated by root exudates: a 33P isotopic dilution study. Soil Biol Biochem 65:254–263. https://doi.org/10.1016/j.soilbio.2013.05.028
Behera BC, Yadav H, Singh SK, Mishra RR, Sethi BK, Dutta SK, Thatoi HN (2017) Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J Genet Eng Biotechnol 15(1):169–178. https://doi.org/10.1016/j.jgeb.2017.01.003
Takeda M, Knight JD (2006) Enhanced solubilization of rock phosphate by Penicillium bilaiae in pH-buffered solution culture. Can J Microbiol 52(11):1121–1129. https://doi.org/10.1139/w06-074
Pachikin K, Erokhina O, Funakawa S (2014) Soils of Kazakhstan, their distribution and mapping. In: Mueller L, Saparov A, Lischeid G (eds) Novel measurement and assessment tools for monitoring and management of land and water resources in agricultural landscapes of Central Asia. Springer, Cham. https://doi.org/10.1007/978-3-319-01017-5_32
Yapiyev V, Gilman C, Kabdullayeva T, Suleimenova A, Shagadatova A, Duisembay A, Naizabekov S, Mussurova S, Sydykova K, Raimkulov I, Kabimoldayev I, Abdrakhmanova A, Omarkulova S, Nurmukhambetov D, Kudarova A, Malgazhdar D, Schönbach C, Inglezakis V (2018) Top soil physical and chemical properties in Kazakhstan across a north-south gradient. Sci Data 5:180242. https://doi.org/10.1038/sdata.2018.242
Zúñiga-Silgado D, Rivera-Leyva JC, Coleman JJ, Sánchez-Reyez A, Valencia-Díaz S, Serrano M, de-Bashan LE, Folch-Mallol JL (2020) Soil type affects organic acid production and phosphorus solubilization efficiency mediated by several native fungal strains from Mexico. Microorganisms 8(9):1337. https://doi.org/10.3390/microorganisms8091337
Belimov AA, Safronova VI, Mimura T (2002) Response of spring rape (Brassica napus var. oleifera L.) to inoculation with plant growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase depends on nutrient status of the plant. Can J Microbiol 48(3):189–199. https://doi.org/10.1139/w02-007
Peix A, Rivas-Boyero AA, Mateos PF, Rodriguez-Barrueco C, Martı́nez-Molina E, Velazquez E, (2001) Growth promotion of chickpea and barley by a phosphate solubilizing strain of Mesorhizobium mediterraneum under growth chamber conditions. Soil Biol Biochem 33:103–110. https://doi.org/10.1016/S0038-0717(00)00120-6
Canbolat MY, Bilen S, Çakmakçı R, Şahin F, Aydın A (2006) Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol Fertil Soils 42:350–357. https://doi.org/10.1007/s00374-005-0034-9
Zhao L, Zhang Y-Q (2015) Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J Integr Agric 14(8):1588–1597. https://doi.org/10.1016/S2095-3119(14)60966-7
Lubna AS, Hamayun M, Gul H, Lee I-J, Hussain A (2018) Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J Plant Interact 13:100–111. https://doi.org/10.1080/17429145.2018.1436199
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156(3):989–996. https://doi.org/10.1104/pp.111.175448
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571. https://doi.org/10.3389/fpls.2016.00571
Belimov AA, Dodd IC, Dumova SVI, VA, Shaposhnikov AI, Ladatko AG, Davies WJ, (2014) Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol Biochem 74:84–91. https://doi.org/10.1016/j.plaphy.2013.10.032
Ribot C, Wang Y, Poirier Y (2008) Expression analyses of three members of the AtPHO1 family reveal differential interactions between signaling pathways involved in phosphate deficiency and the responses to auxin, cytokinin, and abscisic acid. Planta 227(5):1025–1036. https://doi.org/10.1007/s00425-007-0677-x
Acknowledgements
We are very grateful to Vera I. Safronova for valuable consultations and advices during this study. The research was supported using equipment of the research resource center “Genomic Technologies, Proteomics and Cell Biology” of the All-Russia Research Institute for Agricultural Microbiology.
Funding
This work was supported by the Ministry of Education and Science of Kazakhstan (Grant No. APO9261262). The Russian Science Foundation supported deposition of strains to the RCAM collection, determination of phytohormone production (Grant No. 21-16-00084), and genetic identification (Grant No. 20-76-10042) of the studied strains.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and experimental analysis, and methodology were performed by YVB, AIS, ALS, TDM, and LVI. The first draft of the manuscript was written by YVB. Andrey AB made review and critical supervision. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict and competing interests.
Deposition in Repositories
The most effective strains deposited to the Republican Collection of Microorganisms (RCM, Nur-Sultan, Kazakhstan, http://www.rcm.kz/) and/or Russian Collection of Agricultural Microorganisms (RCAM, St.-Petersburg, Russian Federation, http://www.arriam.ru/kollekciya-kul-tur1/).
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brazhnikova, Y.V., Shaposhnikov, A.I., Sazanova, A.L. et al. Phosphate Mobilization by Culturable Fungi and Their Capacity to Increase Soil P Availability and Promote Barley Growth. Curr Microbiol 79, 240 (2022). https://doi.org/10.1007/s00284-022-02926-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02926-1