Abstract
The aim of this work was to study phosphate (P) solubilization (and the processes involved in this event) by Talaromyces flavus (BAFC 3125) as a function of carbon and/or nitrogen sources. P solubilization was evaluated in NBRIP media supplemented with different carbon (glucose, sorbitol, sucrose, and fructose) and nitrogen (l-asparagine, urea, ammonium sulfate (AS), and ammonium nitrate (AN) combinations. The highest P solubilization was related to the highest organic acid production (especially gluconic acid) and pH drop for those treatments where glucose was present. Also P solubilization was higher when an inorganic nitrogen source was supplemented to the media when compared to an organic one. Although not being present an organic P source, phosphatase activity was observed. This shows that P mineralization and P solubilization can occur simultaneously, and that P mineralization is not induced by the enzyme substrate. The combination that showed highest P solubilization was for AN-glucose. The highest acid phosphatase activity was for AS-fructose, while for alkaline phosphatase were for AS-fructose and AN-fructose. Acid phosphatase activity was higher than alkaline. P solubilization and phosphatase activity (acid and alkaline) were influenced by the different carbon–nitrogen combinations. A better understanding of phosphate-solubilizing fungi could bring a better use of soil P.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key nutrient for sustained agriculture productivity. Although total P is present in high concentration in most soils, a vast majority remains unavailable and only less than 10 % enters the biogeochemical cycle [15]. Apart from being an essential nutrient for plant development, there are other reasons which make P a major agricultural research topic: (1) the price of fertilizers sky-rocketed in recent times, making P fertilizers unaffordable for farmers in developing countries, (2) sources of high quality P are rapidly depleted and expected to be exhausted in less than 100 years [16].
Microorganisms have a fundamental role in P biogeochemical cycle and bioavailability [14]. Some fungi and bacteria can solubilize P from unavailable forms in the rhizosphere. These P solubilizers can be used directly as biofertilizers in the soil or in bioreactors for the processing of rock P [1].
Gerretsen [7] demonstrated for the first time that microbiological activity in the rhizosphere can dissolve insoluble P through organic acid release and pH drop. Proton extrusion through ammonium uptake is also a mechanism involved in inorganic P solubilization [12]. Wenzel et al. [29] reported that the secretion of protons could solubilize tricalcium P by calcium exchange.
Sugars available in the medium are used by microorganisms in different metabolic pathways, generating organic acids [28]. Some of these acids are found in living cells as intermediates of tricarboxylic acid cycle. On the other hand, when carbohydrates are available in the medium, they are taken up into the cell, and their utilization is dependent on an enzymatic system that is constitutive in some organisms and inducible in others [4]. In addition, many fungal and bacterial species appear to have the capacity to use glucose by direct oxidation, producing gluconic acid [2, 18].
Fungi produce a vast quantity of enzymes involved in P mineralization. This event is carried out by a group of enzymes known as phosphatases or phosphohydrolases that catalyze the hydrolysis of esters and phosphoric acid anhydrides [17]. Beever and Burns [3] suggested the existence of three types of phosphatases: acid, alkaline, and phosphatases with a high specificity for substrate. Acid phosphatases are more abundant in fungi than the alkaline ones [20]. They play an important role in the mineralization of organic C, organic P, and low levels of free inorganic ions [27]. Extracellular acid phosphatases hydrolyze external phosphate esters [22]. Inorganic phosphate is reported in other fungal species to be an inhibitor of extracellular phosphatase activity [8, 10].
In the particular case of Talaromyces flavus, it has been reported that this fungus is a biocontroler of the phytopathogenic fungus Verticillium dahliae [26] and Sclerotinia sclerotiorum [23], an efficient P solubilizer [24] and to improve the efficiency of the symbiosis between the arbuscular mycorrhizal fungus Gigaspora rosea and wheat plants [6].
Although many mechanisms underlying the P solubilization and mineralization process were proposed, the combined effect of carbon (C) and/or N on organic acid production and extracellular phosphatase activity, and the influence of inorganic/organic N sources on P solubilization have not been analyzed yet. Therefore, the aim of this work was to evaluate how different combinations of C and N sources influence T. flavus growth, P solubilization, organic acids, and phosphatases production.
Materials and Methods
Fungal Strain
Talaromyces flavus strain S73 BAFC 3125 (BAFC: Mycological Culture Collection of the Department of Biodiversity and Experimental Biology, Faculty of Exact and Natural Sciences, University of Buenos Aires) was used in this study. It was isolated from soils of the Buenos Aires Province (Argentina) [23]. Stock cultures were maintained on malt agar (12.7 g l−1), agar (2 % w/v) (MEA) slants at 4 °C with periodic transfer.
Fungal Growth and Culture Conditions
P solubilization was evaluated in 100 ml Erlenmeyer flasks containing 20 ml National Botanical Research Institute (NBRIP) broth [21], containing MgSO4 (0.12 g l−1), KCl (0.20 g l−1), MgCl2 6H2O (5.00 g l−1), and Ca3(PO4)2 (5.00 g l−1), the latter insoluble at neutral and alkaline pH. The medium was also amended with different combinations of C and N sources. For the C sources, d-glucose, d-fructose, sucrose, and sorbitol were tested, and for the N sources two organic (l-asparagine and urea) and two inorganic sources ((NH4)2SO4 (AS) and NH4NO3 (AN)) were tested. The N concentration in the medium was 7.54 × 10−2M, according to the original concentration of AS in the NBRIP broth. The C concentration was 10 g l−1 for every C source. All C–N combinations (16 treatments) were tested. Before sterilization, the medium pH was adjusted to 7.00 with HCl (1.00 M). Each flask was inoculated with a block of agar malt medium (4 mm side) containing the fungus T. flavus (BAFC 3125) and incubated for 85 h under agitation. Uninoculated flasks were used as controls. After incubation, the final pH was measured. The pH drop was calculated as the difference on the final pH between treatments and controls. Also after incubation the medium was filtered. The mycelia were oven-dried (80 °C) for 48 h for fungal dry weight (FDW) measurement (data not shown). The supernatant was separated for further analyses. In order to avoid FDW overestimation, HCl (0.10 M) was used to wash the mycelia and solubilize the remaining insoluble tricalcium P adhered to the fungus. Each treatment had three replicates.
P Solubilization
Soluble P concentration (mg l−1) was achieved by spectrophotometric measurement using a Merck Phosphate Test VM Spectroquant® for both treatments and controls. The fungal P solubilization efficiency (mg P l−1 mg−1 mycelia) was calculated as the difference for soluble P between each treatment and control over FDW.
Organic Acid Analysis
The supernatant was filtered through a 0.22-µm pore-size syringe filter (Chrom Tech Inc., Apple Valley, MN) and stored at 4 °C for high-pressure liquid chromatography analysis (LC-20AT Prominence, Shimadzu, Kyoto, Japan), equipped with an Aminex column HPX-87-H (Cat no. 125-0140; Bio Rad Laboratories Inc., Hercules, CA) at 50 °C. An UV detector (SPD-20AV Shimadzu, Kyoto, Japan, set to 215 nm) was used for the quantification of organic acids (injection volume 20 µl). The mobile phase consisted of 5 mM H2SO4, run at a flow rate of 0.6 ml min−1. Peaks were identified by their characteristic retention times against a set of standards of known organic acids (Sigma-Aldrich Co., St. Louis, MO).
Phosphatase Activity Assays
Acid phosphatase activity was measured at 25.5 °C using acetate buffer (pH 4.50), according to Pawar and Thaker [22]. The reaction was stopped after 30 min with 2.00 ml NaOH (1.00 M). Alkaline phosphatase activity was measured using universal buffer (pH 9.00) [13]. The reaction was stopped after 60 min with 2.00 ml NaOH (1.00 M). Para-nitrophenolphosphate (PNPP) suspended in acetate buffer was used as enzyme substrate for both acid and alkaline phosphatases. Heat denaturation was used as control for both enzymes. An enzymatic unity (EU) was defined as the amount of enzyme that hydrolyzes 1 μmol PNPP in 1 l of supernatant per minute produced per gram of FDW under the experimental conditions described.
Statistical Analysis
The results were analyzed by one- and two-way ANOVA, analyzing C × N factors. Normality and homogeneity of variances were checked. Organic acids and pH were analyzed by rank transformation, due to the lack of homoscedastic. The contrasts among treatments were analyzed by Fisher LSD test. All the statistical tests were done using the software STATISTICA 7. Data are presented as mean ± standard deviation.
Results
pH of T. flavus in Different C and N Sources
The variation in pH was analyzed to know if this event had a relationship with P solubilization. The statistical analysis showed that there were C–N combinations that led to the highest pH drop (interaction P < 0.05) (Table 1). Although a decrease in pH was observed in all treatments, the lowest pH was found in urea-glucose treatment (3.32 ± 0.01), followed by l-asparagine-glucose, AS-glucose, and AN-glucose (2.37 ± 0.14, 2.38 ± 0.02 and 2.27 ± 0.06, respectively) (Table 1). The highest decrease of pH was present when glucose was the C source. In contrast, the lowest pH drop was for the treatment AS-fructose (0.33 ± 0.17).
P Solubilization
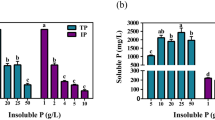
P solubilization over FDW was measured to analyze the influence of C and/or N sources in this phenomenon. A statistical analysis revealed those C–N combinations that enabled the highest P solubilization (interaction P < 0.05). The highest soluble P concentration was for both AS-glucose and AN-glucose (569.45 ± 1.14 and 561.93 ± 4.56 mg P l−1 mg−1 FDW, respectively), followed by l-asparagine-glucose and urea-glucose (7.22 ± 0.49 and 5.53 ± 0.50 mg l−1 mg−1 FDW, respectively) (Fig. 1). These soluble P levels were achieved by AN-sucrose, AS-sucrose, asparagine-sucrose, AS-fructose, AN-sorbitol, and AN-fructose (3.18 ± 0.50, 3.00 ± 0.93, 1.74 ± 0.39, 1.82 ± 0.69, 1.56 ± 0.50, and 1.70 ± 0.31 mg l−1 mg−1 FDW, respectively). The lowest P efficiency values were for asparagine-sorbitol, asparagine-fructose, urea-sorbitol, urea-sucrose, urea-fructose, and AS-sorbitol, (0.06 ± 0.01, 0.22 ± 0.14, 0.09 ± 0.01, 0.09 ± 0.03, 0.18 ± 0.02, and 0.39 ± 0.17 mg l−1 mg−1 FDW, respectively).
Soluble P concentration (mg l−1 mg−1 FDW). Treatments with the same letter have no significant differences, according to Fisher LSD test (P < 0.05). C sources are glucose (white bars), sorbitol (black bars), sucrose (light gray bars), and fructose (dark gray bars). Vertical bars are ± SD of the mean
Organic Acids Analysis
The organic acids found in the culture media were gluconic and oxalic acid, the former in a higher concentration than the latter. Neither citric nor fumaric acid were detected. The only factor that influenced fungal production of gluconic acid was the C source (Fig. 2), especially when it was glucose (793.5 ± 194.5 mM g−1 FDW). The lowest concentration was found when sucrose and fructose were used (27.96 ± 8.13 and 25.70 ± 14.32 mM g−1 FDW). Gluconic acid was not detected when sorbitol was the C source.
Interaction was found between C and N factors (P < 0.05) for oxalic acid production. The highest concentrations were found in AN-glucose, urea-fructose, urea-sucrose, and AS-glucose treatments (33.10 ± 13.30, 19.36 ± 4.83, 12.04 ± 0.89, and 11.59 ± 1.32 mM g−1 FDW, respectively) (Table 2). On the other hand, this acid was not detected in AN-fructose and the lowest concentrations were found in l-asparagine-sorbitol and AS-sorbitol (3.02 ± 0.23 and 3.26 ± 1.03 mM g−1 FDW, respectively).
Acid and Alkaline Phosphatase Activity Assays
Acid Phosphatase Activity
Interaction between C and N sources was statistically significant (P < 0.05). The highest acid phosphatase activity was found in asparagine-fructose and AS-fructose (6.39 ± 0.60 and 7.00 ± 0.76 EU). The lowest activity was found in asparagine-glucose, asparagine-sorbitol, urea-sorbitol, and AS-glucose (3.80 ± 0.28, 2.28 ± 0.30, 3.14 ± 0.17, and 3.41 ± 0.60 EU, respectively) (Fig. 3a).
Phosphatase activity (EU). a Acid. b Alkaline. Each bar represents a C–N combination. C sources are glucose (white bars), sorbitol (black bars), sucrose (light gray bars), and fructose (dark gray bars). Treatments with the same letter have no significant differences, according to the Fisher LSD test (P < 0.05). Vertical bars are ± SD of the mean
Alkaline Phosphatase Activity
Interaction between C and N sources was statistically significant (P < 0.05). The highest alkaline phosphatase activity was observed in asparagine-fructose, AS-fructose, and AN-fructose (2.10 ± 0.41, 2.24 ± 0.24 and 2.03 ± 0.07 EU, respectively) (Fig. 3b). The lowest values were found in asparagine-sorbitol, asparagine-sucrose, and urea-sorbitol (0.41 ± 0.05, 0.79 ± 0.04, and 0.63 ± 0.09 EU, respectively) (Fig. 3b).
Discussion
Growth and/or development of T. flavus are a response to the interaction of multiple variables analyzed in this study. Although an interaction between C and N sources was found for P solubilization, glucose is the outstanding factor for this event as previously reported [5, 30]. However, these differences were lower than the ones seen in this work. On the other hand, it was found that when other C sources, as sorbitol, were present, T. flavus solubilized less P than other fungal strains [25] showing that the results of this work cannot be extended to other fungal strains. In general, P solubilization was lower when organic N sources (urea and asparagine) were present. These results suggest that the nature and availability of N could modify the P solubilization mechanism.
P solubilization was accompanied by an acidification of the culture media as previously reported [12, 30]. The results obtained in this work showed that the pH drop and gluconic acid production were related to the presence of glucose rather than a particular C–N combination, since glucose is the substrate for the production of this acid.
On the other hand, the maximum drop of pH did not coincide with the highest production of oxalic acid, suggesting that this acid did not have a high contribution to the decrease of pH. Interestingly, the highest P solubilization and oxalic acid production were found in the same treatment (AN-glucose), suggesting that this acid is an important factor in tricalcium P solubilization, probably as a chelating element. Previous studies showed that the oxalate presence promoted calcium phosphate salts solubilization, coinciding with our results [9, 24]. The production of oxalic acid was increased in AN-glucose and urea-fructose treatments, while in AN-fructose this acid was not detected. It is clear that oxalate production by T. flavus was influenced by specific C–N combinations rather than a specific C or N source effect.
To our knowledge, the studies where the effect of C and/or N sources was analyzed on phosphatases activity are scarce [20]. The treatments where phosphatase activity reached its highest activity (l-asparagine-fructose and AS-fructose for acid phosphatase, and l-asparagine-fructose, AS-fructose, and AN-fructose for alkaline phosphatase) were those where P solubilization was low. This might be due to a higher phosphatase activity when available P is limited, as it was reported in Aspergillus niger [11]. Also the presence of fructose (or its concentration) could increase phosphatase activity or its production by T. flavus. Similar results were observed by Pawar and Thaker [22] where sucrose concentration increased the acid phosphatase activity in A. niger. All together, these results suggest that different C sources could benefit phosphatases production or activity of different species. On the other hand, acid phosphatase activity was higher than alkaline, maybe because of the low pH found in the culture media. Previous studies showed that acid phosphatase activity was higher than alkaline in soil fungi, coinciding with our results [17, 19].
Although an organic P source was not used in this work, phosphatase activity was found in all the C–N combinations. Having found soluble P and phosphatase activity (both acid and alkaline) in all the treatments lets us assume that both P solubilization and P mineralization can occur simultaneously. Also, the optimal C–N combinations were different for P solubilization and phosphatase activity, giving to this fungus a wide range of conditions to make P available.
In conclusion, the presence of glucose as a C source was the main factor that influenced P solubilization, pH drop, and gluconic acid production by T. flavus. Also a higher efficiency was obtained when an inorganic N source was amended to the culture medium. The oxalic acid and biomass production were influenced by specific C–N combinations. The highest phosphatase activity occurred when P solubilization was low, suggesting that P mineralization could be the main mechanism of making P available when inorganic P solubilization is not enough for fungal development. This finding shows a wide range of conditions where T. flavus can make P available, either by solubilization or mineralization. Therefore, this fungus could be used as an efficient bioinoculant in soils with high insoluble P concentration (either organic or inorganic forms) and low bioavailable P condition.
References
Ahuja A, Ghosh SB, D’Souza SF (2007) Isolation of a starch utilizing, phosphate solubilizing fungus on buffered medium and its characterization. Bioresour Technol 98:3408–3411
Bankar SB, Bule MV, Singhal RS, Ananthanarayan L (2009) Glucose oxidase—an overview. Biotechnol Adv 27:489–501
Beever RE, Burns DJW (1980) Phosphorus uptake, storage and utilization by fungi. Adv Bot Res 8:127–219
Brock TD, Madigan MT, Martinko JM, Parker J (1994) Biology of microorganisms, 7th edn. Prentice, New Jersey
Chai B, Wu Y, Liu P, Gao M (2011) Isolation and phosphate-solubilizing ability of a fungus, Penicillium sp. from soil of an alum mine. J Basic Microb 51:5–14
Della Mónica IF, Stefanoni Rubio PJ, Cina RP, Recchi M, Godeas AM, Scervino JM (2014) Effects of the phosphate-solubilizing fungus Talaromyces flavus on the development and efficiency of the Gigaspora rosea-Triticum aestivum symbiosis. Symbiosis 64:25–32
Gerretsen FC (1948) The influence of microorganisms on the phosphorus uptake by plant. Plant Soil 1:51–81
Guimarães LHS, Terenzi HS, Jorge JA, Leone FA, Polizeli MLTM (2004) Characterization and properties of acid phosphatases with phytase activity produced by Aspergillus caespitosus. Biotechnol Appl Bioc 40:201–207
Gyaneshwar G, Kumar N, Parekh LJ (1998) Effect of buffering on the phosphate-solubilizing ability of microorganisms P. J Microbiol Biotechnol 14:669–673
Haas H, Redl B, Leitner E, Stöffler G (1991) Penicillium chrysogenum extracellular acid phosphatase: purification and biochemical characterization. Biochim Biophys Acta 1074:392–397
Hidayat BJ, Eriksen NT, Wiebe MG (2006) Acid phosphatase production by Aspergillus niger N402A in continuous flow culture. FEMS Microbiol Lett 254:324–331
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem 24:389–395
Johnson WC, Lindsey AJ (1939) An improved universal buffer. Analyst 64:490–492
Khan MS, Zaidi A, Ahemad M, Oves M, Wani PA (2010) Plant growth promotion by phosphate solubilizing fungi- current perspective. Arch Agro Soil Sci 56:73–98
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42:199–228
Glenn CR, Garrison RE (2003) Phosphorites. In: Middleton GV (ed) Encyclopedia of sediments and sedimentary rocks: Encyclopedia of Earth Sciences Series. Kluwer Academic Publishers, Dordrecht, pp 519–526
Nahas E (2002) Microrganismos do solo produtores de fosfatases em diferentes sistemas agrícolas. Bragantia 61:267–275
Nahas E (2007) Phosphate solubilizing microorganisms: Effect of carbon, nitrogen and phosphorus sources. In: Velazquez E, Rodriguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization. Dordrecht, pp 111–115
Nahas E, Centurion JF, Assis LC (1994) Microganismos solubilizadores de fosfato e produtores de fosfatases de vários solos. R bras Ci Solo 18:43–48
Nahas E, Terenzi HF, Rossi A (1982) Effect of carbon source and pH on the production and secretion of acid phosphatase (EC 3.1.3.2) and alkaline phosphatase (EC 3.1.3.1) in Neurospora crassa. J Gen Microbiol 128:2017–2021
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Pawar VC, Thaker VS (2009) Acid phosphatase and invertase activities of Aspergillus niger. Mycoscience 50:323–330
Rodriguez MA (2004) Hongos del suelo antagonistas de Sclerotinia sclerotiorum. Selección y estudio de potenciales agentes de biocontrol. PhD thesis, Universidad de Buenos Aires, Argentina
Scervino JM, Prieto Mesa M, Della Mónica I, Recchi M, Sarmiento Moreno N, Godeas A (2010) Soil fungal isolates produce different organic acid patterns involved in phosphates salts solubilization. Biol Fert Soils 46:755–763
Seshadri S, Ignacimuthu S, Lakshminarasimhan C (2004) Effect of nitrogen and carbon sources on the inorganic phosphate solubilization by different Aspergillus niger strains. Chem Eng Commun 191:1043–1052
Stosz SK, Fravel DR, Roberts DP (1996) In vitro analysis of the role of glucose oxidase from Talaromyces flavus in biocontrol of the plant pathogen Verticillium dahliae. Appl Environ Microb 62:3183–3186
Straker CJ, Mitchell DT (1986) The activity and characterization of acid phosphatases in endomycorrhizal fungi of the Ericaceae. New Phytol 104:243–256
Vinopal RT, Romano AH (2000) Carbohydrate synthesis and metabolism. In: Lederberg J (ed) Encyclopedia of microbiology, vol 1, 2nd edn. Academic, San Diego, pp 647–668
Wenzel CL, Ashford AE, Summerell BA (1994) Phosphate-solubilizing bacteria associated with proteoid roots of seedlings of waratah [Telopea speciosissima (Sm.) R.Br.]. New Phytol 128:487–496
Yadav J, Verma JP, Tiwari KN (2011) Solubilization of tricalcium phosphate by fungus Aspergillus niger at different carbon source and salinity. Trends Appl Sci Res 6:606–613
Acknowledgments
This work was supported by the following institutions: Universidad de Buenos Aires (UBA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Statement of Human and Animal Rights
Neither human nor animals are used in this study.
Additional information
P. J. Stefanoni Rubio and M. S. Godoy have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stefanoni Rubio, P.J., Godoy, M.S., Della Mónica, I.F. et al. Carbon and Nitrogen Sources Influence Tricalcium Phosphate Solubilization and Extracellular Phosphatase Activity by Talaromyces flavus . Curr Microbiol 72, 41–47 (2016). https://doi.org/10.1007/s00284-015-0914-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-015-0914-7