Abstract
Inoculants are of great importance in sustainable and/or organic agriculture. In the present study, plant growth of barley (Hordeum vulgare) has been studied in sterile soil inoculated with four plant growth-promoting bacteria and mineral fertilizers at three different soil bulk densities and in three harvests of plants. Three bacterial species were isolated from the rhizosphere of barley and wheat. These bacteria fixed N2, dissolved P and significantly increased growth of barley seedlings. Available phosphate in soil was significantly increased by seed inoculation of Bacillus M-13 and Bacillus RC01. Total culturable bacteria, fungi and P-solubilizing bacteria count increased with time. Data suggest that seed inoculation of barley with Bacillus RC01, Bacillus RC02, Bacillus RC03 and Bacillus M-13 increased root weight by 16.7, 12.5, 8.9 and 12.5% as compared to the control (without bacteria inoculation and mineral fertilizers) and shoot weight by 34.7, 34.7, 28.6 and 32.7%, respectively. Bacterial inoculation gave increases of 20.3–25.7% over the control as compared with 18.9 and 35.1% total biomass weight increases by P and NP application. The concentration of N and P in soil was decreased by increasing soil compaction. In contrast to macronutrients, the concentration of Fe, Cu and Mn was lower in plants grown in the loosest soil. Soil compaction induced a limitation in root and shoot growth that was reflected by a decrease in the microbial population and activity. Our results show that bacterial population was stimulated by the decrease in soil bulk density. The results suggest that the N2-fixing and P-solubilizing bacterial strains tested have a potential on plant growth activity of barley.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus, one of the major nutrients limiting plant growth, is rapidly immobilized after addition to soil as a soluble fertilizer, and thus, it become less available to plant. Seed or soil inoculation with phosphate-solubilizing bacteria (PSB) such as Bacillus spp. can solubilize fixed soil P and applied phosphates, resulting in higher crop yields (Yadav and Dadarwal 1997; Puente et al. 2004a,b) and also in increased inorganic P availability to plant by mineralization of organic P (Kumar and Narula 1999; Rodriguez et al. 2004). The presence of a high number of bacteria in the rhizosphere is important since they may convert organic and inorganic substances into available plant nutrients (Badalucco and Kuikman 2001). Solubilization of insoluble compounds is due to the excretion of microbial metabolites such as organic acids (Puente et al. 2004a; Iyamuremye and Dick 1996). Indeed, the production of microbial metabolites including organic acids may result in a decrease in soil pH, which plays a major role in solubilization of some nutrients (Rodriguez and Fraga 1999; Nautiyal et al. 2000).
Plant growth-promoting bacteria (PGPB) are classified into two different groups (Bashan and Holguin 1998). The first group includes bacterial strains that have the capability of synthesizing plant growth-promoting substances (i.e. phytohormones, vitamins, enzymes and siderophores); inhibiting ethylene synthesis; fixing atmospheric nitrogen; solubilizing inorganic phosphate and iron; mineralizing organic phosphate and improving plant stress tolerance to drought, salinity, metal toxicity, and pesticide load (Glick and Bashan 1997; Lucy et al. 2004). A second group is able to decrease or prevent the deleterious effects of phytopathogenic microorganisms (Bashan and Holguin 1998).
The aim of the study was to evaluate the efficiency of novel P-solubilizing and N2-fixing bacterial strains isolated from barley and wheat rhizosphere soils so as to asses their possible use as inoculants for increasing productivity of agricultural crops by minimizing the need for chemical fertilizers.
Materials and methods
Bacterial strains
Three bacterial strains were isolated from the rhizosphere field-grown crops and identified as Bacillus RC01, Bacillus RC02, and Bacillus RC03 based on fatty acid methyl ester analysis using MIDI System (Sherlock Microbial Identification System version 4.5, MIDI, Inc., Newark, DE). The bacterial strain Bacillus M-13 was originally isolated from pepper plants at Atatürk University, Erzurum, Turkey (Şahin et al. 2004). In the present study, P-solubilizing activities of three new (RC01, RC02 and RC03) and a previously isolated strain (M-13, positive control) were measured according to the qualitative and quantitative methods (Mehta and Nautiyal 2001). The bacterial strains were characterized by morphological, biochemical and physiological tests, including pigment production on nutrient agar medium and the Gram reaction (Forbes et al. 2002). These bacterial strains were also able to grow in N-free basal medium (Table 1).
Isolation and identification of bacteria
Bacillus RC01 and Bacillus RC03 were initially isolated from the rhizosphere of wheat grown in alkaline soil. The strain Bacillus RC02 was isolated from the rhizosphere of barley grown in sandy loam. Plants were removed from a field site with non-rhizosphere soil, brought immediately to the laboratory in polythene bags and air-dried. The non-rhizosphere sandy-loam soil was removed by gentle shaking, whereas the soil adhering strongly to the root was referred to as rhizosphere soil. Ten grams soil from each sample was aseptically weighed, transferred to an Erlenmeyer flask with 100 ml sterile water and shaken for 30 min at 150 rpm. Immediately after shaking, a series of tenfold dilutions of the suspension was made for each sample by pipetting 1-ml aliquots into 9 ml sterile water. The final dilution was 105-fold; 0.1 ml of each dilution of the series was placed onto a Petri dish with nutrient agar (NA) (Difco). Three replicate dishes were made for each dilution. Dishes were placed in an incubator at 28°C for 7 days (aerobically). Rhizobacteria isolates were selected to represent distinct types based on differences in colony morphology, including colony form, elevation and pigment production. Isolates were re-streaked on NA and checked for purity. Isolated bacterial strains were identified based on whole-cell fatty acids methyl esters (FAMEs) analysis (De Freitas et al. 1997) performed according to the method described by the MIDI System manufacturer's manual. The identified bacterial strains at the genus level were maintained in nutrient broth (NB) with 15% glycerol at −86°C for further tests.
Seed inoculation
For this experiment, pure cultures were grown in NB at 28°C and diluted to a final concentration of 109 colony-forming units (CFU) ml−1 in sterile distilled water containing 0.025% Tween 20. Barley seeds were sterilized in 70% ethanol for 2 min and 1.2% sodium hypochlorite for 10 min and rinsed ten times in sterile tap water. Seeds were then treated with the bacterial suspensions at the concentration of 108 CFU ml−1 for 30 min under sterilized conditions.
Microbial populations
Determinations of viable microbial bacteria and fungi counts were carried out 15, 30 and 45 days after sowing. The rhizosphere soil, which was adhering to the root, was separated by gentle tapping, air-dried at room temperature (25°C) and analysed for the content of culturable bacteria, fungi and P-solubilizing bacteria by the CFU according to the pour plate method (Salle 1973) in asparagine–mannitol agar, dextrose–rose bengal agar (Martin 1950) and sucrose–tricalcium phosphate agar (Pikovskaya 1948) media, respectively. Ten grams soil from each sample was aseptically weighed, transferred to an Erlenmeyer flask with 100 ml sterile water and shaken for 30 min at about 150 rpm. Immediately after shaking, each suspension was tenfold diluted by pipetting 1-ml aliquots into 9 ml sterile water. Thus, a 108-fold final dilution was obtained; 0.1 ml of each dilution of the series was placed onto a Petri dish with NA. Three replicate dishes were made for each dilution. The agar plates were incubated at 30°C for 7 days (aerobically). After the incubation period, the CFU of the bacteria developed on the respective agar plates were counted by the standard method. The averaged CFU per gram of oven-dried soil was calculated for each soil sample.
Soil sampling and laboratory analysis techniques
The soil was sampled from the Ap horizon, dried indoors until it could be crumbled to pass through 4 mm for pots experiment and 2 mm sieves for analyses of physicochemical properties. The soil is classified as Ustorthents according to USDA soil taxonomy (Soil Survey Staff 1999). The loam soil had an organic matter content of 1.8%, pH 6.9, available P content of 13.7 mg kg−1 (Olsen and Sommers 1982), NH4 +-N and NO3 −-N contents of 8.3 and 7.6 mg kg−1, respectively, cation exchange capacity and exchangeable K and Ca+Mg of 24.9, 1.6 and 17.8 cmol kg−1, respectively. Soil samples were sterilized by autoclaving at 121°C at 1.1 atm pressure in a Pyrex beaker for 2 h and then cooled for 24 h.
Soil compression
Approximately 2.5 kg of loose sterile soil at 15% water content was placed in PVC cylindrical pots, and the bulk density was 1.1 Mg m−3 in the non-compacted pots. The compacted pot was prepared by packing soil in a Proctor hammer (5 cm i.d., 30 cm drop height, 2.5 kg weight) in three layers by giving a sufficient number of blows using a flat-bottom hammer to reach the target bulk density (approximately 1.25 and 1.4 Mg m−3). Soil bulk densities were determined by the core method (Blake and Hartge 1986).
Pot experiment
Pots were sterilized with 20% sodium hypochlorite solution, filled with 2.5 kg soil and seeded. The following treatments with three replicates were investigated: (1) control (without bacteria inoculation and mineral fertilizers), (2) N fertilizer (40 mg N kg−1 soil), (3) P fertilizer (20 mg P kg−1 soil), (4) NP fertilizer (40 mg N kg−1 soil+20 mg P kg−1 soil), (5) Bacillus RC01, (6) Bacillus RC02, (7) Bacillus RC03, (8) Bacillus M-13. There were eight treatments, three soil compaction (1.1, 1.25 and 1.40 Mg m−3), three harvest times (15, 30 and 45 days) and three replicates totalling 216 pots. Pots were arranged on a bench in the greenhouse according to a randomized complete block design with three blocks (i.e. each block contained all 8 treatments×3 soil compaction×3 harvests). Barley seeds were placed at the same depth (approximately 2.5 cm below the soil surface) in all pots.
The seedlings were grown in a greenhouse under a day–night cycle of 15–9 h natural light (ranged from 900 to 1,200 μmol/m−2 s−1), 25–16°C and 55% relative humidity. Plants were thinned at the earliest to maintain the desired number of uniform plants (five seedlings per pot) in each pot. The pots were watered to 60% water-holding capacity and were maintained at this moisture content by watering to weight every 2–3 days. Sterile water was slowly added over the top soil in each pot. The treated plants were uprooted after 15, 30 and 45 days of sowing, with minimal damage to the root system, and were washed gently under running tap water to remove the adhering soil particles. At harvest, the root system was separated from the shoots, which were oven-dried for 3 days at 70°C. Total root length was measured as described by Farrell et al. (1993). Macronutrient (N, P, K, Ca and Mg) and micronutrient (Fe, Mn, Zn and Cu) of barley seedlings were determined according to the Association of Official Analytical Chemists (AOAC 1990). An analysis of variance (ANOVA) and Duncan's multiple range test (at P<0.05) were performed to analyse statistical differences and to discriminate between means.
Results
Table 2 showed that sampling time and bulk density affected soil properties. In general, soil pH and organic matter values were reduced when increasing bulk density and time. Maximum available P in soil was observed in the P and NP fertilizer treatments followed by the previously isolated strain P-solubilizing Bacillus M-13 and new strain Bacillus RC01. Bacillus M-13 and Bacillus RC01 inoculations increased available P, respectively, by 16.9 and 8.8% compared with control pots. Inoculation with other bacterial strain did not show significant differences in available P with respect to the control, and it was not affected by bulk density. Bacterial inoculations significantly affected the contents of both NO3 −-N and total mineral N content of soil. Among the bacterial inoculants, the maximum NO3 −-N content of soil was seen in Bacillus RC01, followed by RC03, RC02 and M-13. There was no significant difference in the concentration of extractable NH4 +-N among the various treatments, except for the N and NP fertilizer treatments. Concentration of NO3 −-N was greater than that of NH4 +-N. Maximum concentration of NO3 −-N and NH4 +-N was observed on the 15th and 30th day, respectively, and then declined. Soil NO3 −N and NH4 +N contents were reduced by soil compaction (Table 2).
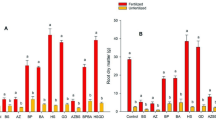
In general, time and bulk density significantly affected all the tested parameters in barley. Root length and/or dry weight increased in all treatments with time, whereas they were decreased with soil compaction (Table 3). Of the bacterial inoculation, root length was only affected by Bacillus RC01 compared to the control. Mineral fertilizer and bacterial inoculation significantly increased root and shoot dry weight of barley plants. The results showed that there was numerical but no statistical difference between bacterial inoculation and P fertilizer in terms of root and shoot dry weight. As bulk density decreased and harvest time increased, bacterial count and seedling growth increased. Soil compaction caused low root and shoot dry weight, but the effect depended on the treatment. Of the treatments, the maximum barley root dry weight was observed in the NP fertilizer treatment, followed by the Bacillus RC01 and N fertilizer treatments.
Total culturable bacteria varied from 0.9 to 10.5×106 CFU g−1 of soil depending on the treatment. The bacterial and fungal population at day 45 was significantly greater than at days 15 or 30 in all treatments (Table 4). The highest number of total culturable bacteria and PSB was observed in pots inoculated with P-solubilizing Bacillus M-13. The counts of PSB ranged between 1.2×104 and 6.7×106 CFU g−1 dry weight soil. The treatments, either that based on fertilizer application or those with PSB inoculations, were affected by both bacterial and fungal population. On the other hand, total number of bacteria and fungi decreased with increasing bulk density in all treatment groups as well as in the control (Table 4). The number of days between sowing and seedling harvest time was positively and significantly correlated (p<0.01) with total culturable bacteria (r=0.53), fungi (r=0.48) and PSB (r=0.19) counts, but they were negatively and significantly correlated (p<0.01) with soil compaction (r=−0.63, r=−0.63 and r=−0.26, respectively).
Both inoculations with PGPB and fertilizer application increased the N and P contents of barley seedlings compared to the untreated control. Inoculation with the P-solubilizing bacteria Bacillus M-13 also significantly enhanced Mn, Zn and Cu contents of barley seedling. Both NP and P applications increased P content up to 40%, whereas there were no significant differences in K, Ca and Fe concentrations between inoculation and fertilizer treatments. Bulk density did not affect K, Mg and Zn contents of barley, whereas high bulk density decreased N, P and Ca contents. The Fe, Mn and Cu contents of barley were higher in compacted than in loose soil (Table 5).
Discussion
The soil NO3 −-N content and bacterial and fungal counts at 15, 30 and 45 days were influenced significantly (p<0.05) by the application of chemical fertilizers, PGPB inoculation and/or soil bulk density. Seed inoculation resulted in an increased content of soil NO3 −-N as compared to the control. Available amount of P by Bacillus M-13 and newly isolated Bacillus RC01 was higher than that of the control and the other strains tested. Bacterial inoculation did not influence soil pH. Similarly, Deubel et al. (2000) could not find a clear connection between the decrease in pH value and bacterial P mobilization. Generally, it is believed that solubilization of insoluble compound including P from rock phosphate is due to the excretion of microbial metabolites such as organic acids (Gyaneshwar et al. 1998; Carrillo et al. 2002; Rodriguez et al. 2004). However, in addition to acid production, other mechanisms can cause phosphate solubilization (Nautiyal et al. 2000).
All bacterial inoculations caused significant differences on the tested plant parameters, such as root and shoot dry weight, with respect to the control, although differences between various bacterial strains were insignificant. Interestingly, only strain Bacillus RC01 had a prolonged effect on the root length, whereas PGPB enhanced the early seedling vigour, and the root and shoot dry weights of plants inoculated with the PGPB were equal to those of plants receiving N and P fertilizers. Our data showed that a higher nutrient uptake by inoculated roots significantly improved seedling growth. This result confirmed that the PGPB inoculations may effectively increase the surface area of roots (Bashan et al. 2004) and the root weight (Bertrand et al. 2001). Similar findings were reported by Puente et al. (2004b), who showed that the separate inoculation of cactus seedling with Bacillus pumilus and Bacillus subtilis changed several plant growth parameters.
At the end of the pot experiment (7 weeks after inoculation), total P-solubilizing bacteria differed significantly between control and the PGPB-inoculated pots bearing Bacillus M-13 and RC02, the most effective viable population. In addition, all the PGPB strains affected the abundance of bacteria, fungi and PSB in soil (Table 4). It has been shown that the PSB application can increase the PSB population and the amount of plant available P in the rhizosphere soil (Sundara et al. 2002). It has been reported that the growth and metabolic activity of the PGPB population in soil declined probably due to nutrient exhaustion (Rojas et al. 2001; Welbaum et al. 2004). Rojas et al. (2001) demonstrated a synergism between N2-fixing bacteria and P-solubilizing bacterium. Bacterial and fungal counts of sterile soil were strongly affected by both fertilizer and PGPB inoculation. Indeed, the PBS strain can significantly affect the abundance of bacteria (Shishido and Chanway 1998) and fungal population in soil (Vivas et al. 2003). However, soil commonly reacts as a “biological buffer”, and thus, any change in the composition and abundance of microbial population can be temporary (Bashan 1998).
The soil microbial biomass appears to increase with both mineral fertilization and bacterial inoculation, and the microbial community structure (total culturable bacteria and fungi and PSB) changed consistently according to the treatments applied. The beneficial effect of inoculation on microbial population may be direct, due to an increased supply of available P and N, or indirect, through changes in the growth rate and metabolic activities of crop. Microbial growth in the rhizosphere may be limited by available N and P. The nutrient competition between plant and bacteria population in control pots (without bacteria and fertilizer application) probably limited both bacteria and plant growth. Nutritional deficiencies can take place at the acclimatization period of micropropacted sugarcane plants; it is possible that such deficiencies may have influenced the composition of soil microflora (Oliveira et al. 2002).
Inoculation of barley with the Bacillus RC01 and M-13 significantly increased concentration of P in plants and in soil. Also, bacteria inoculation increased the N content in soil and plant. The higher total N and P uptake of barley indicated that Bacillus RC01 and M-13 were able to fix N and solubilize P, with consequent promotion of plant growth. Increased P uptake by plants and plant growth as the result of the PSB inoculation have been already reported (Pal 1998; Rodriguez and Fraga 1999; Puente et al. 2004b). Probably, phosphate-solubilizing and N2-fixing bacteria improved the N and P nutrition of plants and stimulated of plant growth. Azospirillum brasilense and Azospirillum irakense strains stimulated overall plant growth, including root development and grain yield of spring wheat and maize, but both rhizobacteria did not change the N concentration in plants or grains (Dobbelaere et al. 2002). By contrast, plants inoculated with the PGPB generally have a higher N content than the uninoculated plants (Puente et al. 2004b).
In the case of Bacillus RC02 and RC03 inoculants, stimulation of barley growth probably occurred through the release of plant growth substances by these bacteria since an increase in the available P content of soil was not observed. The production of hormones has been suggested to be one of the mechanisms by which PGPB including Bacillus species stimulate plant growth (Amer and Utkheda 2000; Steenhoudt and Vanderleyden 2000). However, only a few experiments with the PSB have been concluded, and relative results are quite diverse, varying according to plant or bacterial species. Bacillus megaterium and Paenibacillus polymyxa were able to enhance growth and yield but not the P uptake of canola, indicating that P solubilization is not the main mechanism responsible for positive plant response (De Freitas et al. 1997).
Our results have also showed that root length and root and shoot weight of plants were reduced by soil compaction due to increased resistance to root penetration and decreased the effects of fertilizer and bacterial strains. Root growth and soil inorganic N (NH4 +-N+NO3 −-N) content generally decreased with increasing soil bulk density. The most pronounced effect of soil compaction was the reduction of root growth, and this result confirmed previous reports (Oussible et al. 1992; Groleau-Renaud et al. 1998). The limited root growth caused by soil compaction was accompanied by a decrease in the microbial population and by changes in the composition of microbial communities of rhizosphere soil. Soil compaction can also affect microbial activity (Jordan et al. 2003).
In summary, this study showed that the each isolated bacterial strains had a potential as a PGPB, fixed N2, dissolved P and increased growth of barley seedlings. Inoculation study carried out under sterile soil conditions with barley and the selected rhizobacteria indicated that all inoculant strains increased total bacteria and the PSB population, root and shoot dry weight, and N and P uptake by plants. This study also showed that root length and root and shoot weight of plants were decreased by soil compaction. The results suggest that the bacterial strains tested in this study have a potential to be formulated and used as inoculants in sustainable and organic agriculture.
References
Amer GA, Utkheda RS (2000) Development of formulations of biological agents for management of root rot of lettuce and cucumber. Can J Microbiol 46:809–816
AOAC (1990) Official methods of analysis of the Association of Official Analytical Chemists. In: Helrich K (ed) Association of Official Analytical Chemists (AOAC) Inc., vol 1, 15th edn. AOAC, Arlington, VA, USA
Badalucco L, Kuikman PJ (2001) Mineralization and immobilization in the rhizosphere. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil–plant interface. Marcel Dekker, New York, pp 159–196
Bashan Y (1998) Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv 16:729–770
Bashan Y, Holguin G (1998) Proposal for the division of plant growth-promoting rhizobacteria into two classifications: biocontrol-PGPB (plant growth-promoting bacteria) and PGPB. Soil Biol Biochem 30:1225–1228
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances. Can J Microbiol 50:521–577
Bertrand H, Nalin R, Bally R, Cleyet-Marel JC (2001) Isolation and identification of the most efficient plant growth-promoting bacteria associated with canola (Brassica napus). Biol Fertil Soils 33:152–156
Blake GR, Hartge KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis. Part 1. ASA-SSSA, Madison, WI, pp 363–375
Carrillo AE, Li CY, Bashan Y (2002) Increased acidification in the rhizosphere of cactus seedlings induced by Azospirillum brasilense. Naturwissenschaften 89:428–432
De Freitas JR, Banerjee MR, Germida JJ (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol Fertil Soils 24:358–364
Deubel A, Gransee A, Merbach W (2000) Transformation of organic rhizodepositions by rhizosphere bacteria and its influence on the availability of tertiary calcium phosphate. J Plant Nutr Soil Sci 163:387–392
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Okon Y, Vanderleyden J (2002) Effect of inoculation with wild type Azospirillum brasilense and A. irakense strains on development and nitrogen uptake of spring wheat and grain maize. Biol Fertil Soils 36:284–297
Farrell RE, Walley FL, Lukey AP, Germida JJ (1993) Manual and digital line-intercept methods for measuring root length: a comparison. Agron J 85:1233–1237
Forbes BA, Sahm DF, Weissfeld AS (2002) Bailey and Scott's diagnostic microbiology, 11th edn. Mosby, St. Louis, MO, USA
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15:353–378
Groleau-Renaud V, Plantureux S, Guckert A (1998) Influence of plant morphology on root exudation of maize subjected to mechanical impedance in hydroponic conditions. Plant Soil 201:231–239
Gyaneshwar P, Kumar GN, Parekh LJ (1998) Effect of buffering on the phosphate-solubilizing ability of microorganisms. W J Microbiol Biotechnol 14:669–673
Iyamuremye F, Dick RP (1996) Organic amendments and phosphorus sorption by soils. Adv Agron 56:139–185
Jordan D, Ponder F Jr, Hubbard VC (2003) Effects of soil compaction, forest leaf litter and nitrogen fertilizer on two oak species and microbial activity. Appl Soil Ecol 23:33–41
Kumar V, Narula N (1999) Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum. Biol Fertil Soils 28:301–305
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86:1–25
Martin JP (1950) Use of acid rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci 69:215–232
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R, Verma D (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett 182:291–296
Oliveira ALM, Urquiaga S, Döbereiner J, Baldani JI (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242:205–215
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2. ASA-SSSA, Madison, WI, pp 403–427
Oussible M, Crookston PK, Larson WE (1992) Subsurface compaction reduces the root and shoot growth and grain yield of wheat. Agron J 84:34–38
Pal SS (1998) Interactions of an acid tolerant strain of phosphate solubilizing bacteria with a few acid tolerant crops. Plant Soil 198:169–177
Pikovskaya RE (1948) Mobilization of phosphates in soil in connection with vital activities of some microbial species. Mikrobiologia 17:362–370
Puente ME, Bashan Y, Li CY, Lebsky VK (2004a) Microbial populations and activities in the rhizoplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biol 6:629–642
Puente ME, Li CY, Bashan Y (2004b) Microbial populations and activities in the rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings. Plant Biol 6:643–650
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rodriguez H, Gonzalez T, Goire I, Bashan Y (2004) Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 91:552–555
Rojas A, Holguin G, Glick BR, Bashan Y (2001) Synergism between Phyllobacterium sp. (N2-fixer) and Bacillus licheniformis (P-solubilizer), both from a semiarid mangrove rhizosphere. FEMS Microbiol Ecol 35:181–187
Şahin F, Çakmakçı R, Kantar F (2004) Sugar beet and barley yields in relation to inoculation with N2-fixing and phosphate solubilizing bacteria. Plant Soil 265:123–129
Salle AJ (1973) Laboratory manual on fundamental principles of bacteriology. McGraw-Hill, New York, USA
Shishido M, Chanway CP (1998) Forest soil community responses to plant growth-promoting rhizobacteria and spruce seedlings. Biol Fertil Soils 26:179–186
Soil Survey Staff (1999) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys, 2nd edn. USDA-SCS Agriculture Handbook 436. US Government Printing Office, Washington, DC
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Sundara B, Natarajan V, Hari K (2002) Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yield. Field Crops Res 77:43–49
Vivas A, Marulanda A, Ruiz-Lozano JM, Barea JM, Azcon R (2003) Influence of a Bacillus sp on physiological activities of two arbuscular mycorrhizal fungi and plant responses to PEG-induced drought stress. Mycorrhiza 13:249–256
Welbaum GE, Sturz, AV, Dong ZM, Nowak J (2004) Managing soil microorganisms to improve productivity of agro-ecosystems. Crit Rev Plant Sci 23:175–193
Yadav KS, Dadarwal KR (1997) Phosphate solubilization and mobilization through soil microorganisms. In: Dadarwal KR (ed) Biotechnological approaches in soil microorganisms for sustainable crop production. Scientific, Jodhpur, India, pp 293–308
Acknowledgements
This study was supported by The Turkish Academy of Sciences- TUBA- GEBIP programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canbolat, M.Y., Bilen, S., Çakmakçı, R. et al. Effect of plant growth-promoting bacteria and soil compaction on barley seedling growth, nutrient uptake, soil properties and rhizosphere microflora. Biol Fertil Soils 42, 350–357 (2006). https://doi.org/10.1007/s00374-005-0034-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0034-9