Abstract
Thermotolerant bacteria producing medium-chain-length polyhydroxyalkanoate (mcl-PHA) were isolated from various thermal sites, including palm oil mill effluent, textile wastewater, and hot spring water, in Thailand. Fifteen strains were isolated at 45 °C using nutrient-rich (NR) medium. However, only six isolates produced mcl-PHA at 0.41 ± 0.01 g/L to 0.80 ± 0.01 g/L, representing a mcl-PHA content of 29.44% to 50.77% of the dry cell weight (DCW). The six strains of bacterial isolates could utilise a variety of substrates; all were identified as Bacillus thermoamylovorans. The highest mcl-PHA content (50.77% of the DCW) was accumulated by the B. thermoamylovorans strain PHA005 isolated from palm oil mill effluent. The mcl-PHA from strain PHA005 was composed of five different monomers, 3-hydroxyoctanoate (3HO), 3-hydroxydecanoate (3HD), 3-hydroxytetradecanoate (3HTD), 3-hydroxyhexadecanoic acid (3HHD), and 3-hydroxyoctadecanoic (3HOD), with a monomer content of 24.12, 15.50, 13.00, 39.25, and 8.13 mol%, respectively. The optimum temperature for B. thermoamylovorans strain PHA005 growth is 45 °C, and it can survive at up to 60 °C. This is a first report of PHA synthesis by a thermotolerant B. thermoamylovorans. Moreover, the high content of 3HHD monomers (39.25 mol%) has never been reported in Bacillus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are bio-polyesters synthesized by bacteria as intracellular storage reserves of carbon and energy. PHAs have attracted great interest due to the similarities between their material properties and the material properties of thermoplastics and elastomers, as well as their origination from renewable resources and their complete biodegradability [1]. PHA can be divided into two groups, (i) short-chain-length PHA (scl-PHA) and (ii) medium-chain-length PHA (mcl-PHA), with 3–5 and 6–14 carbon atoms in their monomeric units, respectively [2]. The mcl-PHA and its copolymers are more suitable for a wide range of applications than scl-PHA due to their structural diversity, which allows for the tailoring of their physical and mechanical properties [3]. However, the production of PHA on an industrial scale is still a major concern due to the higher production cost compared to conventional plastic. The high production cost of PHA comes from (i) the cost of the substrate and (ii) the running cost of maintaining a temperature suitable for microbial growth. Therefore, the screening of novel PHA-producing bacteria, which can be utilised as low-cost substrates and produce PHA under high temperature of fermentation (> 40 °C), is a major challenge for the economical production of PHA [4, 5]. Satoh et al. [2] reported that the thermotolerant Pseudomonas sp. strain SG4502, isolated from biodiesel fuel (BDF) by-product, is able to accumulate mcl-PHA at a 45 °C cultivation temperature. The maximum amount of mcl-PHA (40.6% of the dry cell weight [DCW]), with 0–8 mol% of 3-hydroxybutyrate (3HB), 5–7 mol% of 3-hydroxyhexanoate (3HHx), 1–25 mol% of 3–hydroxyoctanoate (3HO), 8–59 mol% of 3-hydroxydecanoate (3HD), and 6–8 mol% of 3-hydroxydodecanoate (3HDD), was observed in a medium containing BDF as the sole substrate. Pantazaki et al. [6] found that when using sodium gluconate or sodium octanoate as the substrate, Thermus thermophilus, a thermophilic bacterium, produced PHA at 35–40% of the DCW. The monomer composition of mcl-PHA was mainly 3HD (64 mol%).
A rapid, simple, and reliable screening method for PHA-producing bacteria is key for a successful fermentation process. Many screening methods to detect microorganisms that accumulate PHA have been developed using PHA-detecting dyes, such as Nile red, Nile blue A, and Sudan black [7,8,9]. Moreover, a number of methods are available for screening, such as spectrophotometric analysis, Fourier transform infrared (FTIR) spectroscopy, high-performance liquid chromatography (HPLC), flow-cytometry and spectrofluorometry, gas chromatography-mass spectrometry (GC–MS), nuclear magnetic resonance (NMR), molecular weight determination, and thermal analysis of the extracted polymer [10]. Therefore, the goal of this study was to isolate thermotolerant bacteria capable of producing mcl-PHA under high temperatures (> 40 °C). Two screening methods, inducing Nile red fluorescence and spectrophotometry, were also applied, and their screening accuracy was calculated compared to gas chromatography (GC) analysis as a control method. The biochemical characteristics of PHA-producing bacteria were also investigated to determine their ability to consume a wide range of substrates. The superior isolates were selected and identified for their potential to produce mcl-PHA in a medium containing sodium octanoate as a carbon source. Moreover, the monomer composition of mcl-PHA was analysed by GC–MS.
Materials and Methods
Collection and Characterisation of Samples
High-temperature sediment and water samples were collected from three sites included palm oil mill effluent (Univanich Palm Oil Public Company Limited, Krabi, Thailand), hot spring water (Khaochaison Hot Spring, Phatthalung, Thailand), and textile wastewater (Ban Phreak weaving group, Phatthalung, Thailand). Water (50 mL) was collected at the middle of the pond using sterile bottles, and sediment (50 g) was collected at the edge of the pond by digging 5–10 cm deep into the land shore at different sites using sterile spoons. All samples were kept in 100 mL sterile bottles, transported to the biochemical laboratory (Thaksin University, Phatthalung) on ice, and stored at − 20 °C until use [11]. The water and sediment were subjected to pH determination using a pH meter, temperature determination using a thermometer, and chemical oxygen demand (COD) and total solid (TS) determination using the following standard method.
The COD value was measured by the closed reflux method using a mixture of potassium dichromate as a strong chemical oxidant. The COD was determined by a method of colorimetric determination using a HACH DR3900 spectrophotometer, and the results are displayed in mg/L. The dilution of the sample can be made if necessary in order to obtain the COD in the range; however, the content should be multiply by dilution multiple before the report. The TS content was determined by a gravimetric method at a temperature of 103–105 °C. Clean crucibles were weighed using an analytical balance. The crucible dish was heated at 103 °C in an oven for 1 h, allowed to cool in a desiccator, and then reweighed. This is recognised as the initial weight (B). Then, 50 mL of each sample was added to the weighed crucibles and evaporated using a water bath for 24 h. Afterward, the residual samples remaining in the crucibles were dried in an oven at 103 °C for 2 h and then cooled in a desiccator and dry weight determination. This was recorded as the final weight (A). The TS concentration was calculated using the following Eq. (1):
Culture Media and Screening of Thermotolerant Bacteria
Mineral salt (MS) medium and nutrient-rich (NR) medium were used as the screening and cultivating medium, respectively. The MS medium contained 9.0 g/L of Na2HPO4⋅12H2O, 1.5 g/L of KH2PO4, 0.1 g/L of NH4Cl, and 0.2 g/L of MgSO4⋅7H2O, and the NR medium contained 5.0 g/L of peptone and 3.0 g/L of yeast extract. For the agar medium, 1.5% agar was added. Moreover, the NR medium containing 3.32 g/L of sodium octanoate was utilised as the mcl-PHA-producing medium [2].
One millilitre of water and 0.5 g of sediment from each site were first inoculated into 50 mL of MS medium and incubated at 45 °C with agitation at 150 rpm for 48 h. Afterward, 100 μL of each sample was serial diluted, spread in a NR agar plate and incubated at 45 °C for 48 h. All of the cream- or yellow-pigmented colony was selected and streaked onto new NR plates. Streak plating was repeated until a single colony was obtained. Then, a single colony was transferred into a 5 mL NR medium and incubated 45 °C, 150 rpm for 48 h. Thereafter, a culture was sequentially transferred to 25 mL and 125 mL of NR medium. The culture was cultivated at 45 °C, 150 rpm for 48 h for each step. After 48 h, each culture was collected and studied for the ability of the isolated strain to produce PHA. All isolated bacteria were kept frozen in 10% glycerol in Eppendorf tube and stored in refrigerator at − 20 °C. The culture were recultured on NR medium every month [12].
Isolation of Polyhydroxyalkanoate (PHA)-Producing Bacteria from Isolated Thermotolerant Culture
The isolated strains were tested for their ability to produce mcl-PHA. Two screening methods, including Nile red fluorescence and UV–visible spectrophotometric methods, were used to detect the accumulation of PHA. Moreover, the screening accuracy using Nile red and spectrophotometric method was calculated and compared with GC analysis as a control method. For the PHA screening procedures use in this current study were described as below.
The Nile red fluorescence method was used following Spiekermann et al. [7]. First, 1 mL of the suspension was centrifuged at 12,000×g for 5 min; only the pellet was collected and resuspended in 1 mL of distilled water. Afterward, 40 μL of dimethyl sulphoxide (DMSO) containing 80 μg/mL Nile red was added. Therefore, a final concentration of 3.1 μg Nile red per millilitre of suspension was obtained. The sample was incubated at room temperature (30 °C) for 30 min. Afterward, the suspension was centrifuged at 12,000×g for 5 min, and the pellet was collected. The pellet was resuspended in 1 mL of distilled water, and the suspension was vortexed. The suspension was transferred to a 96-well microplate, and the existing of PHA was detected at excitation and emission wavelengths of 535 nm and 605 nm, respectively [13]. A known concentration of PHA was utilised as a standard to generate a calibration curve. The calibration curve was used to estimate the accumulated PHA, both as a concentration (g/L) and as content (%DCW), following Eqs. (2) and (3), as follows:
where Kc is the calibration constant, IPHA is the fluorescence intensity of the stained suspension, Ccell is the cell concentration (mg/mL), and DCW is the dry cell weight (mg/mL).
The UV–visible spectrophotometric method was evaluated following Mojaveryazdia et al. [14]. One millilitre of cell suspension was centrifuged at 12,000×g for 5 min, and the pellet was collected and resuspended in 1 mL of distilled water. Afterwards, 2 mL of concentrated H2SO4 was added. The sample was mixed and hydrolysed in a water bath at 100 °C for 20 min to convert PHA in the pellet into crotonic acid. PHA was determined with a UV–visible spectrophotometer at 235 nm. H2SO4 and a known concentration of PHA were used as the blank and standard, respectively.
The production of PHA from all isolated were determined using both Nile red fluorescence and spectrophotometric methods. The PHA production determined from both method were compared and the accuracy of each method was compared with the data analysed by GC as a control method. The accuracy was calculated by subtracting each PHA value from the PHA production obtained by GC method.
Determination of Medium-Chain-Length Polyhydroxyalkanoate (mcl-PHA) Content and Composition by Gas Chromatography–Mass Spectroscopy (GC–MS) Analysis
GC analysis was used to determine the PHA content in a cell-containing polymer or purified PHA. First, 5–8 mg of lyophilised cells or 1–2 mg of purified PHA was methanolised by adding the sample into a mixture of chloroform and methanol containing 15% (v/v) H2SO4. THE PHA was converted into hydroxyacyl methyl ester and subjected to GC–MS analysis [15, 16]. GC analysis was performed on a Hewlett Packard GC-6890 system equipped with a mass spectrometer 5973 and HP-INNOWax capillary column (length, 30 m; internal diameter, 0.25 mm; film thickness, 0.25 μm). The injection was done in splitless mode with Tinjector at 230 °C. The temperature program used was as follows: 40 °C to 240 °C at 20 °C/min, 240 °C for 10 min. Helium was used as carrier gas (3 mL/min). The MS was operated in scanning mode between 40 and 360 m/z. The identification of compounds was performed by comparison of mass spectra with standard ones. The MS data was searched in the NIST database to determine the monomer structure and the corresponding monomer content was calculated from the peak area of the GC spectrum. The isolated strain that was able to produce mcl-PHA was selected and characterised the 16S rRNA and biochemical properties.
16S Ribosomal RNA Sequences
Six pure bacterial cultures able to produce mcl-PHA were obtained. The nucleotide sequence of the 16S rRNA gene of isolated bacteria was amplified by polymerase chain reaction (PCR) employing DNA polymerase. The universal primers I-179L (5′-ACAGATCAAGTTCTACATCTTCGAC-3′) and I-179R (5′-GGTGTTGTCGTTCCAGTAGAGGATGTC-3′) were used. The reaction mixture contained 10 μL of template DNA, 5 μL of reaction buffer, 2 μL of Taq DNA polymerase (3 U/μL), 5 μL of the four deoxynucleotide triphosphates (dNTPs) (200 μM each) (Amersham Biosciences, USA), 4 μL of MgCl2, and 2 μL of universal primer (20 μM); the final volume 50 μL. The thermal PCR profile was operated according to Porwal et al. [17], as follows: initial denaturation at 94 °C for 1 min and primer annealing at 55 °C for 1.30 min. The elongation step was extended at 72 °C for 10 min. The dideoxy chain termination method was used for DNA sequencing. The sequence was analysed and aligned using the National Center for Biotechnology Information (NCBI) BLAST tool. The 16S rRNA gene partial sequences were deposited in the NCBI database under accession numbers MK622835, MK622836, MK622837, MK622838, MK622840, and MK622843.
Biochemical Characterisation
Six bacterial isolates were also tested for biochemical characterisation with KB009 and KB003 Hi Carbohydrate™ kits (Himedia). The test kit containing 33 different carbohydrates as the carbon source, 5 proteins as the nitrogen source, and 7 enzyme activity were tested and used as described by the manufacturer [11].
Cloning and Sequencing of PHA Synthase Gene of B. thermoamylovorans
PHA synthase gene (phaC) was analysed following Yang et al. [18] with a modification. The polymerase chain reaction (PCR) targeted at a part of the PHA synthase gene (phaC) was performed with the forward primer CF1: 5′-ATCAACAARTWCTACRTCYTSGACCT-3′ and the reverse primer CR4: 5′-AGGTAGTTGTYGACSMMRTAGKTCCA-3′using a PCR mixture consisting of 0.2 mM of deoxynucleotide (dNTP), 2.5 units of DNA polymerase (Fermentas), 0.03% of DMSO, and 2.5 μM of each primer. The amount of DNA used as a template was 50 ng in 100 μL of PCR mixture. The PCR mixture was pre-incubated at 94 °C for 10 min, 51 °C for 2 min, and 72 °C for 2 min. The PCR cycle consisted of 20 s of denaturation at 94 °C, 45 s of annealing at 57 °C, and 1 min of extension at 72 °C. This cycle was repeated 35 times and then incubated at 72 °C for 10 min for the final extension [19]. The PCR products were fractionated on 1% agarose gel, and DNA from appropriate band was recovered using a DNA gel purification kit (Sigma-Aldrich, USA). These fragments were subcloned into the pGEM-T Easy plasmid (Promega) and subsequently subcloned into the vector pBBR1MCS-5. Possitive colonies were screened by colony PCR using M13 forward and reverse primers (ThermoFisher, USA). Plasmid DNA was purified from overnight bacterial cultures and sequenced by an automatic DNA sequencer. A similarity search of nucleotide sequences was performed using the BLASTN non-redundant database. The nucleotide sequences of the phaC genes have been submitted to the GenBank database. The phaC partial sequences were deposited in the GenBank database.
Statistical Analysis
All experiments were performed in triplicate, and the data is expressed as mean values ± SD. One-way analysis of variance (ANOVA) at a 95% confidence interval was performed on data. The statistical package was installed directly in Excel through the Add-In function of Microsoft Word 2010.
Results and Discussion
Isolation of Thermotolerant Bacterial Strains
Water and sediment from thermal sites, including palm oil mill effluent, hot spring water, and textile wastewater, were collected and screened for thermotolerant bacteria. The partial characteristics of the samples, such as temperature, pH, TS, and COD, were determined (Table 1). The samples were collected in different (from palm oil mill effluent, hot spring water and textile wastewater) and represent moderate thermophilic to thermophilic (26–85 °C) and acidic to alkalophilic (pH 4.7–9.4) environments, with variable COD and TS at 42 to 55,000 mg/L and 6 to 40,000 mg/L, respectively. Fifteen bacterial strains were isolated using NR medium at 45 °C. The textile wastewater yielded the highest number of bacterial isolates, about 54% of the total isolates, followed by the palm oil mill effluent (46%). However, no isolates were obtained from hot springs. The number of isolates in this study may be correlated to the environmental conditions, such as temperature, pH, COD, and the nutritional status available. The ecological environment established in textile wastewater was suitable for microorganisms, while the hot springs was long-known to be moderate to high temperature and nutrient poor. All bacterial isolates were designated as strains PHA001–015 (Supplementary Table S1); all isolates grew well at 45 °C. Morphological characterisation revealed that the colonies were small, white and cream, and lens-shaped. The cells were 0.45–0.50 mm wide and 3.0–4.0 mm long. Based on Gram staining, isolates can be divided into Gram-positive and Gram-negative. Sixty-seven percent of the total isolates showed a Gram-positive character, while the other strains were Gram-negative, as shown in Supplementary Table S1.
All isolated strain were transferred to NR medium and cultured at 45 °C for 48 h. Thereafter, bacterial cells were harvested and subjected to Nile red fluorescence and UV–visible spectrophotometric methods to detect the accumulation of PHA. GC was also used to confirm the accumulation of PHA in microbial cells. The cell-produced PHA was selected and used throughout this study.
Screening of PHA-Producing Bacteria from 15 Isolated Strains
To investigate the PHA content of the 15 isolated strains, they were cultivated on NR medium supplemented with sodium octanoate (3.32 g/L) as a carbon source. Nile red fluorescence and spectrophotometric analysis was first used to identify and quantify the PHA produced by the isolated bacteria. Only 6 (PHA001, PHA003, PHA005, PHA007, PHA009, and PHA012) of the 15 strains showed the ability to produce PHA, as detected by the GC method (Supplementary Table S1). Thereafter, the efficiency of PHA-screening methods, including Nile red fluorescence and spectrophotometric methods, was also determined. Nile red fluorescence showed that the highest PHA content was observed in strain PHA005 (48.45% of the DCW), followed by PHA007 (42.88% of the DCW) and PHA003 (21.23% of the DCW). However, using spectrophotometric methods, the highest PHA was PHA003 (58.11% of the DCW), followed by PHA012 (42.05% of the DCW) and PHA009 (24.29% of the DCW (Table 2). Notably, the PHA content determined by Nile red fluorescence and spectrophotometric methods was different. To test the accuracy of the methods, the PHA content from GC analysis was utilised as a control value. The results showed that the Nile red fluorescence gave a smaller difference in PHA value when compared to GC analysis (Table 2). Only a 1.56–8.35% difference was observed. However, a large difference was observed from the spectrophotometric methods (7.01–2.67% difference). It can be clearly seen that the Nile red fluorescence method is relevant to the investigated GC analysis method. It can be applied as a rapid and reliable method for PHA screening.
Normally, GC analysis is the most well-known method for PHA analysis due to the automated sample analysis, accuracy of PHA quantification, high separation power, detection sensitivity, and GC method enabled both quantitative and qualitative analysis of PHA [20]. However, it still has limitations in that it requires many sample preparation steps, which can take up to 24 h [21]. Moreover, this method is not environmentally friendly due to the production of hazardous waste (solvents and acids). Therefore, the Nile red fluorescence method can be used as a front-line method for the screening and identification of novel microbes with PHA production potential. The advantages of this method include the ease of sample preparation, low cost, use less labour, and short analysis time [22,23,24]. Moreover, the Nile red fluorescence method can be done using wet cells, leading to rapid PHA monitoring. The poor accuracy of the spectrophotometric method in this study may be due to the amount of PHA present. It has been reported that the spectrophotometric method is suitable for samples containing 0.001 to 20 mg of PHA [25]. This method is only applicable to samples with a relatively large quantity of polyesters [26]. Moreover, the presence of endogenous components in the cell can interfere with the result, and matrix interference can result in overestimation of PHA content [20, 21, 23]. The determination of PHA content and composition is the most important issue because it has multiple effects on the PHA yield and recovery efficiency [27]. The research and development of a novel, simple, cheap, and rapid method of PHA detection and quantification for the efficient economic production of PHA is still a challenge. From the results, all methods clearly indicated that only six isolates (including PHA001, PHA003, PHA005, PHA007, PHA009, and PHA012) were able to produce PHA. Therefore, six PHA-producing bacteria were subjected to further analysis.
16S Ribosomal RNA Sequences and Biochemical Characterisation
The 16S ribosomal RNA sequences of six PHA-producing bacteria were determined and compared with the databank contents using Nucleotide BLAST. Using the BLAST program, the 16S rRNA sequence from 6 isolated (PHA001, PHA003, PHA005, PHA007, PHA009 and PHA012) was determined and aligned with the bacterial sequence available in GenBank. A 361 bp of 16S rRNA sequence was obtained from PCR using universal primer (I-179R and I-179L). The result revealed that all isolates belonged to the Firmicutes (Bacillus sp.) and that the closest phylogenetic neighbour of the strains is Bacillus thermoamylovorans, with a 99% identity. Moreover, a phylogenetic tree was constructed based on the 16S rRNA sequence alignment. The result showed that the six strains and B. thermoamylovorans are clustered and are relatively distant from the other Bacillus sp. (Fig. 1). It is not a surprise that all isolates are Bacillus; this may due to the ability of this genus to resist and adapt to extreme environmental condition [28]. Sangkharak and Prasertsan [11] isolated 50 strains of bacteria from palm oil mill effluent (POME), fermented food, and dung under various selective conditions. The results showed that all dominant species belonged to the Bacillus genus.
Phylogenetic tree of six strains and their related microorganisms. The 16S rRNA gene sequence of the six strains was aligned using Molecular Evolutionary Genetics Analysis (MEGA X) software version X. The additional strains of the species B. thermoamylovorans were chosen for comparison. A phylogenetic tree was constructed using the neighbour-joining method. The numbers on the tree indicate the percentages of bootstrap sampling, derived from 1000 replications
Moreover, using KB009 and KB003 Hi Carbohydrate™ kits, six isolates were subjected to biochemical characterisation to determine their ability to utilise different carbon and nitrogen sources (Table 3). The results showed that all isolates had similar phenotypic patterns. Six isolates could utilise various tested compounds, including lactose, xylose, maltose, fructose, dextrose, galactose, trehalose, sucrose, L-arabinose, mannose, sodium gluconate, salicin, d-glucoside, rhamnose, cellobiose, melezitose, and esculin. However, they were not able to use raffinose, melibiose, inulin, glycerol, dulcitol, sorbitol, mannitol, adonitol, arabitol, erythritol, d-mannoside, xylitol, o-Nitrophenyl-β- d-galactopyranoside (ONPG), d-arabinose, malonate, or sorbose. From an economic point of view, all isolates had an interesting profile due to their ability to use a wide range of substrates. Therefore, the strains may possible to utilise the substrate and convert into PHA with a novel PHA monomers. Notably, the isolates can utilise cheap substrates, such as xylose, cellobiose, and glycerol. Xylose and cellobiose are the most abundant sugars in the plant cell wall, whereas glycerol is a main by-product in biodiesel plants. Therefore, the isolated bacteria show great potential for producing PHA in a low-cost substrate, which can reduce PHA production costs because the cost of the substrate is the most important factor for PHA production. Due to the ability to use a wide range of substrates, B. thermoamylovorans may be more advantageous than a commercial PHA producer (Ralstonia eutropha or Pseudomonas putida) since it can use more diversified substrates and applications.
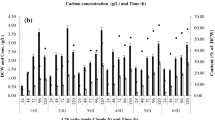
All strains were determined for their ability to grow at 37–60 °C. The highest growth (0.80 ± 0.01 g/L) and PHA production (50.77% of the DCW) was observed in B. thermoamylovorans strain PHA005 at 45 °C (Fig. 2). A similar pattern was observed in all tested strains. Six isolates could grow at an optimum temperature of 45 °C able to survive up to a cultivation temperature of 50 °C and no growth was observed at 60 °C; hence, it is considered a moderately thermophilic bacterium. The result was in agreement with Combet-Blanc et al. [29], who found that the upper temperature limit for B. thermoamylovorans growth is 60 °C. Thermotolerant bacteria are useful in industrial applications using a fermentation process. A seriously increasing of temperature in the recent year make a serious problem to fermentation process since high cost of cooling system was paid for sustaining the optimum temperature. Therefore, the production of PHA by thermotolerant bacteria is of interest due to the economic and thermal advantages [30]. Moreover, cultivation of bacteria at > 45 °C decreases the contamination risk of other microorganisms because only a few microorganisms can grow under these temperatures [6].
Cloning and Sequencing of PHA Synthase Gene of B. thermoamylovorans
PHA synthase gene of B. thermoamylovorans was also isolated and characterized. Dieffenbach and Dveksler [31] have been reported that PHA synthase genes possess high G+C contents. Therefore, DNA templates with a high G+C content usually hamper PCR amplification; the reagents formamide, glycerol, DMSO and betaine are often used as PCR additives to improve the PCR amplification of GC-rich DNA sequences [18]. In this current study, DMSO was added to the PCR reaction mixture. Approximately, 400 bp of PhaC gene was obtained from PCR using CF1 and CR4 primer. The size of PhaC was similar with the PhaC from Yang et al. [18] and Sheu et al. [19]. The PhaC from B. thermoamylovorans was compared with other Bacillus sp. phaC from B. thermoamylovorans showed similarities with other Bacillus sp. up to 91% (Table 4). The data suggested that Class I PHA synthase is the dominant type of PHA synthase in B. thermoamylovorans. However, there are some PCR clones were closer to the Class II PHA synthase (data not show). PHA synthases have been categorized into four major classes based on their primary sequences, substrate specificity, and subunit composition [32]. Class I comprises enzymes consisting of only one type of PhaC, which forms a homodimer, while Class II contains two types of synthases, PhaC1 and PhaC2. Class III and IV synthases form heterodimers, comprising PhaC-PhaE and PhaC-PhaR, respectively. Class I, III and IV synthases tend to favour short-chain-length (SCL) monomers comprising C3-C5 carbon chain lengths. A typical example of a C4 SCL monomer is (R)-3-hydroxybutyrate (3HB), and PhaC polymerizes the acyl moieties of 3-hydroxybutyryl-coenzyme A (3HB-CoA) to the high molecular weight PHA product poly-hydroxybutyrate (PHB). Class II synthases favour medium-chain-length (MCL) monomers comprising C6-C14 carbon chain lengths, such as the C6 monomer 3-hydroxyhexanoate (3HHx) [33].
Production of PHA by Thermotolerant Isolated Bacteria Using Nutrient-Rich (NR) Medium Supplemented with Sodium Octanoate
The production of PHA was observed in six isolated bacteria. Most of the isolates produced PHA during the log phase (48 h) in NR medium supplemented with 3.32 g/L sodium octanoate (Fig. 2). PHA production accumulated in B. thermoamylovorans varied from 29.44% of the DCW by PHA003 to 50.77% of the DCW by strain PHA005. The B. thermoamylovorans strain PHA005 gave the highest values for mcl-PHA production (50.77% of the DCW and 0.8 ± 0.01 g/L). GC analysis was used to determine the composition of PHA. In this current study, five remarkable monomer was accumulated by B. thermoamylovorans PHA005 in medium supplemented with sodium octanoate: 3HO, 3HD, 3-hydroxytetradecanoate (3HTD), 3-hydroxyhexadecanoic acid (3HHD), and 3-hydroxyoctadecanoic acid (3HOD) at 24.12, 15.50, 13.00, 39.25 and 8.13 mol%, respectively. Therefore, this indicated that PHA from B. thermoamylovorans PHA005 was mcl-PHA since it was composed of monomer units with 8–18 carbon atoms. Shahid et al. [34] reported that the B. megaterium strain DSM 509 produces mcl-PHA consisting of 3HB, 3-hydroxyvalerate (3HV), 3HO, 3HD, 3HHD, and 3HTD using octanoic acid as the sole substrate. In this study, the production of mcl-PHA by B. thermoamylovorans PHA005 with a 3HHD monomer proportion as high as 39.25% and using sodium octanoate as a carbon source was reported for the first time. 3HHD was first discovered by Singh and Mallick [35] in P. aeruginosa MTCC7925 when grown in palm oil cake, but only at 14.30 mol%. Gumel et al. [36] and Munawar et al. [37] reported 6.3 mol% 3HHD in P. putida Bet001 grown in palmitic acid and palm kernel oil, respectively. Singh et al. [38] reported 2.70 mol% 3HHD in P. aeruginosa MTCC7925 when grown in jatropha oil. Therefore, the current study is the first report of B. thermoamylovorans PHA005 producing mcl-PHA with a 3HHD monomer proportion as high as 39.25% using sodium octanoate as a carbon source. Bacillus species have not been reported to accumulate the mcl-PHA monomer 3HHD in the production medium. The HHD monomer of mcl-PHA is of special interest due to its good properties. Singh et al. [38] reported that the 3HHD monomer is elastomeric, biodegradable, and biocompatible, with low crystallinity and high elongation to break. Therefore, it shows great potential in a wide range of applications, especially biomedical applications where flexible biocompatible biomaterials are required.
The production of mcl-PHA from B. thermoamylovorans strain PHA005 cultivated in NR medium containing 3.32 g/L of octanoate at pH 7 and 45 °C was also compared with other research (Table 5). Polyhydroxybutyrate and scl-PHA were observed in B. megaterium, B. cereus, B. thuringiensis, and B. mycoides when cultivated in a medium containing glucose, decanoate, glycerol, fructose, sucrose, decanoic, and acetate as the sole substrate [22, 39,40,41,42]. Sangkharak and Prasertsan [11] reported that the accumulation of poly (hydroxybutyrate-co-hydroxyvalerte) [P(HB-co-HV)], a copolymer of scl-PHA, was observed by B. cereus PHA008 when cultivated in a medium supplemented with POME. P. thermotolerans SG4502 and T. thermophiles HB8 produced a smaller amount of mcl-PHA (28.0 to 40.6% of the DCW) at 45 °C [2, 43] when compared to mcl-PHA from B. thermoamylovorans PHA-005 (50.77% of the DCW) (Table 5). Moreover, a different monomer composition was also observed. 3HB, 3HH, 3HO, and 3HD monomers, the high 3HO monomers (82.5 mol%) were accumulated by P. thermotolerans SG4502 using the BDF by-product as a substrate [2]. In addition, T. thermophiles HB8 was able to produce mcl-PHA with a high proportion of 3HD monomers (35.0% of the DCW and 64.0 mol%), with sodium gluconate as a carbon source [42]. The production of PHA and the monomer composition may depend on the bacterial strain and the substrate. This study successfully isolated a thermotolerant bacterium producing a satisfactory amount of mcl-PHA (50.77% of the DCW) when grown in octanoate. Moreover, a high proportion of 3HHD monomers (39.25 mol%) from B. thermoamylovorans PHA005 has never been reported before. B. thermoamylovorans PHA005 is very interesting for industrial applications due to its thermotolerant characteristics and its ability to produce mcl-PHA.
Conclusions
Fifteen strains of thermotolerant mcl-PHA-producing microorganisms were isolated from different environmental sources, with the temperature of each site from 26–85 °C. However, only six strains of bacteria produced PHA at a concentration of 0.41 ± 0.01 to 0.80 ± 0.01 g/L (corresponding to a PHA content of 29.44 to 50.77% of the DCW). Of the six naturally promising strains, PHA005 was found to be the most efficient mcl-PHA producer. Bacterial strain PHA005 produced a high amount of mcl-PHA, with 50.77% of the DCW (0.80 ± 0.01 g/L) with 24.12, 15.50, 13.00, 39.25, and 8.13 mol% of 3HO, 3HD, 3HTD, 3HHD, and 3HOD unit fractions, respectively. The results of the 16S rRNA analysis indicated that all isolated PHA-producers were Gram-positive, belonging to the Firmicutes (Bacillus sp.). The 16S rRNA gene sequence analysis revealed that strain PHA005 was phylogenetically affiliated with the species B. thermoamylovorans. The bacterium is a facultative anaerobe that can grow and produce PHA at an optimum temperature of 45 °C. B. thermoamylovorans PHA005 is a novel thermotolerant bacterium producing mcl-PHA. The production of mcl-PHA by B. thermoamylovorans PHA005 with a 3HHD monomer proportion as high as 39.25% using sodium octanoate as a carbon source has never been reported before. The optimisation and characterisation of PHA, especially mcl-PHA, from B. thermoamylovorans strain PHA005 under various substrates and environmental conditions will be presented in a future study.
References
Numata K, Abe H (2009) Biodegradability of poly (hydroxyalkanoate) materials. Materials 2:1104–1126. https://doi.org/10.3390/ma2031104
Satoh Y, Tajima K, Nakamoto S, Xuerong H, Matsushima T, Ohshima T, Kawano S, Erata T, Dairi T, Munekata M (2011) Isolation of a thermotolerant bacterium producing PHAs-polyhydroxyalkanoate. J Appl Microbiol 1:1364–5072. https://doi.org/10.1111/j.1365-2672.2011.05093.x
Rai R, Yunos DM, Boccaccini AR, Knowles JC, Barker IA, Howdle SM, Tredwell GD, Keshavarz T (2011) Poly-3-hydroxyoctanoate P (3HO), a medium chain length polyhydroxyalkanoate homopolymer from Pseudomonas mendocina. Biomacromol 12:2126–2136. https://doi.org/10.1021/bm2001999
Oshima T, Imahori K (1974) Description of thermos thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int J Syst Evol Microbiol 24(1):102–112. https://doi.org/10.1099/00207713-24-1-102
Fischer CR, Klein-Marcuschamer D, Stephanopoulos G (2008) Selection and optimization of microbial hosts for biofuels production. Metab Eng 10:295–304. https://doi.org/10.1016/j.ymben.2008.06.009
Pantazaki AA, Tambaka MG, Langlois V, Guerin P, Kyriakidis DA (2003) Polyhydroxyalkanoate (PHA) biosynthesis in Thermus thermophilus: purification and biochemical properties of PHA synthase. Mol Cell Biochem 254:173–183. https://doi.org/10.1023/A:1027373100955
Spiekermann P, Rehm BH, Kalscheuer R, Baumeister D, Steinbüchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol 171:73–80. https://doi.org/10.1007/s002030050681
Ostle AG, Holt JG (1982) Nile blue-a as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol 44(1):238–241
Steinbuchel A, Frund C, Jendrossek D, Schlegel HG (1987) Isolation of mutants of alcaligenes-eutrophus unable derepress the fermentative alcohol-dehydrogenase. Arch Microbiol 148:178–186. https://doi.org/10.1007/BF00414809
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megateriumstrain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 156:201–207. https://doi.org/10.1078/0944-5013-00104
Sangkharak K, Prasertsan P (2012) Screening and identification of polyhydroxyalkanoates producing bacteria and biochemical characterization of their possible application. J Gen Appl Microbil 58:173–182. https://doi.org/10.2323/jgam.58.173
Higuchi-Takeuchi M, Morisaki NK (2016) A Screening method for the isolation of polyhydroxyalkanoate-producing purple non-sulfur photosynthetic bacteria from natural seawater. Front Microbiol 7:1509. https://doi.org/10.3389/fmicb.2016.01509
Zuriani R, Vigneswari S, Azizan MNM, Majid MIA, Amirul AA (2013) A High throughput Nile red fluorescence method for rapid quantification of intracellular bacterial polyhydroxyalkanoates. Biotechnol Bioprocess Eng 18:472–478. https://doi.org/10.1007/s12257-012-0607-z
Mojaveryazdia FS, Muhamada II, Rezaniab S, Benhamc H (2014) Importance of glucose and Pseudomonas in producing degradable plastics. Teknologi 69:7–10. https://doi.org/10.11113/jt.v69.3194
Brandl H, Gross RA, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly (β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol 54:1977–1982
Timm A, Byrom D, Steinbüchel A (1990) Formation of blends of various poly(3-hydroxyalkanoic acids) by a recombinant strain of Pseudomonas oleovorans. Appl Microbiol Biotechnol 33(3):296–301. https://doi.org/10.1007/BF00164525
Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC (2008) Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol 99:5444–5451. https://doi.org/10.1016/j.biortech.2007.11.011
Yang C, Zhang W, Liu R, Zhang C, Gong T, Li Q, Wang S, Song C (2013) Analysis of polyhydroxyalkanoate (PHA) synthase gene and PHA-producing bacteria in activated sludge that produces PHA containing 3-hydroxydodecanoate. FEMS Microbiol Lett 346:56–64. https://doi.org/10.1111/1574-6968.12201
Sheu DS, Wang YI, Lee CY (2000) Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiology 146:2019–2025. https://doi.org/10.1099/00221287-146-8-2019
Amy Tan GY, Chen CL, Li L (2014) Start a research on biopolymer polyhydroxyalkanoate (PHA): a review. Polymers 6(3):706–754. https://doi.org/10.3390/polym6030706
Karr DB, Waters JK, Emerich DW (1983) Analysis of poly-β-hydroxybutyrate in Rhizobium japonicum bacteroids by ion-exclusion high-pressure liquid chromatography and UV detection. Appl Environ Microbiol 46(6):1339–1344
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47. https://doi.org/10.1016/j.mimet.2009.01.001
Guillermo RC, Bridget KL, Bruce P, David LK (2005) Emulsan quantitation by Nile red quenching fluorescence assay. Appl Microbiol Biotechnol 67:767–770. https://doi.org/10.1007/s00253-004-1849-2
Huang GH, Gu C, Feng C (2009) Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass Bioenergy 33:1386–1392. https://doi.org/10.1016/j.biombioe.2009.05.022
Sulol P, Hudecovg D, Propperov A (1996) Rapid and simple analysis of poly-β-hydroxybutyrate content by capillary isotachophoresis. Biotechnol Tech 10:413–418. https://doi.org/10.1007/BF00174225
Patil DY (2016) Methods for identification, quantification and characterization of polyhydroxyalkanoates a review. Int J Bioassays 5:4977–4983. https://doi.org/10.21746/ijbio.2016.04.005
Godbole S (2016) Methods for identification, quantification and characterization of polyhydroxyalkanoates. Int J Bioassays. https://doi.org/10.21746/ijbio.2016.04.005
Aanniz T, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M, Amar M (2015) Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Braz J Microbiol 46(2):443–453. https://doi.org/10.1590/S1517-838246220140219
Combet-Blanc Y, Olivier G, Steicher C, Patel BKC, Dwivedi PP, Pot B (1995) Bacillus thermoamylovorans sp. Nov., a moderately thermophilic and amylolytic bacterium. Int J Syst Evol Microbiol 45:9–16. https://doi.org/10.1099/00207713-45-1-9
Moryadee A, Pathom-Aree W (2008) Isolation of thermotolerant acetic acid bacteria from fruits for vinegar production. Research J Microbiology 3:209–212. https://doi.org/10.3923/jm.2008.209.212
Dieffenbach CW, Dveksler GS (1995) PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33. https://doi.org/10.1042/bj20031254
Chek MF, Kim SY, Mori T, Arsad H, Samian MR, Sudesh K, Hakoshima T (2017) Structure of polyhydroxyalkanoate (PHA) synthase PhaC from Chromobacterium sp. USM2, producing biodegradable plastic. Sci Rep 7:5312. https://doi.org/10.1038/s41598-017-05509-4
Shahid S, Mosrati R, Ledauphin J, Amiel C, Fontaine P, Gaillard JL, Corroler D (2013) Impact of carbon source and variable nitrogen conditions on bacterial biosynthesis of polyhydroxyalkanoates: Evidence of an atypical metabolism in Bacillus megaterium DSM 509. J Biosci Bioeng 116:302–308. https://doi.org/10.1016/j.jbiosc.2013.02.017
Singh AK, Mallick N (2009) Exploitation of inexpensive substrates for production of a novel SCL-LCL-PHA co-polymer by Pseudomonas aeruginosa MTCC 7925. J Ind Microbiol Biotechnol 36:347–354. https://doi.org/10.1007/s10295-008-0503-x
Gumel AM, Annuar MSM, Heidelberg T (2012) Biosynthesis and characterization of polyhydroxyalkanoates copolymers produced by Pseudomonas putida BET001 isolated from palm oil mill effluent. PLoS ONE 7(2):1–8. https://doi.org/10.1371/journal.pone.0045214
Munawar KMM, Simarani K, Annuar MSM (2016) Bioconversion of mixed free fatty acids to poly-3-hydroxyalkanoates by Pseudomonas putida BET001 and modeling of its fermentation in shake flasks. Electron J Biotechnol 19:50–55. https://doi.org/10.1016/j.ejbt.2015.07.005
Singh AK, Bhati R, Mallick N (2015) Pseudomonas aeruginosa MTCC 7925 as a biofactory for production of the novel SCL-LCL-PHA thermoplastic from non-edible oils. Curr Biotechnol 4:65–74. https://doi.org/10.2174/2211550104666150414194835
Sangkharak K, Prasertsan P (2013) The production of polyhydroxyalkanoate by Bacillus licheniformis using sequential mutagenesis and optimization. Biotechnol Bioprocess Eng 18:272–279. https://doi.org/10.1007/s12257-012-0615-z
Liu Y, Shaobin H, Zhang Y, Xu F (2014) Isolation and characterization of a thermophilic Bacillus shackletonii K5 from a biotrickling filter for the production of polyhydroxybutyrate. J Environ Sci 26(7):1453–1462. https://doi.org/10.1016/j.jes.2014.05.011
Tajima K, Igari T, Nishimura D, Nakamura M, Satoh Y, Munekata M (2003) Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J Biosci Bioeng 95(1):77–81. https://doi.org/10.1016/S1389-1723(03)80152-4
Ibrahim HA, Steinbu-chel A (2009) Poly(3-Hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl Environ Microbiol 75:6222–6231. https://doi.org/10.1128/AEM.01162-09
Xiao Z, Zhang Y, Xi L (2015) Thermophilic production of polyhydroxyalkanoates by a novel Aneurinibacillus strain isolated from Gudao oilfield, China: thermophilic production of PHA by a novel Aneurinibacillus strain. J Basic Microbiol 55(9):125–133. https://doi.org/10.1002/jobm.201400843
Giedraityte G, Kalėdiene L (2015) Purification and characterization of polyhydroxybutyrate produced from thermophilic Geobacillus sp. AY 946034 strain. Chemija 26(1):38–45
Sheu DS, Chen WM, Lai YW, Chang RC (2012) Mutations derived from the thermophilic polyhydroxyalkanoate synthase PhaC enhance the thermostability and activity of PhaC from Cupriavidus necator H16. J Bacteriol 194(10):2620–2629. https://doi.org/10.1128/JB.06543-11
Cui B, Huang S, Xu F, Zhang R, Zhang Y (2015) Improved productivity of poly (3-hydroxybutyrate) (PHB) in thermophilic Chelatococcus daeguensis TAD1 using glycerol as the growth substrate in a fed-batch culture. Appl Microbiol Biotechnol 99:6009–6019. https://doi.org/10.1007/s00253-015-6489-1
Pantazaki AA, Papaneophytou CP, Agathi Maria GP, Kyriakides L, Kyriakidisa DA (2009) Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem 44(8):847–853. https://doi.org/10.1016/j.procbio.2009.04.002
Ibrahim MHA, Steinbüchel A (2010) High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl Environ Microbiol 76(23):7890–7895. https://doi.org/10.1128/AEM.01488-10
Sheu DS, Chen WM, Yang JY, Chang RC (2009) Thermophilic bacterium Caldimonas taiwanensis produces poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from starch and valerate as carbon sources. Enzyme Microb Technol 44(5):289–294. https://doi.org/10.1016/j.enzmictec.2009.01.004
Taran M (2011) Utilization of petrochemical wastewater for the production of poly(3-hydroxybutyrate) by Haloarcula sp. IRU1. J Hazard Mater 188(1):26–28. https://doi.org/10.1016/j.jhazmat.2011.01.036
Acknowledgements
The authors would like to thank the Thailand Research Fund (TRF) for a research grant (Project Number RSA 6180066) and the Energy Policy and Planning Office (EPPO), Ministry of Energy, Thailand, and the Development Institute at Thaksin University for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choonut, A., Prasertsan, P., Klomklao, S. et al. Bacillus thermoamylovorans-Related Strain Isolated from High Temperature Sites as Potential Producers of Medium-Chain-Length Polyhydroxyalkanoate (mcl-PHA). Curr Microbiol 77, 3044–3056 (2020). https://doi.org/10.1007/s00284-020-02118-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02118-9