Abstract
Cyanobacteria produce some carotenoids. We identified the molecular structures, including the stereochemistry, of all the carotenoids in the terrestrial cyanobacterium, Nostoc commune NIES-24 (IAM M-13). The major carotenoid was β-carotene. Its hydroxyl derivatives were (3R)-β-cryptoxanthin, (3R,3′R)-zeaxanthin, (2R,3R,3′R)-caloxanthin, and (2R,3R,2′R,3′R)-nostoxanthin, and its keto derivatives were echinenone and canthaxanthin. The unique myxol glycosides were (3R,2′S)-myxol 2′-fucoside and (2R,3R,2′S)-2-hydroxymyxol 2′-fucoside. This is only the second species found to contain 2-hydroxymyxol. We propose possible carotenogenesis pathways based on our identification of the carotenoids: the hydroxyl pathway produced nostoxanthin via zeaxanthin from β-carotene, the keto pathway produced canthaxanthin from β-carotene, and the myxol pathway produced 2-hydroxymyxol 2′-fucoside via myxol 2′-fucoside. This cyanobacterium was found to contain many kinds of carotenoids and also displayed many carotenogenesis pathways, while other cyanobacteria lack some carotenoids and a part of carotenogenesis pathways compared with this cyanobacterium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nostoc, a genus of cyanobacteria, is one of the most widespread phototrophic bacteria. According to Rippka et al. [15], it is classified into subsection IV together with Anabaena and some other genera. They are filamentous and heterocystous cyanobacteria, commonly observed in both aquatic and terrestrial habitats.
The terrestrial cyanobacterium Nostoc commune (N. commune) is well known and highly drought tolerant. Because it sustains the capacity for cell growth for over 100 years in a dry state, it is considered an anhydrobiotic microorganism with oxygenic photosynthetic capabilities. Since N. commune does not differentiate into akinetes (spores), the mechanisms of its extreme desiccation tolerance most likely involve multiple processes including the cessation of photosynthesis and the accumulation of trehalose in response to desiccation [16]. Although some cyanobacteria have been investigated during the sequencing of their genome and the development of gene manipulation techniques, N. commune has not been well characterized in terms of its physiological or genetical attributes.
We have previously identified the molecular structures of the carotenoid and the glycoside moieties in some Anabaena and Nostoc species. Anabaena sp. PCC 7120 (also known as Nostoc sp. PCC 7120) and Nostoc punctiforme (N. punctiforme) PCC 73102 contain β-carotene, echinenone, canthaxanthin, myxol 2′-fucoside, and 4-ketomyxol 2′-fucoside, but not 2′-rhamnoside [25]. Anabaena variabilis (A. variabilis) ATCC 29413 contains β-carotene, echinenone, canthaxanthin, free myxol, and 4-hydroxymyxol but not myxol glycosides [26], while A. variabilis IAM M-3 (PCC 7118) contains β-carotene, echinenone, canthaxanthin, myxol 2′-fucoside, and 4-ketomyxol 2′-fucoside [25]. Based on functional analyses of their carotenogenesis genes, we have previously proposed the biosynthetic pathways for the carotenoids, their enzymes, and their genes, in these cyanobacteria [25, 26], and further we have summarized the biosynthetic pathways and their enzymes and genes, in cyanobacteria [22, 23].
In another recent study, we have reported that the unicellular thermophilic cyanobacterium Thermosynechococcus elongatus (T. elongatus) strain BP-1 also contains two polyhydroxyl carotenoids, caloxanthin and nostoxanthin [8]. In T. elongatus strain BP-1, 2,2′-β-hydroxylase, CrtG, produced nostoxanthin from zeaxanthin via caloxanthin and 2-hydroxymyxol from myxol. This represented the first functional identification of this enzyme in cyanobacteria [8].
In this study, we identified the molecular structures, including the stereochemistry, of all the carotenoids in N. commune NIES-24. The major carotenoid was β-carotene, and its hydroxyl or keto derivatives. The myxol glycosides were myxol 2′-fucoside and 2-hydroxymyxol 2′-fucoside. This species is only the second one found to contain 2-hydroxymyxol. We proposed the carotenogenesis pathways based on the identification of the carotenoids, including the hydroxyl, keto, and myxol pathways. Thus, this bacterium was found to contain many kinds of carotenoids compared with other cyanobacteria, and also many carotenogenesis pathways.
Materials and Methods
Strain and Culturing Conditions
The cyanobacterium N. commune NIES-24 (IAM M-13) was grown in BG-11 medium with continuous shaking (110 rpm) at 26–28°C under continuous illumination from white fluorescent light (30–40 μE m−2 s−1) for 2 weeks. The grown cells were then collected by centrifugation [25].
Purification and Identification of Pigments
The carotenoids were isolated and purified as follows (supplemental Fig. 1). We extracted the pigments with acetone/methanol (7:2, v/v) using an ultra sonicator, evaporated the solvent, dissolved them in a small volume of acetone, and loaded them on a column of DEAE-Toyopearl 650 M (Tosoh, Japan). A broad orange–yellow carotenoid band and then chlorophyll a (Chl a) were eluted with n-hexane/acetone (1:1, v/v), and a second polar orange carotenoid band was eluted with acetone. The first orange–yellow carotenoids were loaded on a column of silica gel 60 (Merck, Germany), and orange carotenoids were eluted with n-hexane/acetone (9:1, v/v), while the other orange carotenoids were eluted with n-hexane/acetone (7:3, v/v). Each fraction was further separated with KC-18 thin layer chromatography (TLC) (Whatman, USA) developed with methanol, and each band was collected. Each carotenoid was finally collected from the high performance liquid chromatography (HPLC) apparatus (described below) and eluted with methanol or methanol/water (9:1, v/v). The second polar orange carotenoids were purified with silica gel TLC (Merck) developed with dichloromethane/ethyl acetate/acetone/methanol (2:4:2:1, by vol), and then each carotenoid was collected from the HPLC apparatus eluted with methanol/water (9:1, v/v) [25].
Spectroscopic Analysis
The HPLC system was equipped with a μBondapak C18 column (8 × 100 mm, RCM type; Waters, USA) eluted with methanol/water (9:1, v/v) for the first 20 min and then with 100% methanol (2.0 ml min−1) [8, 25].
We measured the absorption spectra of the pigments using an MCPD-3600 photodiode array detector (Otsuka Electronics, Japan) attached to the HPLC apparatus [21]. For quantitative analysis, the molar extinction coefficient in the HPLC eluent at the maximum wavelengths of each carotenoid was assumed to be the same, 140 mM−1 cm−1, and that at 618 nm of Chl a was 16.8 mM−1 cm−1 [8]. The circular dichroism (CD) spectra of the carotenoids were measured using a J-820 spectropolarimeter (JASCO, Japan) in diethyl ether/2-pentane/ethanol (5:5:2, by vol) at room temperature. The relative molecular masses of the carotenoids were measured using an FD-MS; M-2500 double-focusing gas chromatograph-mass spectrometer (Hitachi, Japan) equipped with a field-desorption apparatus. The proton nuclear magnetic resonance (1H-NMR) (500 MHz) spectra of the carotenoids, which were further purified using a column of DEAE-Toyopearl eluted with n-hexane/acetone (1:1, v/v), in CDCl3 at 24°C were measured using the UNITY INOVA-500 system (Varian, USA).
Results
Separation and Identification of Carotenoids
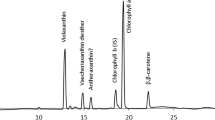
Figure 1 shows an elution profile of HPLC for the organic solvent-soluble pigments extracted from N. commune NIES-24. The peak-7 pigment (Fig. 1) was identified as Chl a by its absorption spectrum and the specific retention time on HPLC as compared with Chl a from T. elongatus strain BP-1 [8]. One minor peak eluted just before peak 7 had a Chl a-like spectrum.
The first orange–yellow carotenoids eluted from the DEAE-Toyopearl column (supplemental Fig. 1) were loaded on the column of silica gel 60, and orange carotenoids, which corresponded to peaks 6, 8, and 9 on HPLC (Fig. 1), were eluted with n-hexane/acetone (9:1, v/v); the next orange carotenoids, which corresponded to peaks 2, 4, and 5, were eluted with n-hexane/acetone (7:3, v/v). Each fraction was further purified with KC-18 TLC developed with methanol, and then each band was collected. Finally, each carotenoid was collected from the HPLC apparatus eluted with methanol for peaks 6, 8, and 9 or with methanol/water (9:1, v/v) for peaks 2, 4, and 5.
The absorption maxima of the peak-9 carotenoid in methanol were 273, 342, 426 (shoulder), 449, and 475 nm, and the spectral fine structure of %III/II was 16; this is the ratio of the peak heights of the longest and the middle wavelength absorption bands from the trough between the two peaks [21]. It had a relative molecular mass of 536, and hence this peak-9 carotenoid was identified as β-carotene (Fig. 3). The peak-6 (Fig. 2, dashed line) and -8 carotenoids showed broad absorption spectra in methanol, and their absorption maxima were around 475 and 460 nm, respectively. They had relative molecular masses of 564 and 550, respectively, and hence these peak-6 and -8 carotenoids were identified as canthaxanthin (Fig. 3) and echinenone, respectively. A minor carotenoid in this fraction was identified as β-cryptoxanthin based on the compatibility of its absorption spectrum with that of β-carotene, and its relative molecular mass of 552. Further, it eluted at around 31 min on HPLC and was hidden under Chl a (see Fig. 1). The specific retention times on HPLC of these carotenoids were also compatible with those from N. punctiforme PCC 73102 [25].
Absorption spectra of 2-hydroxymyxol 2′-fucoside (1, solid line) and nostoxanthin (2, dotted line) in methanol/water (9:1, v/v), and canthaxanthin (6, dashed line) in methanol. Numbers in parentheses indicate the peak numbers in Fig. 1
Structures of selected carotenoids. Numbers in parentheses indicate the peak numbers in Fig. 1
The absorption maxima of the peak-2 carotenoid in methanol/water (9:1, v/v) were 275, 344, 428 (shoulder), 451, and 477 nm (Fig. 2, dotted line), and the spectral fine structure of %III/II was 38, which were compatible with those of β-carotene. Its CD spectrum in diethyl ether/2-pentane/ethanol (5:5:2, by vol) was compatible with that of (2R,3R,2′R,3′R)-nostoxanthin from T. elongatus strain BP-1 [8]. It had a relative molecular mass of 600, and its 1H-NMR spectrum (Table 1) was compatible with that of nostoxanthin from T. elongatus strain BP-1 [8]. Hence, this peak-2 carotenoid was identified as (2R,3R,2′R,3′R)-nostoxanthin (Fig. 3). The absorption spectra of the peak-4 and -5 carotenoids were compatible with that of nostoxanthin (Fig. 2, dotted line). The CD spectrum of the peak-4 carotenoid was compatible with that of the peak-2 carotenoid. [Note: (2R,3R,2′R,3′R)-nostoxanthin, (2R,3R,3′R)-caloxanthin, (3R,3′R)-zeaxanthin, and (3R)-β-cryptoxanthin have the same CD spectra [2].] The peak-4 and -5 carotenoids had relative molecular masses of 584 and 568, respectively, and were thus identified as (2R,3R,3′R)-caloxanthin and (3R,3′R)-zeaxanthin (Fig. 3), respectively. The specific retention times on HPLC of these carotenoids were also compatible with those from T. elongatus strain BP-1 [8].
Separation and Identification of Polar Carotenoids
The second polar orange carotenoids eluted from the DEAE-Toyopearl column (supplemental Fig. 1) were purified with silica gel TLC (Merck) developed with dichloromethane/ethyl acetate/acetone/methanol (2:4:2:1, by vol). We collected one broad polar orange band, separated it via HPLC, and it was eluted with methanol/water (9:1, v/v). The products corresponded to the peak-1 and -3 carotenoids on HPLC (Fig. 1). Based on their characteristics on the silica gel TLC and C18-HPLC, they were considered to be carotenoid glycosides [24].
The absorption maxima of the purified peak-1 carotenoid in methanol/water (9:1, v/v) were 296, 366, 451, 475, and 506 nm (Fig. 2, solid line), and the spectral fine structure of %III/II was 56. Based on these results, the carotenoid moiety was identified as a derivative of γ-carotene with 12 conjugated double bonds [21]. Its CD spectrum was compatible with that of (2R,3R,2′S)-2-hydroxymyxol 2′-fucoside from T. elongatus strain BP-1 [8]. The relative molecular mass was 746. The 1H-NMR spectrum (Table 1) was compatible with that of 2-hydroxymyxol 2′-α-L-fucoside from T. elongatus strain BP-1 [8]. Hence, the structure of the peak-1 carotenoid was identified as 2-hydroxymyxol 2′-fucoside, (2R,3R,2′S)-2-hydroxymyxol 2′-α-L-fucoside (Fig. 3). The IUPAC–IUBMB semi-systematic name is (2R,3R,2′S)-2′-(α-L-fucopyranosyloxy)-3′,4′-didehydro-1′,2′-dihydro-β,ψ-carotene-2,3,1′-triol.
The absorption spectrum of the purified peak-3 carotenoid was compatible with that of the peak-1 carotenoid (Fig. 2, solid line). Its CD spectrum was compatible with that of the peak-1 carotenoid. The relative molecular mass was 730. The 1H-NMR spectrum (Table 1) was compatible with that of myxol 2′-α-L-fucoside from T. elongatus strain BP-1 [8] and Anabaena sp. PCC 7120 [25]. Thus, the structure of the peak-3 carotenoid was identified as myxol 2′-fucoside, (3R,2′S)-myxol 2′-α-L-fucoside (Fig. 3). The IUPAC-IUBMB semi-systematic name is (3R,2′S)-2′-(α-L-fucopyranosyloxy)-3′,4′-didehydro-1′,2′-dihydro-β,ψ-carotene-3,1′-diol.
Composition of Pigments
The composition of the pigments in the cells cultured under light for 2 weeks was 50% β-carotene (mol% of total carotenoids), <1% β-cryptoxanthin, 2% zeaxanthin, 5% caloxanthin, 11% nostoxanthin, 20% echinenone, 2% canthaxanthin, 4% myxol 2′-fucoside, and 5% 2-hydroxymyxol 2′-fucoside. The molar ratio of total carotenoids to Chl a was 0.44, which was comparable to those of some Anabaena and Nostoc species [25]. These values varied slightly as a function of the batch and the culture conditions.
Colonies of N. commune collected from a natural field (Kanazawa, Japan) contained the same pigments, but the composition was different from that of N. commune NIES-24: 18% β-carotene, 1% zeaxanthin, 3% caloxanthin, 4% nostoxanthin, 43% echinenone, 12% canthaxanthin, 9% myxol 2′-fucoside, and 11% 2-hydroxymyxol 2′-fucoside. The molar ratio of total carotenoids to Chl a was 0.53. The growth conditions in the natural field might be very different from the laboratory conditions.
Discussion
In this study, we identified the molecular structures, including the stereochemistry, of all the carotenoids in N. commune NIES-24 (IAM M-13). The major carotenoid was β-carotene. Its hydroxyl derivatives were (3R)-β-cryptoxanthin, (3R,3′R)-zeaxanthin, (2R,3R,3′R)-caloxanthin, and (2R,3R,2′R,3′R)-nostoxanthin. The unique keto derivatives were echinenone and canthaxanthin; the unique myxol glycosides were (3R,2′S)-myxol 2′-fucoside and (2R,3R,2′S)-2-hydroxymyxol 2′-fucoside. This is only the second species found to contain 2-hydroxymyxol [1, 8]. Although these carotenoids, including their stereochemistry, have been found in other cyanobacteria, only the species of the study contained nine kinds of carotenoid or had such a variety of carotenoids compared with other cyanobacteria [22, 23]. 4-Ketomyxol 2′-methylpentoside was, however, absent.
The carotenoids found in Nostoc were only reported in a few species with insufficient identification [5, 7]. Nostoc commune strain B 1453-5 was reported to contain β-carotene, zeaxanthin, caloxanthin, nostoxanthin, echinenone, canthaxanthin, 4-hydroxy-4′-keto-β-carotene, and myxoxanthophyll [20]. Since 4-hydroxy-4′-keto-β-carotene was only found in animals [1], this structure might be disputable. The structure of myxoxanthophyll (myxol glycoside) was unknown, and 2-hydroxymyxol 2′-methylpentoside was absent. Further, a Nostoc sp. was reported to contain β-carotene, zeaxanthin, echinenone, and myxoxanthophyll [4]. We have previously found that N. punctiforme PCC 73102 and Anabaena sp. PCC 7120 (also known as Nostoc sp. PCC 7120) contain β-carotene, echinenone, canthaxanthin, myxol 2′-fucoside, and 4-ketomyxol 2′-fucoside [25].
The presence of glycosides (mostly methylpentoside) of myxol and its derivatives is limited to cyanobacteria, with no reports of their presence in any other bacteria or eukaryotic algae [1, 5, 22, 23]. Further free myxol has been found in only two marine bacteria, strain P99-3 (MBIC 03313; previously Flavobacterium sp.) [28] and strain YM6-073 (MBIC 06409, Flavobacteriaceae) [17].
The biosynthesis of carotenoids in some cyanobacteria has been previously reported [22, 23]. In the cyanobacterium N. commune NIES-24, the following pathway is proposed from its carotenoid composition (Fig. 4). Geranylgeranyl diphosphate synthase (CrtE) and phytoene synthase (CrtB) produce phytoene, and then phytoene desaturase (CrtP), ζ-carotene desaturase (CrtQ), and cis-carotene isomerase (CrtH) produce lycopene as in other cyanobacteria. Lycopene cyclase(s) produces γ-carotene and/or β-carotene from lycopene, but the enzyme(s) is unknown, except for Synechococcus sp. PCC 7002 (CruA and CruP) [11], Synechococcus elongatus PCC 7942 and Prochlorococcus marinus MED4 (CrtL) [3, 19]. β-Carotene is changed to zeaxanthin via β-cryptoxanthin by β-carotene hydroxylase (CrtR), and then changed to nostoxanthin (Fig. 3) via caloxanthin by 2,2′-β-hydroxylase (CrtG) in the hydroxyl pathway. CrtG has been recently functionally confirmed in Brevundimonas sp. SD212 (MBIC 03018) and in T. elongatus strain BP-1 [8, 14, 27]. β-Carotene is also changed to canthaxanthin (Fig. 3) via echinenone by β-carotene ketolase (CrtO or CrtW) in the keto pathway [12]. Although the synthesis of myxol 2′-fucoside (the myxol pathway) has yet to be elucidated [23], at least CrtR for myxol, which is the same enzyme described above, and GDP-fucose synthase (WcaG) for myxol 2′-fucoside are known to play a role [13]. Recently, the functions of 1′,2′-hydratase (CruF) and GDP-fucosyl transferase (CruG) have been confirmed in Synechococcus sp. PCC 7002 [6]. Finally, myxol 2′-fucoside is changed to 2-hydroxymyxol 2′-fucoside (Fig. 3) by the same CrtG described above, which has been functionally confirmed in T. elongatus strain BP-1 [8]. The functions of two CrtWs for canthaxanthin and 4-ketomyxol from N. punctiforme PCC 73102 [18]; those of CrtQ, CrtR for only myxol, CrtO for canthaxanthin, CrtW for 4-ketomyxol, and WcaG from Anabaena sp. PCC 7120 [9, 10, 12, 13]; and that of CrtR for myxol from A. variabilis ATCC 29413 [10] have been confirmed. Thus, this cyanobacterium contains three carotenogenesis pathways (Fig. 4). Further studies are needed for identification of these enzymes and genes.
Proposed biosynthetic pathways of carotenoids and their enzymes in N. commune NIES-24. Numbers in parentheses indicate the peak numbers in Fig. 1. See the text for further details
Variation in carotenoid compositions have been attributed to be due to the presence or absence of specific carotenogenesis genes and pathways, as well as to the characteristics of specific enzyme(s) [22, 23]. Nostoc commune NIES-24 contains many functional carotenogenesis enzymes and pathways, while other cyanobacteria may lack some enzymes. This species could be standard species for carotenoids of Nostoc and related genera, and may be useful for studying the characteristics of carotenoids and carotenogenesis enzymes, and regulation of carotenogenesis in each pathway. Further studies of carotenoids in some Nostoc species are in progress.
References
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook. Birkhäuser Verlag, Basel
Buchecker R, Liaaen-Jensen S, Borch G, Siegelman HW (1976) Carotenoids of Anacystis nidulans, structures of caloxanthin and nostoxanthin. Phytochemistry 15:1015–1018
Cunningham FX Jr, Sun Z, Chamovitz D, Hirschberg J, Gantt E (1994) Molecular structure and enzymatic function of lycopene cyclase from the cyanobacterium Synechococcus sp strain PCC7942. Plant Cell 6:1107–1121
Goodwin TW (1957) The nature and distribution of carotenoids in some blue-green algae. J Gen Microbiol 17:467–473
Goodwin TW (1980) The biochemistry of the carotenoids, vol 1: Plants, 2nd edn. Chapman and Hall, London
Graham JE, Bryant DA (2009) The biosynthetic pathway for myxol 2′-fucoside (myxoxanthophyll) in the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol 191:3292–3300
Hirschberg J, Chamovitz D (1994) Carotenoids in cyanobacteria. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, pp 559–579
Iwai M, Maoka T, Ikeuchi M, Takaichi S (2008) 2,2′-Hydoxylase (CrtG) is involved in carotenogenesis of both nostoxanthin and 2-hydroxymyxol 2′-fucoside in Thermosynechococcus elongatus strain BP-1. Plant Cell Physiol 49:1678–1687
Linden H, Vioque A, Sandmann G (1993) Isolation of a carotenoid biosynthesis gene coding for ζ-carotene desaturase from Anabaena PCC 7120 by heterologous complementation. FEMS Microbiol Lett 106:99–104
Makino T, Harada H, Ikenaga H, Matsuda S, Takaichi S, Shindo K, Sandmann G, Ogata T, Misawa N (2009) Characterization of cyanobacterial carotenoid ketolase CrtW and hydroxylase CrtR by complementation analysis in Escherichia coli. Plant Cell Physiol 50:1867–1878
Maresca JA, Graham JE, Wu M, Eisen JA, Bryant DA (2007) Identification of a forth family of lycopene cyclases in photosynthetic bacteria. Proc Natl Acad Sci USA 104:11784–11789
Mochimaru M, Masukawa H, Takaichi S (2005) The cyanobacterium Anabaena sp. PCC 7120 has two distinct β-carotene ketolases: CrtO for echinenone and CrtW for ketomyxol synthesis. FEBS Lett 579:6111–6114
Mochimaru M, Masukawa H, Maoka T, Mohamed HE, Vermaas WFJ, Takaichi S (2008) Substrate specificities and availability of fucosyltransferase and β-carotene hydroxylase for myxol 2′-fucoside synthesis in Anabaena sp. strain PCC 7120 compared with Synechocystis sp. strain PCC 6803. J Bacteriol 190:6726–6733
Nishida Y, Adachi K, Kasai H, Shizuri Y, Shindo K, Sawabe A, Komemushi S, Miki W, Misawa N (2005) Elucidation of a carotenoid biosynthesis gene cluster encoding a novel enzyme, 2,2′-β-hydroxylase, from Brevundimonas sp. strain SD212 and combinatorial biosynthesis of new or rare xanthophylls. Appl Environ Microbiol 71:4286–4296
Rippka R, Castenholz RW, Herdman M (2001) Subsection IV. (Formerly Nostocales Castenholz 1989b sensu Rippka, Deruelles, Waterbury, Herdman and Stanier 1979). In: Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology, vol 1, 2nd edn. Springer, New York, pp 562–566
Sakamoto T, Yoshida T, Arima H, Hatanaka Y, Takani Y, Tamaru Y (2009) Accumulation of trehalose in response to desiccation and salt stress in the terrestrial cyanobacterium Nostoc commune. Phycol Res 57:66–73
Shindo K, Kikuta K, Suzuki A, Katsuta A, Kasai H, Yasumoto-Hirose M, Matuo Y, Misawa N, Takaichi S (2007) Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl Microbiol Biotechnol 74:1350–1357
Steiger S, Sandmann G (2004) Cloning of two carotenoid ketolase genes from Nostoc punctiforme for the heterogous production of canthaxanthin and astaxanthin. Biotechnol Lett 26:813–817
Stickforth P, Steiger S, Hess WR, Sandmann G (2003) A novel type of lycopene ε-cyclase in the marine cyanobacterium Prochlorococcus marinus MED4. Arch Microbiol 179:409–415
Stransky H, Hager A (1970) The carotenoid pattern and the occurrence of the light induced xanthophylls cycle in various classes of algae. IV. Cyanophyceae and Rhodophyceae. Arch Mikrobiol 72:84–96
Takaichi S, Shimada K (1992) Characterization of carotenoids in photosynthetic bacteria. Methods Enzymol 213:374–385
Takaichi S, Mochimaru M (2007) Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides. Cell Mol Life Sci 64:2607–2619
Takaichi S, Mochimaru M (2009) Carotenoids, their diversity and carotenogenesis in cyanobacteria. In: Gault PM, Marler HJ (eds), Handbook of cyanobacteria: biochemistry, biotechnology and applications. Nova Science Publishers, New York, in press (https://www.novapublishers.com/catalog/product_info.php?products_id=9524)
Takaichi S, Maoka T, Masamoto K (2001) Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2′-dimethyl-fucoside, (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-L-fucoside), not rhamnoside. Plant Cell Physiol 42:756–762
Takaichi S, Mochimaru M, Maoka T, Katoh H (2005) Myxol and 4-ketomyxol 2′-fucosides, not rhamnosides, from Anabaena sp. PCC 7120 and Nostoc punctiforme PCC 73102, and proposal for the biosynthetic pathway of carotenoids. Plant Cell Physiol 46:497–504
Takaichi S, Mochimaru M, Maoka T (2006) Presence of free myxol and 4-hydroxymyxol and absence of myxol glycosides in Anabaena variabilis ATCC 29413, and proposal of a biosynthetic pathway of carotenoids. Plant Cell Physiol 47:211–216
Tao L, Rouvière PE, Cheng Q (2006) A carotenoid synthesis gene cluster from a non-marine Brevundimonas that synthesizes hydroxylated astaxanthin. Gene 379:101–108
Yokoyama A, Miki W (1995) Isolation of myxol from a marine bacterium Flavobacterium sp. associated with a marine sponge. Fish Sci 61:684–686
Acknowledgments
The authors wish to thank Professors M. Tanaka and T. Sanji, Tokyo Institute of technology, for their help in measuring CD spectra, and Dr. T. Sakamoto, Kanazawa University, for his gift of N. commune natural field sample.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takaichi, S., Maoka, T. & Mochimaru, M. Unique Carotenoids in the Terrestrial Cyanobacterium Nostoc commune NIES-24: 2-Hydroxymyxol 2′-Fucoside, Nostoxanthin and Canthaxanthin. Curr Microbiol 59, 413–419 (2009). https://doi.org/10.1007/s00284-009-9453-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9453-4