Abstract
Even though organisms with squalene hopene cyclase activity involved in hopanoid synthesis has been reported earlier, their existence along with carotenoid synthesis is rarely reported. Here, we report the existence of hopanoid and C30 carotenoid biosynthetic pathway in Pseudomonas mendocina, the squalene hopene cyclase producing endophyte of the medicinal plant Murraya koenigii. The enzyme squalene hopene cyclase from Pseudomonas mendocina is involved in the synthesis of dehydrosqualene-mediated alternate pathway for carotenoid biosynthesis. The hopanoids are involved in membrane stability and integrity, and the carotene chromophores are involved in the photo protection of the cell. The orange-colored C30 carotenoid pigment 4–4′ diaponeurosporenic acid in the extracellular extract of Pseudomonas mendocina with squalene cyclase activity was detected by the combination of UV/Vis spectrometry, FTIR, and Mass Spectrometry. 4–4′ diaponeurosporenic acid could be traced as the end product of the carotenoid pathway and belonged to the xanthophyll group of carotenoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triterpenoids and their precursor isoprenoids constitute a large group of natural products. The two major classes of bacterial triterpenoids are the acyclic triterpenoid carotenoids and the cyclic hopanoids [1]. These two groups of compounds and their derivatives are emerging as major bacterial natural products. The terpenoid biosynthesis occurs by either of the two pathways: the C30 pathway in which two molecules of farnesyl pyrophosphate (C15) undergo head-to-head condensation and reductive dimerization to form the first acyclic parent triterpenoid (C30) or the C40 pathway by the dimerization of two molecules of geranyl geranyl pyrophosphate (C20) to form the first acyclic tetraterpenoid (C40).
Squalene is the C30 triterpenoid precursor for the acyclic carotenoids and the cyclic hopanoids. Of the cyclic triterpenoids, pentacyclic compounds are the major group in bacteria. Carotenoids have been known to occur in bacteria for long and are being studied for their structural diversity and unique biosynthetic pathway. The C30 carotenoids are relatively restricted in nature and are confined to minor groups of bacteria.
The first C30 carotenoid reported was the conjugated triene 4–4′- diapophytoene from Staphylococcus aureus 209P [2]. Later, the C30 carotenoids were identified from different bacterial species like Streptococcus faecium [1], Pseudomonas rhodos [3], Methylomonas sp. strain 16a [4], Bacillus firmus [5], and Pantoea stewartii [6]. The gene cluster for C30 carotenoid biosynthesis was well studied in Staphylococcus aureus in which all genes were functionally assigned [7].
Squalene hopene cyclases are enzymes involved in the pathway for hopanoid biosynthesis in bacteria. The synthesis of hopene involves the formation of five ring structures from the acyclic linear precursor, squalene. Studies on structural and functional aspects of squalene hopene cyclase from different bacterial system like Alicyclobacillus acidocaldarius [8], Zymomonas mobilis [9], Rhodopseudomonas palustris [10], Bradyrhizobium japonicum [11], Methylococcus capsulatus (Bath) [12], and Streptomyces peucetius [13] have been characterized.
The hopanoids act as architectural membrane components of the lipid bilayer by imparting phase transitions and stability of the membrane into an intermediate fluidity state. This stabilization is associated with a variety of membrane functions including permeability, nutrient transport, osmotic stability, etc. The C30 carotenoids are involved in photoprotective functions or energy transfer reactions by acting as accessory light-gathering pigments in photosynthesis. The carotenoids also protect other molecules against light-induced destruction of molecular oxygen by quenching free radicals. The colorless strains of bacteria are more susceptible to photo-induced destruction compared to the pigmented strains that prevent photosensitization in the presence of oxygen-free radicals [1].Endophytes are fascinating chemical partners within the host plants in synthesizing bioactive molecules or secondary metabolites that contribute to structural integrity, plant growth, and defense responses against biotic and abiotic stress [14]. The endophytic bacterium Pseudomonas sp.102525 from the leaves of yew tree was being reported to produce the carotenoid zeaxanthin diglucoside, a promising natural antioxidant that belonged to the family of carotenoids [15].Methylomonas sp. strain 16a is reported to possess both carotenoid and hopanoid pathway. In this organism, the genes for squalene synthase and squalene hopene cyclase are present along with carotenoid desaturase gene CrtN [4]. In our present study, the extraction of C30 carotenoid pigment 4–4′ diaponeurosporenic acid from the endophytic bacteria, Pseudomonas mendocina with squalene cyclase activity, can be attributed to the squalene route for C30 carotenoid synthesis rather than the normal carotenoid synthesis involving dehydrosqualene saturase.
Materials and Methods
Source of Bacterial Strain and Culture Conditions
The novel pigmented bacteria Pseudomonas mendocina was isolated as an endophyte of the medicinal plant Murraya koenigii and the identity was confirmed by morphological, biochemical, and molecular screening using 16SrDNA sequencing. The sequence was deposited in Gen Bank with accession number MFO99411 [16]. The isolate was inoculated in 20 mL fresh sterile nutrient broth in 100 mL Erlenmeyer flask and incubated at 30 °C and 200 rpm agitation speed until the optical density reached 1.0 at 600 nm (OD600 = 1). For cultivation of the isolate, 2 mL of the above culture was inoculated into 100 mL of fresh sterile nutrient broth medium taken in a 500 mL flask and incubated under similar conditions. Harvesting of the cells was done by centrifugation of the 48 h grown culture at 800×g for 10 min at 4 °C.
Enzyme Assay and Hopanoid Detection
Squalene cyclase activity in the isolate Pseudomonas mendocina was detected according to the procedure described by Ghimire et al. 2009. The cyclic product hopene was extracted with hexane and characterized by HPLC and GCMS analysis. The overexpression studies of the enzyme squalene hopene cyclase were conducted by the extraction of shc gene from the organism and cloning it in pET28a vector. The vector construct contained cloning sites for restriction enzymes BamH1 and Xho1with the kanamycin resistance gene, T7 promoter, and lac Z sequence. The 1980 bp shc gene was incorporated into the vector and over expressed in E.coli BL 21(DE3). The enzyme squalene hopene cyclase was purified by affinity chromatography using Ni sepharose beads and run on SDS-PAGE for determining the molecular weight as reported in our previous studies [16, 17].
Extraction and Purification of the Pigment
The pigment from the organism was extracted and purified according to the protocol described by Venil et al. [18]. The culture broth was centrifuged at 8000×g for 10 min at 4 °C. The supernatant was discarded and the pigmented pellet was used for carotenoid detection. The orange-colored cell pellet was rinsed with deionized water followed by centrifugation at 7000×g for 5 min. The recovered cells were extracted with 70% (v/v) acetone. The mixture of cells and acetone was vortexed for 5 min until the cells were completely bleached. The pigment was then separated from cells by centrifugation at 8500×g for 5 min. The extracted pigment was concentrated using the rotary evaporator (Heidolph Rotovac, Germany) at 40 °C and dried for 48 h. The crude pigment extract was subjected to thin-layer chromatography (Merck Silica Gel F254). Most carotenoids are readily detected as colored spots or bands. Spots or bands from thin-layer plates were recovered by scraping the appropriate portion of silica gel into a tube and eluted with acetone.
Structural Characterization of the Pigment
The purified orange pigment from the isolate was determined for maximum wavelength (λmax) using UV–Vis Spectrophotometer (UV-2600, Shimadzu, Japan) between 800 and 200 nm. The acetone extract containing the pigment was subjected to FTIR(Shimadzu, IR Spectrometer) between 4000 cm−1 and 400 cm−1 to determine the functional groups. The mass analysis of the pigment was carried out using LC–MS analysis with acetonitrile: water (99:1) as solvent system and a flow rate of 1 mL/min. The molecular ion mass was further confirmed by detailed LC–MS/MS-based analysis using Acquity H-Class (Waters) Ultra performance liquid chromatography with BEH C18 column (50 mm × 2.1 mm × 1.7 μm) and Xevo G2 (Waters) Quadruple Time of Flight (Q-TOF) mass spectrometer. The molecular weight of the compound was further confirmed by the fragmentation pattern analysis.
Result and Discussion
Hopanoid Activity of the Organism
Triterpenoids in the endophytic isolate Pseudomonas mendocina were studied by determining the squalene hopene cyclase activity in the organism. The product hopene formed after conversion of the substrate squalene with the enzyme in assay mixture was determined by the unique mass fragmentation at m/z 191 in the GC–MS spectra after the disintegration of the parent compound with a mass of m/z 410.71. Squalene hopene cyclases are enzymes that convert the linear compound squalene to a pentacyclic triterpenoid hopene. The SDS-PAGE analysis gave a 75 KDa band of the purified enzyme in gel [16, 17].
Pigment Characterization
The endophytic isolate Pseudomonas mendocina gave bright orange, smooth, elevated colonies when streaked on nutrient agar plates. The nutrient broth culture gave pigmented cell pellets after centrifugation. When extracted with 70% (v/v) acetone, the cells were completely bleached and the pigment was extracted by centrifugation and concentrated in rotary evaporator (Fig. 1, supplementary data). The concentrated acetone extract when spotted to TLC gave three distinct bands. The band at Rf 0.9 was scrapped out and eluted with acetone.
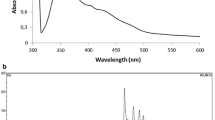
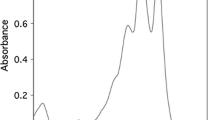
The maximum wavelength absorption of the extracted pigment with acetone was at 470 nm (Fig. 2, supplementary data). The FTIR of the pigment (Fig. 1) showed peaks above 3000 cm−1 which contributed to the unsaturation of the compound and the peak at 3269.34 cm−1 contributed to the OH stretch corresponding to carboxylic acid. In the spectra, the peaks between 1699.29 cm−1and 1230.58 cm−1 are attributed to C-O stretching and the band at 1645 cm−1 represented a conjugated keto group.
From the liquid chromatogram of the pigment, the peak at 6.38 min (Fig. 2a) was selected that gave a mass spectra very similar to that of 4–4′-diaponeurosporenic acid, a C30 carotenoid at m/z 432.21(M + H+) (Fig. 2b) corresponding to a molecular formula of C30H40O2. Tandem mass fragmentation showed fragments at m/z 415.21 and 385.2 which indicate the loss of –OH and –COOH group from the parent ion which confirmed the presence of an acidic functional group. In addition, a number of ions were observed indicating fragment loss from the parent ion at m/z 281.13, 250.15, 135.08, 119.08, 98.94, and 69.03 (Fig. 2c).
Squalene and reduced squalene have been observed in divergent bacterial groups. But the presence of sterols and hopanoids are restricted to certain bacterial groups especially the aerobic or facultative anaerobic bacteria. Members of Pseudomonas family are mostly pigment producers. The fluorescent pigment pyoverdine (fluorescein) imparts fluorescence properties to Pseudomonas fluorescens under UV light. Pyoverdine is the main siderophore of the strain essential for growth and survival [19].
C30 carotenoids like a glycosylated derivative of 4–4′ diapolycopene have been reported in Pseudomonas rhodos [3]. Pseudomonas N842 and its mutant C45 strains accumulated hop-22-ene, hopan-22-ol, squalene, and a C31 homolog of hopanol with m/z 424 instead of m/z 410 of hopene [20]. The first C30 carotenoid reported was the conjugated triene 4–4′- diapophytoene from Staphylococcus aureus 209P [2]. A series of carotenoids has been reported from wild-type and mutant strains of Pseudomonas rhodos. The major pigment isolated is glycosyl esters of 4–4′ diapocarotene- 4–4′- dioic acid [3]. The genes encoding the dehydrosqualene synthase (Crt M) and dehydrosqualene desaturase (Crt N) were discovered in Staphylococcus aureus [21, 22]. The biosynthesis of 4–4′- diaponeurosporene started with the condensation of two molecules of farnesyl diphosphate by dehydrosqualene synthase (CrtM) to form dehydrosqualene which is dehydrogenated by a desaturase (CrtN) to form the yellow intermediate 4–4′-diaponeurosporene [22]. CrtN was evolved to optimize 4,4′-diaponeurosporene or 4,4′-diapo-ζ-carotene biosynthesis pathway [23] Methylomonas sp. strain 16a has been reported to possess both carotenoid and hopanoid pathway. In this organism, the genes coding for squalene synthase and squalene hopene cyclase are present along with carotenoid desaturase gene CrtN. The CrtM gene (dehydrosqualene synthase) is not present in Methylomonas sp. strain 16a.[4]. The orange pigment isolated from Pseudomonas mendocina with an absorption maxima at 475 nm, FTIR spectral representation of –COOH together with the mass spectral data confirmed the pigment as 4–4′-diaponeurosporenic acid with molecular ion mass of m/z 432.21 and molecular formula C30H40O2. The presence of hopanoids in the organism was confirmed by the representation of hopene at m/z 191 in the GCMS spectra after squalene cyclase assay [16]. Both carotenoids and hopanoids are involved in membrane stabilization and structural rigidity. The carotenoid chromophore containing conjugated carbon–carbon double bonds imparts photoprotective function to the cell. Since both hopene and carotenoids existed in the organism, the pathway for carotenoid synthesis could be the C30 pathway which is restricted in bacterial carotenoid synthesis (Fig. 3). The enzyme squalene hopene cyclase in Pseudomonas mendocina could contribute to the production of dehydrosqualene from squalene in an alternate pathway other than the conventional pathway involving the enzyme dehydrosqualene desaturase (CrtM). Moreover, this is the first report for the existence of C30 carotenoid 4–4′ diaponeurosporenic acid in an endophytic organism. This could be considered as the first report for the existence of the pigment 4–4′ diaponeurosporenic acid in a bacterium exhibiting hopanoid activity. The endophyte Pseudomonas mendocina with such a metabolic versatility is a promising tool in terpene production and color production. This pigment could be used as a substitute for synthetic colorants in food and pharmaceutical industries.
Conclusion
The endophyte-plant interactions are usually focussed on the plant growth promotion properties. The present study opens a new angle of research into the role of endophytes in maintaining the structural integrity of the cell membrane.C30 carotenoid biosynthesis is restricted only to a few bacterial groups and the existence of squalene hopene cyclase activity in the pigmented endophytic isolate Pseudomonas mendocina opened a new focus of research into the squalene-mediated pathway for carotenoid biosynthesis. This can provide a valuable platform for screening of functional squalene synthase activity by tracing the pigmentation level in the strain. On the other side, the system can also be exploited for the large-scale synthesis of the colored pigment. The negative impact of synthetic coloring agents on human health like toxicity, hyper allergenicity, and carcinogenicity had increased the consumer demand for natural and health-promoting food ingredients. The utilization of carotenoids as colorants and supplements in food, cosmetics, nutraceuticals, and pharmaceuticals can lead to large-scale production of pigments from bacteria. The current research also opens new channels into the exploration of the biochemical linkages at molecular level between the existence of carotenoid pigments and squalene hopene cyclase activity within the same organism.
References
Taylor R (1984) Bacterial triterpenoids. Microbiol Mol Biol Rev 48:181–198
Suzue G, Tsukada K, Nakai C, Tanaka S (1968) Presence of squalene in Staphylococcus. Arch Biochem Biophys 123:644
Kleinig H, Schmitt R, Meister W, Englert G, Thommen H et al (1979) New C30-carotenoic acid glucosyl esters from Pseudomonas rhodos. Z Naturforsch. https://doi.org/10.1515/znc-1979-3-404
Tao L, Schenzle A, Odom J (2005) Novel carotenoid oxidase involved in biosynthesis of 4, 4′-diapolycopene dialdehyde. Appl Environ Microbiol 71:3294–3301. https://doi.org/10.1128/AEM.71.6.3294
Steiger S, Perez-Fons L, Fraser PD et al (2012) Biosynthesis of a novel C 30 carotenoid in Bacillus firmus isolates. J Appl Microbiol 113:888–895. https://doi.org/10.1111/j.1365-2672.2012.05377.x
Mohammadi M, Burbank L, Roper MC (2012) biological role of pigment production for the bacterial phytopathogen Pantoea stewartii subsp. stewartii. Appl Environ Microbiol 78:6859–6865. https://doi.org/10.1128/AEM.01574-12
Pelz A, Wieland KP, Putzbach K et al (2005) Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem 280:32493–32498. https://doi.org/10.1074/jbc.M505070200
Ochs D, Kaletta C, Entian KD et al (1992) Cloning, expression, and sequencing of squalene-hopene cyclase, a key enzyme in triterpenoid metabolism. J Bacteriol 174:298–302. https://doi.org/10.1128/jb.174.1.298-302.1992
Ochs D, Tappe CH, Gärtner P et al (1990) Properties of purified squalene-hopene cyclase from Bacillus acidocaldarius. Eur J Biochem 194:75–80. https://doi.org/10.1111/j.1432-1033.1990.tb19429.x
Kleemann G, Kellner R, Poralla K (1994) Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochim Biophys Acta (BBA)/Lipids Lipid Metab 1210:317–320. https://doi.org/10.1016/0005-2760(94)90235-6
Perzl M, Müller P, Poralla K, Kannenberg EL (1997) Squalene-hopene cyclase from Bradyrhizobium japonicum : cloning, expression, sequence analysis and comparison to other triterpenoid cyclases. Microbiology 143:1235–1242. https://doi.org/10.1099/00221287-143-4-1235
Tippelt A, Jahnke L, Poralla K (1998) Squalene-hopene cyclase from Methylococcus capsulatus (Bath): a bacterium producing hopanoids and steroids. Biochim Biophys Acta 1391:223–232. https://doi.org/10.1016/s0005-2760(97)00212-9
Ghimire GP, Oh T-J, Lee HC, Sohng JK (2009) Squalene-hopene cyclase (Spterp25) from Streptomyces peucetius: sequence analysis, expression and functional characterization. Biotechnol Lett 31:565–569. https://doi.org/10.1007/s10529-008-9903-2
Pacifico D, Squartini A, Crucitti D et al (2019) The role of the endophytic microbiome in the grapevine response to environmental triggers. Front Plant Sci 10:1–15. https://doi.org/10.3389/fpls.2019.01256
Fidan O, Zhan J (2019) Discovery and engineering of an endophytic Pseudomonas strain from Taxus chinensis for efficient production of zeaxanthin diglucoside. J Biol Eng 13:1–18. https://doi.org/10.1186/s13036-019-0196-x
Nair MI, Jayachandran K (2017) A novel strain of Pantoea eucrina endophyte of Murraya koenigii with squalene cyclase activity. LIFE Int J Heal Life Sci 3:161–177. https://doi.org/10.20319/lijhls.2017.32.161177
Nair IM, Kochupurakal J (2019) In silico characterization and over-expression of squalene hopene cyclase from Pseudomonas mendocina. 3 Biotech 9:1–7. https://doi.org/10.1007/s13205-019-1901-7
Venil CK, Zakaria ZA, Usha R, Ahmad WA (2014) Isolation and characterization of flexirubin type pigment from Chryseobacterium sp. UTM-3T. Biocatal Agric Biotechnol 3:103–107. https://doi.org/10.1016/j.bcab.2014.02.006
Cornelis P (2010) Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol 86:1637–1645. https://doi.org/10.1007/s00253-010-2550-2
Rohmer M, Bouvier-Nave P, Ourisson G (1984) Distribution of Hopanoid triterpenes in Prokaryotes. Microbiology 130:1137–1150. https://doi.org/10.1099/00221287-130-5-1137
Ku B, Jeong JC, Mijts BN et al (2005) Preparation, characterization, and optimization of an in vitro C30 carotenoid pathway. Appl Env Microbiol 71:6578–6583. https://doi.org/10.1128/AEM.71.11.6578-6583.2005
Wieland B, Feil C, Gloria-Maercker E et al (1994) Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4’-diaponeurosporene of Staphylococcus aureus. J Bacteriol 176:7719–7726. https://doi.org/10.1128/jb.176.24.7719-7726.1994
Kim SH, Kim MS, Lee BY, Lee PC (2016) Generation of structurally novel short carotenoids and study of their biological activity. Sci Rep. https://doi.org/10.1038/srep21987
Acknowledgements
The current study was supported by KSCSTE-SARD Programme, Kerala State Council for Science, Technology and Environment, DBT-MSUB Common Instrumentation Facility, School of Biosciences, and LC-MS/MS facility at Inter University Instrumentation Facility, School of Environmental Sciences, Mahatma Gandhi University Kottayam.
Author information
Authors and Affiliations
Contributions
The author IMN has contributed to the execution of the work and the preparation of the manuscript. The corresponding author JK contributed to the planning of the work and correction of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nair, I.M., Jayachandran, K. 4–4′ Diaponeurosporenic Acid, the C30 Carotenoid Pigment in Endophytic Pseudomonas Mendocina with Squalene Cyclase Activity. Curr Microbiol 77, 3473–3479 (2020). https://doi.org/10.1007/s00284-020-02180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02180-3