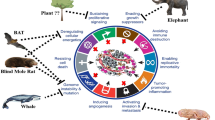
Abstract
Despite advances in therapeutic interventions and supportive care, the morbidity and mortality associated with cancer have remained significant. Thus, there is a need for newer and more powerful anti-tumor agents. The search for new anti-tumor compounds originating from natural resources is a promising research area. Animals living in polluted environments are a potent source of anti-tumor agents. Under polluted milieus, species such as crocodiles, feed on rotten meat, are exposed to heavy metals, endure high levels of radiation, and are among the very few species to survive the catastrophic Cretaceous-Tertiary extinction event with a prolonged lifespan. Thus, it is reasonable to speculate that animals such as crocodiles have developed mechanisms to defend themselves against cancer. The discovery of antitumor activity in animals such as crocodiles, whales, sharks, etc. will stimulate research in finding therapeutic molecules from unusual sources, and has potential for the development of novel antitumor compound(s) that may also overcome current drug resistance. Nevertheless, intensive research in the next few years will be required to realize these expectations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The morbidity and mortality associated with cancer have remained significant, despite advances in therapeutic interventions and supportive care. For example, the International Agency for Research on Cancer reports that in 2012, there were approximately 14.1 million new cancer cases, 32.6 million pre-existing cancer patients and 8.2 million deaths due to cancer worldwide [1]. By 2030, the global cancer burden is expected to almost double, growing to 21.7 million cases and 13 million deaths, in part due to aging population [1,2,3]. Additionally, cancer cell dormancy and the emergence of drug resistance contribute to poor prognosis, resulting in a high number of pre-existing cancer cases. Thus, there is a continuous need to search for anti-cancer therapies.
Natural products have been used widely for medicinal purposes. In particular, natural products derived from plants have led to the identification of anti-cancer agents such as Vinca alkaloids (e.g., Vincristine, Vinblastine) [4], Podophyllotoxin (PPT) derivatives (e.g., etoposide) [5] and Taxol derivatives (e.g., paclitaxel) [6] but drug resistance remains a major challenge and highlights the importance of new compounds. Recently, we hypothesized that crocodiles possess anti-tumor compound(s) and/or mechanisms to counter cancer development [7]. The fact that animals such as crocodiles live in unhygienic conditions, feed on rotten meat, are exposed to heavy metals such as arsenic, cadmium, cobalt, chromium, mercury, nickel, lead, selenium, endure high levels of radiation, are among the very few species to survive the catastrophic Cretaceous-Tertiary extinction event [8,9,10,11,12,13,14], with a prolonged lifespan and rarely develop cancer suggests that they possess mechanisms to counter cancer development. Having visited several crocodile sanctuaries in South-East Asia and working together with expert veterinarians handling crocodiles/alligators over the past few decades, it was intriguing to note the absence of cancer development in these animals (personal communications with expert veterinarians, S. Vellayan, who dissected over 2000 crocodiles, post-mortem, and none showed cancer characteristics). This is corroborated with the absence of scientific evidence on cancer incidence rate in these species. Similarly, other animals such as whales, sharks, turtles, tortoises, elephants, snakes, etc. have long lifespan. Although the incidence rate of cancer is not available for several vertebrates, cancer has been reported in snakes [15], tortoise [16], crocodile [17], monitor lizards [18], whales [19], and sharks [20]. Given the rarity of cancer development or associated complications together with their prolonged life span of up to a 100 years [14, 21,22,23], it is reasonable to speculate that these animals may have developed protective mechanisms or possess bioactive molecules with anti-tumor properties which may prevent them from developing cancer. Here, we review the literature on the occurrence of anti-tumor compounds in animals living in polluted environments and the potential for future investigation in these species.
Animal-based anti-cancer agents
The discovery of anti-cancer agents from animals living in polluted environments is a worthy area of research that offers an untapped biological source for the isolation of novel anti-tumor molecules. Among mammals, animals such as elephants and whales are perceived to be highly resistant to cancer [21,22,23]. Being one of the oldest mammals which have existed since pre-historic times, whales have demonstrated their ability to survive evolution by adaptation [21,22,23]. For example, Bowhead whales (Balaena mysticetus) are able to live up to over 211 years [21,22,23]. Animals with large body size and longer lifespans were presumed to have an increased risk of developing cancer, if organisms possess similar malignant transformation risks and cancer suppression mechanisms when exposed to cancer-causing agents. Therefore, since larger-sized animals contain more cells, they were presumed to have a higher chance of developing cancer compared to smaller sized organisms [21,22,23]. Additionally, animals with longer lifespans were thought to have more time to mount up mutations caused by cancer-causing agents compared to animals with shorter lifespan [21,22,23]. This concept was, however, proven wrong by Peto’s paradox. Cancer was shown to have no correlation with the body size and lifespan of an organism. The concept of Peto’s paradox explains the presence of lower oncogene (anti-apoptotic genes) expression and increase tumor suppressor genes in large, long lived animals [21]. As a result, active apoptosis activity within cells prevents the proliferation or cell division of abnormal cells, leading to a lower chance of developing cancer. Besides, animals such as elephants have lower metabolic rates, leading to reduced free radical accumulation. Additionally, elephants were found to produce ‘cheater’ tumor cells which parasitize the growth of other tumor cells [21]. As a result, tumor cells are unable to grow leading to a reduced risk of developing cancer. Additional studies revealed the possible mechanisms which are involved in lower cancer development in bowhead whales and elephants compared to other mammals [21,22,23]. These animals possess altered gene expression levels which makes them less likely to develop cancer in comparison to other species. The p53 tumor suppressor gene activity was found to be highly expressed in elephants. A higher expression of tumor suppressor gene increases cell sensitivity towards cancer-causing agents, leading to the initiation of apoptosis of tumor cells. Elephants which are the evolved version of mammals from the Proboscideans family are also found to have a very low chance of developing cancer [21, 23]. Studies have shown that the tumor suppressor gene, p53 in elephants was retro-duplicated. This retro-duplicated p53 was highly expressed in elephants leading to enhanced sensitivity of elephant cells towards genotoxic stress, resulting in induction of apoptosis [21]. Although the discovery of multiple p53 genes in elephants partially explains lower cancer risk in elephants and other large-sized animals, the link between the p53 gene expression and cancer suppression as well as the presence of potential anti-tumor molecule(s) is yet to be determined.
Reptiles such as crocodiles are shown to contain many bioactive peptides which exhibit anti-inflammatory, anti-oxidative and anti-microbial characteristics [24,25,26]. Song et al. [27] showed that bile acids from crocodiles and snakes were found to contain anti-cancer properties. Furthermore, ESC-3 was shown to be the active component in crocodile bile that induced apoptosis in Mz-ChA-1 cells through the mitochondria-dependent pathway and it was proposed as a potential chemotherapeutic drug against cholangiocarcinoma [27]. This is consistent with Chinese Traditional Medicine where animal bile acids have been used in the treatment of various diseases including cancer [28]. In particular, Siamese crocodiles (Crocodylus siamensis) are one of the most studied crocodile species in terms for cancer research and their bile acids and white blood cell extracts were shown to exhibit anti-cancer properties [25,26,27,28,29]. In particular, bile acid extract inhibited proliferation of human biliary adenocarcinoma cells (Sk-ChA-1) and several other cholangiocarcinoma cells such as MZ-ChA-1 [27] and QBC939 [29] and human hepatocellular carcinoma cells (SMMC7721) [30] in a dose-dependent manner. Molecular studies revealed that the proliferation of cancer cells was inhibited via the cell cycle arrest mechanism at the G0/G1 phase [28, 29]. Later studies revealed that Siamese crocodile bile extracts induce apoptosis via production of reactive oxygen species, loss of mitochondrial membrane potential, resulting in the release of cytochrome c into the cytosol, up-regulation of pro-apoptotic proteins such as p53 and Bax, and down-regulation of anti-apoptotic proteins such as Bcl-2, Survivin and c-Myc [25,26,27]. In addition to cytotoxic effects of crocodile bile acids on human cells, bile acid extract was found to enhance the sensitivity of drug uptake by human cholangiocarcinoma multidrug resistance cell line (QBC939/5-FU) suggesting that molecular constituents of bile acid extracts of Siamese crocodiles can augment anti-cancer chemotherapeutic properties [31]. Notably, phase III trial of Ursodeoxycholic acid (UDCA) treatment, a component normally present in bile fluid showed a 39% reduction in malignant tumors [29, 32]. This is in contrast to bile from humans where secondary bile acids were shown to play a role in intestinal tumor development [33] suggesting differences in composition of molecular constituents of bile in different species. More recently, white blood cell extracts from Siamese crocodiles are shown to exhibit anti-angiogenic properties in cancer cells by inhibiting the expression of matrix metalloproteinase such as MMP2 and MMP9, suggesting the disruption of vascular endothelial growth factor (VEGF) and integrin-mediated signal transduction [24]. The disruption of MMP2, MMP9 and VEGF activity directly inhibits metastasis among cancer cells [24]. Patathananone et al. [24] demonstrated the anti-motility effects of Siamese crocodile white blood cell extracts against HeLa cells, mediated by disruption of Ras and p38 signaling pathway; however, in vivo studies are needed to determine the translational value of these findings.
Recently, our studies showed that the organ lysates of Crocodylus palustris exhibit antitumor activity against prostate cancer cells (PC3). Among various body organs of crocodile tested including the heart, brain, spleen, gall bladder, lungs, liver, stomach, intestines, blood, cerebrospinal fluid, testis and copulatory organs, the results revealed that 100 µg of sera, bile, gall bladder and heart lysates killed more than 60% PC-3 cells; however, lung, intestine, and brain lysates showed partial cytotoxic effects (unpublished findings). When inoculated in fresh medium, PC3 cells treated with bile, gall bladder, sera, and heart lysates did not revive, while PC3 cells treated with lung, intestine and brain lysates exhibited partial growth. These findings suggest that crocodile organ crude extracts contain active component(s) that affect the viability of PC3 cells. The broad-spectrum antitumor activity of various organ lysates of the crocodile against cancer and primary cells and the chemical identities of the active compound(s) are the subject of future studies. It is hoped that these molecules can eventually be developed into treatments against cancer that are becoming increasingly resistant to current available drugs. Crocodiles are one of the most ancient and hardiest species that have survived millions of years. The ability of crocodiles to survive polluted environments together with the fact that crocodiles are an untapped source of pharmaceutical drug leads suggests such species may possess antitumor compound(s), endogenously and/or mechanisms to counter carcinogenic substance(s); however, further work is needed to realize the potential of these findings.
Snake venom has been tested for therapeutic interventions. Snake venom is made up of a mixture of biologically active components such as neurotoxins, myotoxins, enzymes, and pain inducing agents [34], some of which are shown to be of therapeutic value including captopril (derived from Bothrops jararaca) for renal dysfunction and exenatide (derived from Gila Monster lizard) for diabetes mellitus [34]. For anti-cancer properties, Phospholipase A2 (PLA2) from snake venom was shown to induce apoptosis and inhibition of cell metastasis [35]. This was shown using BnSP-6, an isoform of PLA2 derived from the venom of Bothrops pauloensis that exhibited selective toxicity against MDA-MB-231 breast cancer cells in a dose-dependent manner with lower toxicity against normal breast epithelial cells (MCF10A) [35]. An acidic Asp49PLA2 known as MVL-PLA2, from the venom of Macrovipera lebetina also showed antitumor properties by inhibiting the adhesion and migration of melanoma cells (IGR39) [36]. The molecular mechanism of action of PLA2 indicated the hydrolytic activity of the PLA2 targeting the phospholipid membrane bilayer. The release of lysophospholipids (LysoPL) and fatty acids (FAs) from the membrane results in membrane damage, disruption of membrane surface proteins and cellular cascade functional disruption [34]. Ebrahim et al. [37] demonstrated the cytotoxic effects of cytotoxin, CTX-1 and CTX-11, derived from the Caspian Cobra (Naja oxiana), against tumor cells (liver adenocarcinoma, HepG2, and breast adenocarcinoma, MCF7 cells) and compared with the normal kidney cells (MDCK). It was shown that the cytotoxic effects are mediated via the lysosomal pathway and by entry of cathepsin into the cytosol [37]. Overall, snake venom components such as CTX-1, CTX-11, BnSP-6 are shown to induce apoptosis [37] and inhibit cell adhesion and migration in cancer cell lines [35]. Cardiotoxin III from the venom of Naja naja atra demonstrated anti-metastatic properties against human breast cancer cells by suppressing the expression of hepatocyte growth factor (HGF)-induced c-Met phosphorylation [38]. Besides venom, studies are needed to test snake organ lysates for potential anti-tumor properties and the associated molecular mechanisms. For example, organ extracts of Cryptopodion scabrum, a geckonid lizard and Gekko swinhonis Guenther (GSPP) exhibited anti-proliferative activity and anti-angiogenic effects against cancer cells selectively and in a dose- and time-dependent manner in vitro and in vivo [38, 39]. It was demonstrated that cancer cells were unable to undergo metastasis due to disruption in bFGF function, a growth factor responsible for angiogenesis [38].
Among small mammals, several mechanisms have been proposed that may inhibit cancer development. For example, it was shown that Naked Mole Rats (NMRs; Heterocephalus glaber) exhibit changes in p53 gene [40], and their non-coding RNAs (IncRNAs) interact more with four types of high-molecular-mass hyaluronan (HMM-HA) from the fibroblasts compared to other rodents, which may enable them to inhibit cancer development [41,42,43,44]. Signals from HMM-HA trigger the activation of tumor suppressor INK4 (Inhibitors of cyclin-dependent kinase 4) locus expression [42,43,44]. This results in the activation of an alternate reading frame (ARF) and a novel product, pALTINK4a/b, which is a hybrid of the two tumor suppressor proteins, p15INK4b and p16INK4a. Interestingly, pALTINK4a/b was found to be present in NMRs but absent in humans, which suggests its role in the cancer resistance of NMRs [45]. On the other hand, an equilibrium between cell proliferation and cell death is essential. Extreme expression of cell proliferation proteins may result in tumorigenesis whereas extreme expression of tumor suppressor proteins will contribute in accelerated aging. The tumor suppressor proteins, p15INK4b and p16INK4a, was also found to be highly expressed in NMRs upon low levels of stress in addition to pALTINK4a/b which explains the reason NMRs do not develop cancer [42,43,44,45,46]. However, extreme tumor suppression activities accelerate aging which is not the case among NMRs. Although many studies have been performed to discover the mechanism involved in NMRs cancer resistance, [42,43,44,45,46], Taylor et al. [47] reported the presence of 2 NMRs with tumor. This finding showed that NMRs do develop cancer [47], albeit at a lower rate compared with humans. However, further studies are needed for NMRs with cancer, to investigate the reliability of the anti-cancer mechanism which is believed to protect NMRs against tumorigenesis.
Among Amphibians, the Bufonidae family which generally consists of toad species was found to possess bioactive compounds with anti-cancer activities as well as other therapeutic activities such as anti-microbial and anti-allergy activities [48, 49]. Bufonidae family is able to produce secretions from the parotid glands and skin which is rich in bioactive secondary metabolites with anti-cancer properties [50]. The secretion from the granular glands of the frog (Physalaemus nattereri) is poisonous and is normally used as a defense mechanism against predators. Studies have revealed the anti-cancer ability of the crude skin secretion containing this poisonous substance from Physalaemus nattereri against B16F10 murine melanoma cells [51, 52]. The crude skin extracts were cytotoxic against murine melanoma cells in a concentration-dependent manner via apoptosis and by cell cycle arrest at S phase [42, 51, 52]. This was consistent with the findings by [52]. Cinobufacini compound from the skin of Physalaemus nattereri significantly inhibited the growth of HepG2 via apoptosis, inducing cell cycle arrest at S phase and by downregulating the protein expression of TOPO 1 and TOPO II [51, 52]. Later studies demonstrated the anti-cancer properties of skin extracts from the organisms belonging to the Bufonidae family [53]. The skin extract of the Bufo bufo gargarizans toad exhibited anti-cancer effects against human breast carcinoma cells by inducing apoptosis, cell cycle arrest and inhibiting metastasis via the inhibition of cell migration and cell invasion of cancer cells [50,51,52,53]. The skin of the frog has been used since ancient times in Chinese Traditional Medicines such as Cinobufacini [51,52,53]. This water soluble extract is a cancer treatment compound which is used widely in China and approved by Chinese State Food and Drug Administration (SFDA) (ISO9002) [52]. The active compounds from Cinobufacini such as bufalin and resibufogenin were found to inhibit the proliferation of a wide range of cancer cell lines such as human hepatocellular carcinoma cells (HEPG2) and prostate adenocarcinoma cells (PC3) [50, 52]. Studies also demonstrated the ability of this compound in inducing apoptosis among human hepatocellular carcinoma cells (SMMC-7721) and gastric carcinoma cells by decreasing the expression of certain anti-apoptotic proteins such as Bcl-2 [52]. Notably, the majority of aforementioned studies have been conducted on human cells exposed to variety of cellular extracts from different organisms. Future studies are needed to test the effects of selected compounds in vitro using primary human cells of relevance as well as in vivo using relevant animal models.
Compounds derived from animals are preferred for anti-tumor therapy as they are natural and can be readily synthesized. Being naturally derived molecules, they are more tolerated and potentially non-toxic to normal human cells, albeit there are exceptions. If animal-derived drugs can demonstrate selectivity in research, are non-toxic to primary cells and show cytotoxicity to cancer cell lines, these drugs can be lead into clinical trials for further therapeutic development. Their potential mode of action is methyltransferase inhibitors, DNA damage preventive drugs or antioxidants, histone deacetylases (HDAC) inhibitors and mitotic disruptors.
In summary this review is timely and topical and further investigation is warranted to explore various animals living in polluted environments as a large untapped source of pharmaceutical drug leads that may lead to the identification of novel antitumor compound(s) and/or mechanisms of cancer resistance for the rational development of therapeutic interventions.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Can 136:359–386
Global Cancer facts and figures (2017) American Cancer Society. https://www.cancer.org/research/cancer-facts-statistics/global.html. Accessed 10 Jan 2017
Rolston KVI (2017) Infections in cancer patients with solid tumors: a review. Infect Dis Ther. doi:10.1007/s40121-017-0146-1 (epub ahead of print)
Ehrhardt H, Pannert L, Pfeiffer S, Wachter F, Amtmann E, Jeremias I (2013) Enhanced anti-tumour effects of Vinca alkaloids given separately from cytostatic therapies. Br J Pharmacol 168:1558–1569
Kang K, Oh SH, Yun JH, Jho EH, Kang JH, Batsuren D, Tunsag J, Park KH, Kim M, Nho CW (2011) A novel topoisomerase inhibitor, Daurinol, suppresses growth of HCT116 cells with low hematological toxicity compared to etoposide. Neoplasia 13:1043IN26–1057IN30
Kampan NC, Madondo MT, McNally OM, Quinn M, Plebanski M (2015) Paclitaxel and its evolving role in the management of ovarian cancer. Biomed Res Int 2015:1–25
Siddiqui R, Mansur S, Khan NA (2016) Do crocodiles and alligators hold the key to treat cancer? BMJ 354:i3763
Lehner AF, Rumbeiha W, Shlosberg A, Stuart K, Johnson M, Domenech R, Langner H (2013) Diagnostic analysis of veterinary dried blood spots for toxic heavy metals exposure. J Anal Toxicol 37:406–422
Schneider L, Peleja RP, Kluczkovski A, Freire GM, Marioni B, Vogt PC, Da Silveira R (2012) Mercury concentration in the spectacled caiman and black caiman (Alligatoridae) of the Amazon: implications for human health. Arch Environ Contam Toxicol 63:270–279
Rainwater TR, Millichamp NJ, Barrantes ID, Barr BR, Montero JR, Platt SG, Abel MT, Cobb GP, Anderson TA (2011) Ocular disease in American crocodiles (Crocodylus acutus) in Costa Rica. J Wildl Dis 47:415–426
Vieira LM, Nunes Vda S, Amaral MC, Oliveira AC, Hauser-Davis RA, Campos RC (2010) Mercury and methyl mercury ratios in caimans (Caiman crocodilus yacare) from the Pantanal area Brazil. J Environ Monitor 13:280–287
Campbell JW, Waters MN, Tarter A, Jackson J (2010) Heavy metal and selenium concentrations in liver tissue from wild American alligator (Alligator mississippiensis) livers near Charleston, South Carolina. J Wildlife Dis 46:1234–1241
Janke A, Gullberg A, Hughes S, Aggarwal RK, Arnason U (2005) Mitogenomic analyses place the gharial (Gavialis gangeticus) on the crocodile tree and provide pre-K/T divergence times for most crocodilians. J Mol Evol 61:620–626
Colbert EH (1997) The age of reptiles. Generating Publishing Company (ISBN 0-486-29377-7)
Dietz J, Heckers KO, Aupperle H, Pees M (2016) Cutaneous and subcutaneous soft tissue tumours in snakes: a retrospective study of 33 cases. J Comp Pathol 155:76–87
Eyarefe OD, Antia RE, Oguntoye CO, Abiola OO, Alaka OO, Ogunsola JO (2012) Rhabdomyosarcoma in a terrestrial tortoise (Geochelone nigra) in Nigeria: a case report. J South Afr Vet Assoc. doi:10.4102/jsava.v83i1.300
Janert B (1998) A fibrosarcoma in a Siamese crocodile (Crocodylus siamensis). J Zoo Wildlife Med 29:72–77
Martorell J, Ramis A, Espada Y (2002) Use of ultrasonography in the diagnosis of hepatic spindle-cell sarcoma in a savannah monitor (Varanus exanthematicus). Vet Rec 150:282–284
Leone A, Dark M, Kondo H, Rotstein DS, Kiupel M, Walsh MT, Erlacher-Reid C, Gordon N, Conway JA (2013) Gastrointestinal leiomyosarcoma in a pygmy sperm whale (Kogia breviceps). J Zoo Wildlife Med 44:744–748
Manire CA, Clarke AC, Wert D, Landolfi J (2013) Lymphosarcoma in a captive bonnethead shark, Sphyrna tiburo (L.). J Fish Dis 36:437–440
Sulak M, Fong L, Mika K, Chigurupati S, Yon L, Mongan NP, Emes RD, Lynch VJ (2016) TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. eLife. doi:10.7554/eLife.11994 (epub ahead of print)
Keane M, Semeiks J, Webb AE, Li Y, Quesada V, Craig T, Madsen LB, van Dam S, Brawand D, Marques PI, Michalak P, Kang L, Bhak J, Yim HS, Grishin NV, Nielsen NH, Heide-Jørgensen MP, Oziolor EM, Matson CW, Church GM, Stuart GW, Patton JC, George JC, Suydam R, Larsen K, López-Otín C, O’Connell MJ, Bickham JW, Thomsen B, de Magalhães JP (2015) Insights into the evolution of longevity from the bowhead whale genome. Cell Rep 10:112–122
Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, Kiso WK, Schmitt DL, Waddell PJ, Bhaskara S, Jensen ST, Maley CC, Schiffman JD (2015) Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. JAMA 314:1850–1860
Patathananone S, Thammasirirak S, Daduang J, Gung CJ, Temsiripong Y, Daduang S (2016) Inhibition of HeLa cells metastasis by bioactive compounds in crocodile (Crocodylus siamensis) white blood cells extract. Environ Toxicol 31:1329–1336
Patathananone S, Thammasirirak S, Daduang J, Chung JG, Temsiripong Y, Daduang S (2016) Bioactive compounds from crocodile (Crocodylus siamensis) white blood cells induced apoptotic cell death in hela cells. Environ Toxicol 31:986–997
Theansongnoen T, Maijaroen S, Jangpromma N, Yaraksa N, Daduang S, Temsiripong T, Daduang J, Klaynongsruang S (2016) Cationic Antimicrobial peptides derived from Crocodylus siamensis leukocyte extract, revealing anticancer activity and apoptotic induction on human cervical cancer cells. Protein J 35:202–211
Song W, Shen DY, Kang JH, Li SS, Zhan HW, Shi Y, Xiong YX, Liang G, Chen QX (2012) Apoptosis of human cholangiocarcinoma cells induced by ESC-3 from Crocodylus siamensis bile. World J Gastroenterol 18:704–711
Wang DQ, Carey MC (2014) Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol 20:9952–9975
Kang JH, Zhang WQ, Song W, Shen DY, Li SS, Tian L, Shi Y, Liang G, Xiong YX, Chen QX (2012) Apoptosis mechanism of human cholangiocarcinoma cells induced by bile extract from crocodile. Appl Biochem Biotechnol 166:942–951
Song W, Li SS, Qiu PP, Shen DY, Tian L, Zhang QY, Liao LX, Chen QX (2013) apoptosis induced by aqueous extracts of crocodile bile in human heptacarcinoma SMMC-7721. Appl Biochem Biotechnol 170:15–24
Shen DY, Zhang W, Zeng X, Liu CQ (2013) Inhibition of Wnt⁄b-catenin signaling downregulates P-glycoprotein and reverses multi-drug resistance of cholangiocarcinoma. JCA 104:1303–1308
Albert DS, Martinez ME, Hess LM, Einspahr JG, Green SB, Bhattacharyya AK, Guillen J, Krutzsch M, Batta AK, Salen G, Fales L, Koonce K, Parish D, Clouser M, Roe D, Lance P (2005) Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst 97:846–853
Ajouz H, Mukherji D, Shamseddine A (2014) Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol. doi:10.1186/1477-7819-12-164 (epub ahead of print)
Chaisakul J, Hodgson WC, Kuruppu S, Prasongsook N (2016) Effects of animal venoms and toxins on hallmarks of cancer. J Ca 7:1571–1578
Azevedo FV, Lopes DS, Cirilo Gimenes SN, Achê DC, Vecchi L, Alves PT, Guimarães Dde O, Rodrigues RS, Goulart LR, Rodrigues Vde M, Yoneyama KA (2016) Human breast cancer cell death induced by BnSP-6, a Lys-49 PLA2 homologue from Bothrops pauloensis venom. Int J Biol Macromolec 82:671–677
Bazaa A, Luis J, Srairi-Abid N, Kallech-Ziri O, Kessentini-Zouari R, Defilles C, Lissitzky JC, El Ayeb M, Marrakchi N (2009) MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol 28:188–193
Ebrahim K, Shirazi FH, Mirakabadi AZ, Vatanpour H (2015) Cobra venom cytotoxins; apoptotic or necrotic agents? Toxicon 108:134–140
Tsai PC, Chu CL, Chiu CC, Chang LS, Lin SR (2014) Cardiotoxin III suppresses hepatocyte growth factor-stimulated migration and invasion of MDA-MB-231 cells. Cell Biochem Funct 32:485–495
Ding XL, Man YN, Hao J, Zhu CH, Liu C, Yang X, Wu XZ (2016) The Antitumor Effect of Gekko Sulfated Glycopeptide by Inhibiting bFGF-Induced Lymphangiogenesis. Biomed Res Int. doi:10.1155/2016/7396392 (epub ahead of print)
Keane M, Craig T, Alföldi J, Berlin AM, Johnson J, Seluanov A, Gorbunova V, Di Palma F, Lindblad-Toh K, Church GM, de Magalhães JP (2014) The naked mole rat genome resource: facilitating analyses of cancer and longevity-related adaptations. Bioinformatics 30:3558–3560
Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A (2013) High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499(7458):346–349
Amiri A, Namavari M, Rashidi M, Fahmidekhar MA, Seghatoleslam A (2015) Inhibitory effects of Cyrtopodion scabrum extract on growth of human breast and colorectal cancer cells. APJCP 16:565–570
Zhang SX, Zhu C, Ba Y, Chen D, Zhou XL, Cao R, Wang LP, Ren Y, Wu XZ (2012) Gekko-sulfated glycopeptide inhibits tumor angiogenesis by targeting basic fibroblast growth factor. J Biol Chem 287:13206–13215
Jiang JJ, Cheng LH, Wu H, He YH, Kong QP (2016) Insights into long noncoding RNAs of naked mole rat (Heterocephalus glaber) and their potential association with cancer resistance. Epigenetics Chromatin 9:1–10
Piersigilli A, Meyerholz DK (2016) The ‘‘Naked Truth’’: naked mole-rats do get cancer. Vet Pathol 53:519–520
Tian X, Azpurua J, Ke Z, Augereau A, Zhang ZD, Vijg J, Gladyshev VN, Gorbunova V, Seluanov A (2014) INK4 locus of the tumor-resistant rodent, the naked mole rat, expresses a functional p15/p16 hybrid isoform. PNAS 112:1053–1058
Taylor KR, Milone NA, Rodriguez CE (2017) four cases of spontaneous neoplasia in the naked molerat (Heterocephalus glaber), a putative cancer-resistant species. J Gerontol A Bio Sci 72:38–43
Gonzales JA, Amich F, Postigo-Mota S, Vallejo JR (2016) The use of wild vertebrates in contemporary Spanish ethno veterinary medicine. J Ethnopharmacol 191:135–151
Rodriguez C, Rollins-Smith L, Ibanez R, Durant-Archibold AA, Gutierrez M (2016) Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura). J Ethnopharmacol. doi:10.1016/j.jep.2016.12.021 (epub ahead of print)
Cruz e Carvalho A, Márquez CA, Azevedo RB, Joanitti GA, Pires Júnior OR, Fontes W, Castro MS (2015) Cytotoxic activity and antiproliferative effects of crude skin secretion from Physalaemus nattereri (Anura: Leptodactylidae) on in vitro melanoma cells. Toxins 7:3989–4005
Liu Y, Ban LY, Su X, Gao S, Liu JW, Cui XN (2015) Effects of cinobufacini injection on cell proliferation and the expression of topoisomerases in human HepG-2 hepatocellular carcinoma cells. Mol Med Rep 12:1598–1604
Nakata M, Mori S, Kamoshida Y, Kawaguchi S, Fujita-Yamaguchi Y, Gao B, Tang W (2015) Toad skin extract cinobufatini inhibits migration of human breast carcinoma MDA-MB-231 cells into a model stromal tissue. Biosci Trends 9:266–269
Qi F, Inagaki Y, Kokudo N, Tamura S, Nakata M, Tang W (2011) Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Inter Pharmacol 11:342–349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SJ declares that she has no conflict of interest. NAK declares that he has no conflict of interest. RS declares that she has no conflict of interest.
Funding
This study was funded by Sunway University, Malaysia, grant FST-2015-05.
Ethical approval
This article does not contain any studies with human participants and animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jeyamogan, S., Khan, N.A. & Siddiqui, R. Animals living in polluted environments are a potential source of anti-tumor molecule(s). Cancer Chemother Pharmacol 80, 919–924 (2017). https://doi.org/10.1007/s00280-017-3410-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3410-x