Abstract
Purpose
Fluorouracil and folinic acid with irinotecan (FOLFIRI) plus bevacizumab (BV) is widely used as second-line chemotherapy for patients with metastatic colorectal cancer (mCRC) previously treated with fluoropyrimidines, oxaliplatin, and BV. FOLFIRI requires a CV catheter and an infusion pump, which are inconvenient for patients. Sufficient data are not available for characterizing the effectiveness of fluoropyrimidines beyond first disease progression. In this study, we evaluated the efficacy and safety of irinotecan (CPT-11) plus BV as second-line therapy.
Methods
Patients with mCRC previously treated with at least four courses of a fluoropyrimidine, oxaliplatin, and BV were designated to receive 150 mg/m2 of CPT-11 and 10 mg/kg of BV every 2 weeks as second-line therapy. The primary endpoint was progression-free survival (PFS), and secondary endpoints included response rate (RR), overall survival (OS), and adverse events.
Results
Thirty patients from six institutes were enrolled from March 2011 to January 2014. The median PFS was 5.7 months (95% CI 4.2–7.3 months), and the RR was 6.7% (range 0.8–22.1%). Grades 3–4 adverse events included leucopenia (36.7%), neutropenia (50%), thrombocytopenia (26.7%), anemia (30%), diarrhea (3.3%), anorexia (6.7%), and hypertension (3.3%). Relative dose intensities were 94.5 and 96.3% for CPT-11 and BV, respectively. The median OS was 11.8 months (6.3 months—not reached).
Conclusions
Administration of CPT-11 plus BV to patients with mCRC achieved comparable efficacies with relatively lower toxicities compared with the results of previous studies using FOLFIRI plus BV as second-line therapy. The dose intensity of CPT-11 was judged as satisfactory.
Clinical trial information
UMIN000005228
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Tournigand et al. reported that patients with metastatic colorectal cancer (mCRC) treated with the sequence of fluorouracil (5-FU), folinic acid plus oxaliplatin (FOLFOX), 5-FU and folinic acid plus irinotecan (FOLFIRI) achieved an overall survival (OS) equivalent to that using FOLFIRI followed by FOLFOX [1], FOLFIRI is widely used as second-line chemotherapy for patients with mCRC treated with FOLFOX as first-line therapy. Bevacizumab (BV) is effective when used continuously through first-line to second-line therapies beyond progression [2]. Therefore, FOLFIRI plus BV is regarded as a standard second-line regimen for patients previously treated with FOLFOX plus BV.
However, administration of FOLFIRI and FOLFOX requires a central venous (CV) catheter and continuous infusion of 5-FU, which is inconvenient for patients and diminishes their quality of life. Thus, regimens comprising oral fluoropyrimidines such as capecitabine and S-1 were implemented to replace continuous infusion of 5-FU. A phase III trial found that the S-1 plus irinotecan (CPT-11) regimen achieves noninferior progression-free survival (PFS) compared with FOLFIRI as second-line chemotherapy [3]. Although a CV catheter and an infusion pump are not required for this regimen, they are associated with a high frequency of gastrointestinal toxicities such as diarrhea.
Available information is insufficient to determine whether fluoropyrimidines should be continuously used in second-line chemotherapy, even if previously used in first-line therapy. Long-term administration of fluoropyrimidines causes hand and foot syndromes, skin disorders, and lacrimal drainage disorder, which may diminish a patient’s quality of life [4, 5]. Further, fluoropyrimidines induce neutropenia, particularly when administered as a bolus infusion that is occasionally required to reduce the dose of CPT-11 [3]. If fluoropyrimidines can be omitted from irinotecan-based second-line treatment, the frequency of gastrointestinal toxicities and neutropenia may be reduced, leading to an elevation of the dose intensity of CPT-11. To our knowledge, sufficient data are not available to indicate the efficacy and safety of the combination of CPT-11 plus BV for treating mCRC, although this regimen is described as one of the standard second-line regimens in the National Comprehensive Cancer Network (NCCN) guidelines [6].
To fill this gap in our knowledge, we conducted a phase II study to evaluate a regimen comprising CPT-11 plus BV as second-line chemotherapy for patients with mCRC previously treated with fluoropyrimidine, oxaliplatin, and BV.
Patients and methods
Patient eligibility
Inclusion criteria were as follows: histologically confirmed colorectal adenocarcinoma; unresectable metastatic disease; age >20 years; Eastern Cooperative Oncology Group (ECOG) performance status = 0 or 1; received at least four courses of first-line chemotherapy, including fluoropyrimidine, oxaliplatin, and bevacizumab; withdrawal from first-line chemotherapy because of toxicity or progressive disease and received this protocol as second-line chemotherapy; estimated life expectancy >3 months; and adequate bone marrow, hepatic, and renal functions. Adjuvant chemotherapy was not designated as first-line chemotherapy, and a measurable region was not mandatory. Exclusion criteria were as follows: prior administration of CPT-11; complications such as brain metastasis, ileus, active gastrointestinal ulcer, uncontrollable hypertension, and active infectious disease; severe diarrhea; clinically evident gastrointestinal hemorrhage; ascites or pleural effusion requiring drainage; and history of gastrointestinal perforation.
The study protocol was approved by the institutional ethics committee of the respective institution. The study complied with the Declaration of Helsinki. Written informed consent was obtained from all the participating patients.
Treatment protocol
Patients received a 10–30-min infusion of 10 mg/kg bevacizumab, followed by a 90-min infusion of 150 mg/m2 of CPT-11, which was repeated every 2 weeks until disease progression. The dose of CPT-11 was 150 mg/m2, because this is the maximum dose approved by the Japanese government. The dose of BV was 10 mg/m2 according to the evidence reported by the E3200 study [7]. Patients who harbored three allelic variants of the gene encoding UDP glucuronosyltransferase family 1 member A1 (UGT1A1) (UGT1A1*6, homozygous; UGT1A1*28, homozygous; or UGT1A1*6, 28 double heterozygous) were allowed to decrease the starting dose of CPT-11 to 100 mg/m2. Treatment with CPT-11 was delayed if neutrophil counts fell below 1000/mm3, platelet counts fell below 80,000/mm3, total bilirubin was higher than 2.0 mg/dL, aspartate aminotransferase (AST)/alanine and aminotransferase (ALT) were higher than 100 U/L (>200 U/L if the patient had liver metastasis), or if the patient experienced diarrhea greater than grade 2. If a patient experienced grade-4 neutropenia = thrombocytopenia grade 3 or higher, febrile neutropenia grade 3 or higher, or any nonhematological toxicities greater than grade 3; the dose of CPT-11 was decreased by one level (Level-1, 125 mg/m2; Level-2, 100 mg/m2; or Level-3, 75 mg/m2) for the next course of treatment. BV was temporally discontinued for patients with hypertension grade 3 or higher, proteinuria grade 2 or higher, or hemorrhage grade 2 or higher. If the patient experienced hypertension grade 3 or higher, proteinuria grade 3, the dose of bevacizumab was decreased by one level (Level-1, 7.5 mg/kg; Level-2, 5 mg/kg). When administration of CPT-11 was delayed, BV administration was delayed (administration of BV alone was not allowed). This protocol was terminated if the patient experienced disease progression, unacceptable toxicity, withdrawal of consent, or if any physician decided to terminate the protocol.
Endpoints and procedures
The primary endpoint of this study was PFS. The secondary endpoints were response rate (RR), OS, and the frequency of adverse events. During the 4 weeks before chemotherapy commenced, each patient underwent a physical examination and the analyses as follows: complete blood cell count, hepatic and renal function, urine analysis, electrocardiography, and chest and abdominal computed tomography (CT) or magnetic resonance imaging (MRI). Blood cell count, laboratory tests, and urine analyses were performed every 2 weeks during treatment. CT/MRI was performed every 6 weeks until disease progression. Progression was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, clinical progression judged by the investigator, or death from any cause.
PFS was calculated from the date of registration to the date of progression. OS was defined as the date of registration to the date of death from any cause. OS for first-line treatment was defined as the date of commencement of first-line chemotherapy to death. Tumor response was evaluated according to RECIST version 1.1, and toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
Statistical analysis
This study was a multicenter single-arm phase II trial. A previous clinical trial reported 2.5 months PFS for patients administered with CPT-11 monotherapy as second-line treatment [8]. When bevacizumab was added to CPT-11, we expected a median PFS = 4.0 months. Accordingly, 30 events were required to estimate the 95% confidence interval (CI) of the median PFS within the range 4.0 ± 1.9 months. The number of patients was therefore defined as 33, considering possible ineligibility or exclusion of patients from the analysis. Cumulative survival was estimated using the Kaplan–Meier method. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients’ characteristics
Between April 2011 and March 2015, the 30 patients enrolled in this trial from six institutions in Japan were assessed for responses to therapy and adverse effects. Although the criterion of 33 patients defined by the protocol for statistical analysis was not achieved, the required number of events (n = 30) was observed. Patients’ characteristics are listed in Table 1. Twenty-five (83.3%) patients were refractory to first-line therapy, and five patients (16.6%) did not tolerate first-line therapy. The median follow-up was 17.0 months, and the median number of treatment courses was 9.
Efficacy
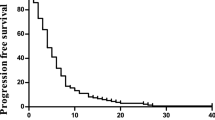
The percentages of the relative dose intensities (RDI) of the planned dose were 100% (median) and 94.5% (mean) for CPT-11, and 100% (median) and 96.3% (mean) for BV. Tumor responses are summarized in Table 2. RR was 6.7% (95% CI 0.8–22.1%), and the disease control rate (DCR) was 86.7% (95% CI 74.5–98.8%). The median PFS was 5.7 months (95% CI 4.2–7.3) (Fig. 1). PFS did not significantly differ between patients who were refractory to first-line therapy (n = 25) and patients who did not tolerate first-line therapy (n = 5) (PFS median 5.86 versus 5.50 months, respectively). Median OS from the start of this protocol was 11.8 months (95% CI 6.7–not reached) (Fig. 2). The median OS from the start of first-line treatment was 30.1 months.
Adverse events
Adverse events are summarized in Table 3. Hematological adverse events ≥ grade 3 were as follows: 36.7% decrease in white blood cell count, 50% decrease in neutrophil count, febrile neutropenia = 6.7, 26.7% decrease in platelet count, anemia = 30%, and 6.7 and 3.3% increases in AST and ALT, respectively. Although there was a relatively high incidence of decreased grade-3 neutrophil counts, grade-4 neutropenia was not observed. The incidence of nonhematological adverse events was relatively low. Nonhematological adverse events ≥grade 3 were fatigue, 3.3%; anorexia, 6.7%; diarrhea, 3.3%; and hypertension 3.3%. No patient died because of treatment.
Discussion
CPT-11 plus BV second-line regimen administered in the present study to patients with mCRC achieved a median PFS = 5.7 months and a median OS = 11.8 months. When this study was planned, the efficacy of the continuous use of BV as second-line therapy beyond tumor progression was not confirmed by a prospective randomized clinical trial, although a large retrospective analysis indicated the effectiveness of the continuous administration of BV [9]. Subsequently, the Arbeitsgemeinschaft Internistische Onkologie and Roche conducted the ML18147 study, which compared chemotherapy (FOLFOX or FOLFIRI) plus BV with chemotherapy alone as second-line therapy of patients with mCRC previously treated with chemotherapy plus BV [2]. The data show that the continuous administration of BV beyond progression is clinically beneficial [2]. Specifically, median PFS and OS were 5.7 months and 11.2 months, respectively, for the chemotherapy plus BV arm, which are very similar to the results of the present study [2]. In the ML 18,147 study, the chemotherapy-alone (BV omitted) arm achieved a median PFS of 4.2 months, suggesting that our regimen provided an additional survival benefit conferred by BV.
Table 4 shows the results of phase II/III studies of second-line regimens comprising fluoropyrimidine and CPT-11 plus BV. The EAGLE trial [10], which compared the effects of the administration of 5 mg/kg of BV and 10 mg/kg of BV with FOLFIRI, reported median PFS times of 6.4 months and 6.1 months for the 10 and 5 mg/kg arms, respectively. The KSCC1102 trial evaluated S-1 and CPT-11 plus bevacizumab and reported a median PFS of 5.6 months [11]. The WJOG 6210G study compared FOLFIRI plus BV with FOLFIRI plus cetuximab in a phase II second-line setting and reported a median PFS of 5.9 months for the BV arm [12]. Although the present phase II study included a small number of subjects, the median PFS of 5.7 months is comparable with these studies, suggesting that there was no severe reduction of PFS, even if fluoropyrimidine was omitted.
In the present study, PFS of patients refractory to first-line therapy and those intolerant did not significantly differ, although the number of the latter was small. We first hypothesized that those who did not tolerate first-line therapy, mainly because of the toxicity of oxaliplatin, might not receive a sufficient dose of fluoropyrimidines during first-line therapy, which may be disadvantageous when fluoropyrimidine was omitted from second-line therapy. Similarly, we were unable to identify any other clinicopathological subgroups of patients who were disadvantaged because of the lack of fluoropyrimidine (data not shown).
In the present study, the median OS of 11.8 months was calculated from the start of second-line regimen to death, which is relatively shorter compared with those of the studies listed in Table 4. However, in the present study, the median OS from the start of first-line therapy was 30.1 months, which is not inferior to the results of the recent SOFT [13] and WJOG 4407 trials [5]. Thus, we assume that the relatively shorter OS reported here cannot be attributed to the lack of effectiveness of this regimen and may be explained by the late timing of the switch from first-line to second-line chemotherapy. Further, 6.7% RR is similar to 5.5% and 5.7% RRs reported by the ML18147 and WJOG 6210G studies, respectively [2, 12]. Therefore, the continuous use of a fluoropyrimidine may not contribute to tumor shrinkage during second-line therapy.
Another option to avoid using a CV catheter and carrying an infusion pump is to administer oral fluoropyrimidines such as capecitabine and S-1 in combination with CPT-11. However, an oral fluoropyrimidine combined with CPT-11 causes a high frequency of adverse gastrointestinal events. Fuchs et al. conducted a phase III study that compared capecitabine+CPT-11 (CapeIRI) with FOLFIRI and irinotecan plus bolus FU/LV (mIFL) [14] and found that CapeIRI was less effective than FOLFIRI because of the high rate of gastrointestinal toxicity. Muro et al. reported that the combination of S-1 and CPT-11 (IRIS) was not less effective than FOLFIRI as second-line therapy [3]; however, the frequency of grade-3 diarrhea was 20.5% in the IRIS regimen, which is fourfold higher compared with FOLFIRI (4.7%). In the present study, 3.3% of patients experienced grade-3 diarrhea, which is much lower compared with the findings of the IRIS or CapeIRI study, and comparable with FOLFIRI. Other gastrointestinal toxicities as well as skin disorders were relatively infrequent, because a fluoropyrimidine was not included.
The frequency of the UGT1A1*6 polymorphism is higher in Asians compared with Caucasians. Specifically, the frequencies of the *6 polymorphism range from 18 to 23%, although the frequencies of the *28 polymorphism range from 4–6% [15–17]. The *6 polymorphism is associated with toxicities caused by CPT-11, particularly severe neutropenia [18]. Thus, the average tolerable dose of CPT-11 may be lower in Asians compared to those tolerated by patients living in western countries. Therefore, the maximum dose of CPT-11 covered by insurance provided by the Japanese government is 150 mg/m2.
In sequential treatment using FOLFOX (+BV) followed by FOLFIRI (+BV), the newly introduced drug in second-line therapy is CPT-11. Therefore, CPT-11 is the most important drug for which the dose intensity should be retained. However, FOLFIRI sometimes causes neutropenia, and therefore the dose of CPT-11 should be reduced. In the present study, because fluoropyrimidine was not included, the mean RDI of CPT-11 was 94.5%, which is satisfactory. Although grade-3 neutropenia occurred in 50% of patients, grade-4 neutropenia was not observed, and dose modification was unnecessary. The satisfactory dose of CPT-11 administered here may have contributed to PFS values similar to those achieved using other fluoropyrimidine-containing regimens (Table 4). We used 150 mg/m2 of CPT-11 here, because this is the maximum dose approved in Japan. However, it might be possible to increase the initial dose of CPT-11, even for Asian patients, because of the low frequency of adverse events.
In the present study, we used 10 mg/kg of BV according to the evidence reported by the E3200 study [7]. Subsequently, Iwamoto et al. reported the results of the EAGLE study that compared 5 versus 10 mg/kg of BV in combination with FOLFIRI in a second-line setting [10]. OS was the primary endpoint and was similar in both arms, leading the investigators to conclude that 5 mg/kg of BV was sufficient. Therefore, when we use CPT-11 plus BV in the clinic, 5 mg/kg of BV may be acceptable.
In conclusion, the CPT-11 plus BV regimen achieved acceptable PFS associated with a decreased frequency of adverse events compared with other regimens containing a fluoropyrimidine, CPT-11, and BV in a second-line setting. Although we are unable conclude from this small single-arm phase II trial that fluoropyrimidine is unnecessary in this setting, we judged the CPT-11 plus BV regimen as acceptable for patients who continuously suffer from fluoropyrimidine-related toxicities or those who cannot continue to receive the prescribed dose of CPT-11 because of bone marrow suppression.
References
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. doi:10.1200/JCO.2004.05.113
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, Schlichting C, Reyes-Rivera I, Bendahmane B, Andre T, Kubicka S (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14(1):29–37. doi:10.1016/S1470-2045(12)70477-1
Muro K, Boku N, Shimada Y, Tsuji A, Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, Yamaguchi K, Takiuchi H, Esaki T, Tokunaga S, Kuwano H, Komatsu Y, Watanabe M, Hyodo I, Morita S, Sugihara K (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11(9):853–860. doi:10.1016/S1470-2045(10)70181-9
Hamamoto Y, Yamaguchi T, Nishina T, Yamazaki K, Ura T, Nakajima T, Goto A, Shimada K, Nakayama N, Sakamoto J, Morita S, Yamada Y (2014) A phase I/II study of XELIRI plus bevacizumab as second-line chemotherapy for Japanese patients with metastatic colorectal cancer (BIX study). Oncologist 19(11):1131–1132. doi:10.1634/theoncologist.2014-0159
Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I (2016) Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 27(8):1539–1546. doi:10.1093/annonc/mdw206
Network NCC (2017) Clinical Practice Guidelines in Oncology, Colon Cancer (Ver.1.2017). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 25 December 2016
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544. doi:10.1200/JCO.2006.09.6305
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA 3rd (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26(14):2311–2319. doi:10.1200/JCO.2007.13.1193
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26(33):5326–5334. doi:10.1200/JCO.2008.16.3212
Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, Hata T, Denda T, Morita Y, Inukai M, Kunieda K, Nagata N, Kurachi K, Ina K, Ooshiro M, Shimoyama T, Baba H, Oba K, Sakamoto J, Mishima H (2015) FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol 26(7):1427–1433. doi:10.1093/annonc/mdv197
Miyamoto Y, Tsuji A, Tanioka H, Maekawa S, Kawanaka H, Kitazono M, Oki E, Emi Y, Murakami H, Ogata Y, Saeki H, Shimokawa M, Natsugoe S, Akagi Y, Baba H, Maehara Y (2016) S-1 and irinotecan plus bevacizumab as second-line chemotherapy for patients with oxaliplatin-refractory metastatic colorectal cancer: a multicenter phase II study in Japan (KSCC1102). Int J Clin Oncol 21(4):705–712. doi:10.1007/s10147-015-0943-z
Shitara S, Yonesaka K, denda T, Yamazaki K, Moriwaki T, Tsuda M, Takano T, Okuda H (2016) A randomized multicenter phase II study of FOLFIRI plus either panitumumab (Pmab) or bevacizumab (Bmab) as second-line treatment for wild-type KRAS exon 2 metastatic colorectal cancer (mCRC) with exploratory biomarker analysis by liquid biopsy: WJOG6210G. J Clin Oncol 34 (suppl; abstr 3567)
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y, Sasaki Y, Satoh T, Takahashi K, Mishima H, Muro K, Watanabe M, Sakata Y, Morita S, Shimada Y, Sugihara K (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14(13):1278–1286. doi:10.1016/S1470-2045(13)70490-X
Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, Barrueco J (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25(30):4779–4786. doi:10.1200/JCO.2007.11.3357
Cheng L, Li M, Hu J, Ren W, Xie L, Sun ZP, Liu BR, Xu GX, Dong XL, Qian XP (2014) UGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: a system review and meta-analysis in Asians. Cancer Chemother Pharmacol 73(3):551–560. doi:10.1007/s00280-014-2382-3
Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Jang IJ, Lee DH, Lee JS (2006) Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol 24(15):2237–2244. doi:10.1200/JCO.2005.03.0239
Han FF, Guo CL, Yu D, Zhu J, Gong LL, Li GR, Lv YL, Liu H, An GY, Liu LH (2014) Associations between UGT1A1*6 or UGT1A1*6/*28 polymorphisms and irinotecan-induced neutropenia in Asian cancer patients. Cancer Chemother Pharmacol 73(4):779–788. doi:10.1007/s00280-014-2405-0
Onoue M, Terada T, Kobayashi M, Katsura T, Matsumoto S, Yanagihara K, Nishimura T, Kanai M, Teramukai S, Shimizu A, Fukushima M, Inui K (2009) UGT1A1*6 polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients. Int J Clin Oncol 14(2):136–142. doi:10.1007/s10147-008-0821-z
Acknowledgements
We thank Naoko Kamba for data management and Edanz for English editing. This study was supported by funds from the Department of Chemotherapy and Palliative Care, Tokyo Women’s Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kuramochi, H., Ando, M., Itabashi, M. et al. Phase II study of bevacizumab and irinotecan as second-line therapy for patients with metastatic colorectal cancer previously treated with fluoropyrimidines, oxaliplatin, and bevacizumab. Cancer Chemother Pharmacol 79, 579–585 (2017). https://doi.org/10.1007/s00280-017-3255-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3255-3