Abstract
Background
Combination chemotherapy with S-1 and irinotecan is one of the standard treatments for metastatic colorectal cancer (mCRC) in Japan. However, there are few alternative practical second-line therapies. We conducted a phase II trial to evaluate the efficacy and safety of the combination of S-1 and irinotecan plus bevacizumab as a second-line treatment for oxaliplatin-refractory mCRC.
Methods
Patients with mCRC who were previously treated with oxaliplatin-containing regimens were enrolled. Oral S-1 at a dose of 80 mg/m2 was administered twice daily for 2 weeks, followed by a 1-week drug-free interval. Irinotecan at a dose of 150 mg/m2 and bevacizumab at a dose of 7.5 mg/kg were administered on day 1. The primary endpoint was progression-free survival (PFS).
Results
Thirty-seven patients were enrolled, and 34 and 36 patients were assessed for response and safety, respectively. The overall response rate was 20.6 % (95 % confidence interval [CI] 8.7–37.9), and the disease control rate was 76.5 % (95 % CI 58.8–89.3). The median PFS was 5.6 months (95 % CI 3.8–7.0). The median overall survival was 16.4 months (95 % CI 8.1–20.0). The most common grade 3/4 adverse events included neutropenia (25.0 %), anorexia (22.2 %), anemia (16.7 %), and fatigue/malaise (16.7 %). The most common grade 3/4 adverse event of special interest for bevacizumab was hypertension (30.6 %). One treatment-related death caused by gastrointestinal bleeding occurred.
Conclusions
The findings suggest that the combination of S-1 and irinotecan plus bevacizumab is effective and tolerable as second-line chemotherapy for patients with oxaliplatin-refractory mCRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S-1 is an oral fluoropyrimidine preparation that combines tegafur with two 5-fluorouracil modulators—gimeracil (5-chloro-2,4-dihydroxypyridine) and oteracil potassium—in a molar ratio of 1.0:0.4:1.0 [1–4]. Several studies suggested that combination treatment with S-1 and irinotecan ± bevacizumab as first-line chemotherapy is an effective, well-tolerated, and convenient regimen in patients with metastatic colorectal cancer (mCRC) [5–8]. Furthermore, phase II/III studies confirmed that the irinotecan and S-1 (IRIS) regimen was equivalent to FOLFIRI as second-line chemotherapy in terms of both efficacy and safety [9]. The IRIS regimen is a current standard therapy for mCRC in Japan and Europe [10].
Currently, bevacizumab is widely used as a chemotherapeutic agent for colorectal cancer globally [11, 12]. However, little is known regarding the use of irinotecan and S-1 plus bevacizumab as second-line chemotherapy. The magnitude of benefit may differ greatly based on the chemotherapy regimen with which bevacizumab is partnered [13].
In this study, we evaluated the combination of S-1 and irinotecan plus bevacizumab as a second-line treatment for mCRC in a phase II study.
Patients and methods
Patient eligibility
All patients entered in this study had histologically confirmed advanced or mCRC measurable on the basis of the Response Evaluation Criteria in Solid Tumors Group (RECIST) criteria. The eligibility criteria included age >20 years, estimated life expectancy of >3 months, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, withdrawal from first-line oxaliplatin-based chemotherapy because of intolerable toxicity or progressive disease or relapse within 180 days after the last dose of oxaliplatin-based adjuvant chemotherapy, the ability to tolerate oral intake, and adequate bone marrow, hepatic, and renal function. Prior bevacizumab was permitted, but not prior irinotecan. Both UGT1A1*28 and *6 polymorphisms were permitted if they were wild-type or heterozygous type.
Patients were excluded from this study for any of the following reasons—symptomatic infectious disease, bleeding tendency, severe heart disease, active double cancer, symptomatic ascites, pregnancy, breast feeding, obstructive bowel disease, previous treatment with irinotecan hydrochloride, history of hemoptysis grade ≥2, radiological evidence of brain tumor or brain metastases, uncontrolled hypertension, severe diabetes mellitus requiring insulin, or past history of drug allergy.
The protocol of this study was approved by the institutional review board or ethics committee of each institution. The study was conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all patients participating in the study.
Treatment plan
Bevacizumab at a dose of 7.5 mg/kg was administered as a 30- to 90-min infusion and irinotecan at a dose of 150 mg/m2 was administered as a 90-min infusion every 3 weeks. The dose of S-1 was determined according to the patient’s body surface area (BSA). Specifically, the drug was administered orally twice daily for 14 consecutive days at a dose that did not exceed 40 mg/m2 based on BSA as follows—BSA <1.25 m2, 40 mg; BSA 1.25–1.5 m2, 50 mg twice daily; and BSA >1.5 m2, 60 mg. Premedication with a 5-hydroxytryptamine-3-receptor antagonist combined with dexamethasone ± an NK-1 antagonist was recommended in patients before the administration of irinotecan. This treatment was administered until disease progression, unacceptable toxicity, withdrawal of consent, or the physician’s decision to terminate treatment.
Dose modification schedule
Treatment with irinotecan or/and S-1 was delayed or modified on the basis of any observed toxicity. If the neutrophil count decreased to <1,000 cells per μL, the platelet count fell to <50,000 per μL, creatinine levels >1.5 mg/dL, infection-associated symptoms were identified, or other non-hematological toxicities of grade >2 according to the National Cancer Institute Common Toxicity criteria scale, version 4.0 occurred, then subsequent courses of treatment were withheld until the resolution of these adverse events to comply with the eligibility criteria. Bevacizumab administration was temporarily discontinued in patients with hypertension grade ≥3, proteinuria grade ≥2, hemorrhagegrade ≥2, or evidence of thrombosis.
The dose was modified for each patient based on hematologic or non-hematologic toxicities. The dose of irinotecan in the subsequent course was reduced to 125 mg/m2 and that of S-1 was reduced by one dose level (from 80 to 60 mg/day, from 100 to 80 mg/day, or from to 100 mg/day) for any of the following reasons—neutrophil count <500/μL, platelet count <10,000/μL, infectious neutrophil count <1,000 IU/L, or grade ≥3 non-hematological toxicity. If irinotecan was not tolerated at a dose of 125 mg/m2, then the dose was reduced to 100 mg/m2. If S-1 was not tolerated even after a dose reduction of one level, the dose was further reduced from 80 to 60 mg/day or from 100 to 80 mg/day. No dose modification of bevacizumab was performed. The treatment cycle could be resumed in subsequent cycles if the adverse events were resolved within 4 weeks after the last dose of S-1 was administered. If the minimum dose of S-1 was poorly tolerated, the patient was excluded from further study. Once lowered, the doses of S-1 and irinotecan were not increased.
Endpoints
The primary endpoint of the study was progression-free survival (PFS), and the secondary endpoints were the objective response rate (ORR), overall survival (OS), time to treatment failure, and adverse effects. During the 4 weeks before chemotherapy commenced, all patients underwent the following examinations—physical examination, complete blood cell count, hepatic and renal function tests, and chest and abdominal computed tomography or magnetic resonance imaging. A physical examination, hepato-renal function tests, and blood counts were performed before every cycle. Patients were assessed before starting each 3-week cycle according to the National Cancer Institute-Common Toxicity Criteria version 3. Tumor evaluation was performed every month for the first 3 months and then every 2 months thereafter using RECIST ver. 1.0. A complete response was defined as the disappearance of all known lesions and the absence of new lesions. A partial response was defined as a reduction of ≥30 % in the sum of the maximum tumor lengths of up to 10 known lesions and the absence of new lesions. Stable disease was defined as a reduction of <30 % or an increase of <20 % in the sum of the maximum tumor lengths of up to 10 known lesions and the absence of new lesions. Progressive disease was defined as an increase of ≥20 % in the sum of the maximum tumor lengths of up to 10 known lesions or as the appearance of at least one new lesion. Treatment was continued until disease progression or unacceptable toxicity occurred or until the patient chose to discontinue treatment. All eligible patients were included in the response and survival analyses on an ‘intent-to-treat’ basis.
Statistical analysis
This study was conducted as a multicenter phase II trial. Several clinical trials reported a PFS of 2.5–3.9 months for FOLFIRI. The null hypothesis median PFS was 3.0 months, and the expected median PFS was 5.0 months. Registration was scheduled to continue for 24 months, and the patients were expected to be followed up for 6 months after the last registration. Assuming a one-sided alpha error of 0.05 and a beta error of 0.1, then 33 patients would be required. The number of patients was set at 37, taking into consideration the possible ineligibility or exclusion of patients from the analysis. The 95 % confidence intervals (CIs) for the response rates were estimated using the exact method. Cumulative proportions concerning survival were estimated using the Kaplan–Meier method, and the CIs were estimated using the Greenwood method. All statistical analyses were performed using the Stata version 10.1 software program (StataCorp, College Station, TX, USA).
Results
Patient characteristics
Between November 2011 and May 2013, a total of 37 patients were enrolled in this trial at 16 institutions in Japan. Two patients did not fulfill the eligibility criteria, and 1 patient was not treated. Thirty-four patients were assessed for response, and 36 patients were assessed for safety. The patient characteristics at study entry and those for eligible patients are listed in Table 1. The median follow-up time was 11.2 (range 3.35–21.39) months.
Efficacy
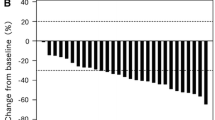
Tumor response is summarized in Table 2. On an intent-to-treat basis, the ORR was 20.6 % (95 % CI 8.7–37.9 %), and the disease control rate was 76.5 % (95 % CI 58.8–89.3 %). The median PFS was 5.6 months (95 % CI 3.8–7.0; Fig. 1). The median overall survival was 16.4 months (95 % CI 8.1–20.0; Fig. 2). In a subgroup of patients who had received prior chemotherapy with or without bevacizumab, the median PFS was 5.5 months (95 % CI 2.7–6.4) and 7.0 months (95 % CI 3.6–8.3), respectively (log-rank P = 0.2678), and the median OS was 16.4 months (95 % CI 7.7–20.0) and 17.5 months (95 % CI 5.1–21.3), respectively (log-rank P = 0.7996; Supplemental Fig. 1).
Dose intensity
The median relative dose intensities to the planned dose were 76.1 % for S-1, 78.8 % for irinotecan and 86.4 % for bevacizumab.
Toxicity
The safety population included 36 patients who completed at least one cycle of chemotherapy. Adverse events are summarized according to the worst grade per patient in Table 3. The most common grade 3 or 4 hematological adverse event was neutropenia (25.0 %), followed by anemia (16.7 %) and leukemia (11.1 %). Grade 3 or 4 non-hematologic toxicities included hypertension (30.6 %), anorexia (22.2 %), fatigue/malaise (16.7 %), and stomatitis (13.9 %). Most adverse events were grade 1 or 2 and manageable. One treatment-related death caused by gastrointestinal bleeding was reported.
Discussion
In this study, our results suggested that the combination of irinotecan and S-1 plus bevacizumab might be an efficient and tolerable second-line regimen for patients with oxaliplatin-refractory mCRC.
This study demonstrated a promising median PFS of 5.6 months, an ORR of 20.6 %, a median time to progression of 5.4 months, and a median survival time of 16.4 months. The most frequent non-hematologic toxicities were hypertension, fatigue/malaise, and anorexia. Most cases of gastrointestinal toxicity were grade 1 or 2, and good oral intake was maintained. To our knowledge, this is the first report regarding S-1 and irinotecan plus bevacizumab as second-line chemotherapy for mCRC.
The FIRIS study of S-1 plus biweekly irinotecan therapy as a second-line treatment for mCRC reported a median PFS of 5.8 months [9]. Moreover, in a subset analysis including patients who had previously received oxaliplatin-containing chemotherapy, better median PFS and OS rates were observed in the IRIS group than in the FOLFIRI group. Baba et al. [14] reported that oxaliplatin-resistant tumor cells displayed high excision repair cross-complementing group 1 and dihydropyrimidine dehydrogenase (DPD) levels and that the IRIS regimen in combination with a DPD-inhibitory fluoropyrimidine may have superior efficacy against tumors with high levels of DPD (e.g., tumors treated with oxaliplatin) compared with FOLFIRI.
There are no randomized data evaluating the effects of bevacizumab in combination with irinotecan-based chemotherapy in the second-line setting. Table 4 shows the results of phase II and III studies of second-line irinotecan-based chemotherapy plus bevacizumab after first-line therapy with oxaliplatin. It also shows the results of phase III studies with second-line irinotecan-based chemotherapy. Although different treatment schedules and doses of irinotecan were used, irinotecan-based combination chemotherapy plus bevacizumab appears to be more effective than irinotecan-based chemotherapy alone. Furthermore, the results of the current study, compared with the results of other second-line studies with bevacizumab, showed almost the same effect. However, PFS (5.6 months) was similar to the FIRIS study (5.8 months) despite the fact that bevacizumab was combined in the current study (although a cross-sectional comparison); the reason seems to be that this study adopted the lowest dose of irinotecan per week (50 mg/m2/week). Thus, further consideration is needed to determine the optimal dose of irinotecan for this regimen.
In the current study, to avoid the onset of diarrhea caused by the administration of irinotecan on day 15 within the S-1 plus triweekly irinotecan regimen, S-1 was administered orally for 2 weeks followed by a drug-free period of 1 week, and irinotecan was administered once every 3 weeks [8]. In our study, all grades of diarrhea were observed in 52.8 % of patients, compared to 55–80 % of patients for other biweekly regimens [7, 9, 15, 16]. Indeed, this triweekly regimen has a lower frequency of diarrhea than other biweekly regimens; thus, it may be superior concerning the balance of efficacy and toxicity, and it is considered suitable for outpatient treatment, thus improving patient convenience [8]. Oh et al. reported another regimen consisting of biweekly irinotecan in combination with S-1 for patients with oxaliplatin-refractory mCRC. The ORR was 20 %, and the disease control rate was 55 %. The median PFS and OS were 3.0 and 9.8 months, respectively. This biweekly regimen resulted in early study termination and modification because of treatment-related mortalities and a high follow-up loss rate [17].
Many studies have reported the safety of bevacizumab. Furthermore, a marked add-on effect of bevacizumab was reported for irinotecan-based regimens [18, 19]. Additionally, continued vascular endothelial growth factor inhibition with bevacizumab beyond the first progression for patients with mCRC has clinical benefits [20]. Adverse events specific for bevacizumab include hypertension, proteinuria, thrombosis, delayed wound healing, and gastrointestinal perforation. In this study, hypertension and thrombosis, which appeared to be caused by bevacizumab administration, were observed at similar levels as reported in other studies. Although serious adverse events such as gastrointestinal bleeding were observed in 1 patient, other serious adverse events that increased the risk of death, such as gastrointestinal perforation, were not observed. In addition, increased toxicity of S-1 or irinotecan attributable to its combination use with bevacizumab was also not observed.
In conclusion, the current study demonstrated that the combination of irinotecan and S-1 plus bevacizumab was efficient and safe as second-line chemotherapy for patients with oxaliplatin-refractory mCRC. The results of this study are extremely promising, and S-1 with irinotecan plus bevacizumab appears to be an appropriate candidate regimen for a phase III comparative study in the future.
Change history
14 November 2017
In the original publication, in Abstract, the sentence that reads as, “Oral S-1 at a dose of 80 mg/m2 was…………. drug-free interval” should read as, “Oral S-1 at a dose of 40 mg/m2 was administered twice daily for 2 weeks, followed by a 1-week drug-free interval.
References
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Shirasaka T, Shimamato Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drug 7:548–557
Miyamoto Y, Sakamoto Y, Yoshida N et al (2014) Efficacy of S-1 in colorectal cancer. Expert Opin Pharmacother 15:1761–1770
Goto A, Yamada Y, Yasui H et al (2006) Phase II study of combination therapy with S-1 and irinotecan in patients with advanced colorectal cancer. Ann Oncol 17:968–973
Yoshioka T, Kato S, Gamoh M et al (2009) Phase I/II study of sequential therapy with irinotecan and S-1 for metastatic colorectal cancer. Br J Cancer 101:1972–1977
Komatsu Y, Yuki S, Sogabe S et al (2012) Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol 51:867–872
Yamada Y, Yamaguchi T, Matsumoto H et al (2012) Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB) as first-line therapy for patients with metastatic colorectal cancer. Invest New Drug 30:1690–1696
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11:853–860
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23:2479–2516
Wagner AD, Arnold D, Grothey AA et al (2009) Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. doi:10.1002/14651858
Strickler JH, Hurwitz HI (2014) Palliative treatment of metastatic colorectal cancer: what is the optimal approach? Curr Oncol Rep 16:363
Welch S, Spithoff K, Rumble RB et al (2010) Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol 21:1152–1162
Baba H, Watanabe M, Okabe H et al (2012) Upregulation of ERCC1 and DPD expressions after oxaliplatin-based first-line chemotherapy for metastatic colorectal cancer. Br J Cancer 107:1950–1955
Komatsu Y, Yuki S, Sogabe S et al (2011) Phase II study of combined treatment with irinotecan and S-1 (IRIS) in patients with inoperable or recurrent advanced colorectal cancer (HGCSG0302). Oncology 80:70–75
Shiozawa M, Akaike M, Sugano N et al (2010) A phase II study of combination therapy with irinotecan and S-1 (IRIS) in patients with advanced colorectal cancer. Cancer Chemother Pharmacol 66:987–992
Oh SY, Ju YT, Choi SK et al (2011) Phase II study of irinotecan/S-1 combination chemotherapy for patients with oxaliplatin-refractory colorectal cancer. Invest New Drug 29:1050–1056
Saltz LB, Cox JV, Blanke C et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786
Bennouna J, Sastre J, Arnold D et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37
Suenaga M, Nishina T, Mizunuma N et al (2015) Multicenter phase II study of FOLFIRI plus bevacizumab after discontinuation of oxaliplatin-based regimen for advanced or recurrent colorectal cancer (CR0802). BMC Cancer 15:176
Tsutsumi S, Ishibashi K, Uchida N et al (2012) Phase II trial of chemotherapy plus bevacizumab as second-line therapy for patients with metastatic colorectal cancer that progressed on bevacizumab with chemotherapy: the Gunma Clinical Oncology Group (GCOG) trial 001 SILK study. Oncology 83:151–157
Bendell JC, Tournigand C, Swieboda-Sadlej A et al (2013) Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX-6 after failure of first-line therapy for metastatic colorectal cancer: a randomized phase II study. Clin Colorectal Cancer 12:239–247
Nakayama G, Uehara K, Ishigure K et al (2012) The efficacy and safety of bevacizumab beyond first progression in patients treated with first-line mFOLFOX6 followed by second-line FOLFIRI in advanced colorectal cancer: a multicenter, single-arm, phase II trial (CCOG-0801). Cancer Chemother Pharmacol 70:575–581
Iwamoto S, Takahashi T, Tamagawa H et al (2015) FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol 26:1427–1433
Suenaga M, Mizunuma N, Matsusaka S et al (2015) A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer. Drug Des Devel Ther 9:1653–1662
Kubicka S, Greil R, André T et al (2014) Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first disease progression (PD) in patients with metastatic colorectal cancer (mCRC) previously treated with BEV-based therapy: outcomes according to KRAS status and first-line CT backbone in the ML18147 study. J Clin Oncol 32(suppl 3):S520
Tabernero J, Yoshino T, Cohn AL et al (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 16:499–508
Van Cutsem E, Tabernero J, Lakomy R et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30:3499–3506
Peeters M, Price TJ, Cervantes A et al (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713
Acknowledgments
We thank all the patients and families who participated in this trial, and we are indebted to the physicians and all of the clinical study teams at the participating institutions. We also thank Ms. Sanae Sakamoto for her excellent secretarial assistance at the data center of Clinical Research Support Center Kyushu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y Miyamoto, H Tanioka, S Maekawa, H Kawanaka, M Kitazono, H Murakami, Y Ogata, H Saeki, M Shimokawa, S Natsugoe and Y Akagi have no conflict of interest. A Tsuji has received honoraria from Taiho Pharmaceutical. E Oki has received fees for promotional materials from Taiho Pharmaceutical, Yakult Honsha, Chugai Pharmaceutical and Merck Serono. Y Emi has received honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical and Yakult Honsha. H Baba has received research funding and honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo. Y Maehara has received research funding and honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Yakult Honsha and Daiichi Sankyo.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10147-017-1212-0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2015_943_MOESM1_ESM.ppt
Supplementary Fig. 1 Kaplan–Meier survival curve for progression-free survival (A) and overall survival (B) among patients receiving prior chemotherapy with or without bevacizumab. Bmab: bevacizumab (ppt 115 kb)
About this article
Cite this article
Miyamoto, Y., Tsuji, A., Tanioka, H. et al. S-1 and irinotecan plus bevacizumab as second-line chemotherapy for patients with oxaliplatin-refractory metastatic colorectal cancer: a multicenter phase II study in Japan (KSCC1102). Int J Clin Oncol 21, 705–712 (2016). https://doi.org/10.1007/s10147-015-0943-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0943-z